Abstract
Introduction:
Few published reports highlight intravenous tissue plasminogen activator use during the first trimester of pregnancy and provide outcomes for mother and fetus. Little guidance is available regarding body weight dosing of intravenous tissue plasminogen activator during pregnancy.
Methods:
Here, we present a patient who received intravenous tissue plasminogen activator in the emergency department during her first trimester of pregnancy for the treatment of an acute ischemic stroke. Outcomes are presented for mother and fetus, as well as discussion about the dosing weight utilized for the intravenous tissue plasminogen activator dose calculation.
Results:
A 35-year-old, Gravida 7 Para 6, presented to the emergency department at 9 weeks gestation with acute stroke symptoms. Her initial National Institutes of Health Stroke Scale was 7. Imaging revealed a hyperdense right middle cerebral artery sign. Intravenous tissue plasminogen activator was administered 57 min after her arrival and based on her actual body weight during pregnancy. Post tissue plasminogen activator imaging revealed recanalization of the vessel and the patient’s National Institutes of Health Stroke Scale was 0. The patient progressed to delivery of a healthy female infant. The patient did not experience any bleeding complications throughout pregnancy.
Conclusion:
We present positive outcomes of a mother and fetus after receipt of intravenous tissue plasminogen activator using actual body weight during the first trimester of pregnancy for an acute ischemic stroke. Additional information is necessary to provide recommendations for the application to future patients in early pregnancy.
Keywords: Ischemic, pregnancy, stroke, tissue plasminogen activator
Introduction
Pregnant patients were excluded from large clinical trials examining the use of intravenous (IV) tissue plasminogen activator (tPA) for acute ischemic stroke.1 Up to 58% of pregnant or postpartum patients with acute ischemic stroke do not receive IV tPA due to pregnancy itself as a reported exclusion.2 Few published reports highlight IV tPA use in early pregnancy and provide outcomes for mother and fetus. Furthermore, little guidance is available regarding body weight dosing of IV tPA during pregnancy. Here, we present a patient who received IV tPA in the emergency department (ED) during her first trimester of pregnancy for the treatment of an acute ischemic stroke. Outcomes are presented for mother and fetus, as well as discussion about the dosing weight utilized for the IV tPA dose calculation.
Case report
A 35-year-old, Gravida 7 Para 6, presented to the ED at 9 weeks gestation after losing balance and being unable to move the left side of her body. Past medical history included pre-eclampsia, gestational diabetes, depression, and history of postpartum hemorrhage requiring blood transfusion. Her medications included prenatal vitamins and acetaminophen, and she denied using any tobacco products. On presentation, blood pressure was 133/83 mmHg and labs and other vital signs were within normal limits. Immediate computed tomography (CT) imaging revealed a hyperdense right middle cerebral artery (MCA) sign ischemic infarct (Figure 1). Per family and patient, left upper and lower extremity weakness improved upon presentation to the ED, and left facial droop and left visual field deficits persisted. The neurology consultant did not appreciate limb weakness or ataxia. Her National Institutes of Health Stroke Scale (NIHSS) was 7 and Glasgow Coma Scale (GCS) was 15. Scoring for NIHSS included complete hemianopia (2 points), partial facial paralysis (2 points), mild-to-moderate dysarthria (1 point), and profound hemi-inattention or extinction to more than one modality and does not recognize own hand or orients to one side of space (2 points). Prior to symptoms, her Modified Rankin Scale (mRS) was 0.
Figure 1.

CT without contrast of the brain completed upon presentation to the emergency department. Hyperdense right middle cerebral artery sign consistent with a right middle cerebral artery ischemic infarct.
IV tPA 0.9 mg/kg was administered based on her actual body weight (63.6 kg) as 10% bolus and 90% by infusion over 60 min, initiated 87 min after her last known normal and 57 min after her arrival to the ED. Head CT angiography identified a filling defect in the right MCA M1 segment (Figure 2). On admission, further magnetic resonance imaging (MRI) without contrast approximately 5 h post-tPA showed interval recanalization of the right MCA M1 segment and no acute hemorrhage (Figure 3). Diffusion weighted imaging (DWI) MRI showed only faint hyperintense DWI signal involving the right insula (Figure 4). Hypercoagulable workup returned unremarkable, including homocysteine, antiphospholipid antibodies, prothrombin 20210, factor V Leiden, methylene tetrahydrofolate reductase, antithrombin III, protein C, and protein S. A laboratory D-dimer was elevated at 39.43 mg/L, and subsequent lower extremity ultrasound showed no significant findings. A transthoracic echocardiogram with bubble study showed a right to left shunt. Ultimately, the etiology of her stroke was unclear and classified as cryptogenic. The patient was discharged on post-stroke day 3 with an NIHSS of 0 and mRS of 1. She was prescribed aspirin 325 mg daily and enoxaparin 1 mg/kg subcutaneous every 12 h for the duration of her pregnancy.
Figure 2.

CT angiography of the brain completed during the administration of intravenous tissue plasminogen activator. Filling defect in the right distal middle cerebral artery (MCA) M1 segment. The superior division of the right MCA is not seen.
Figure 3.
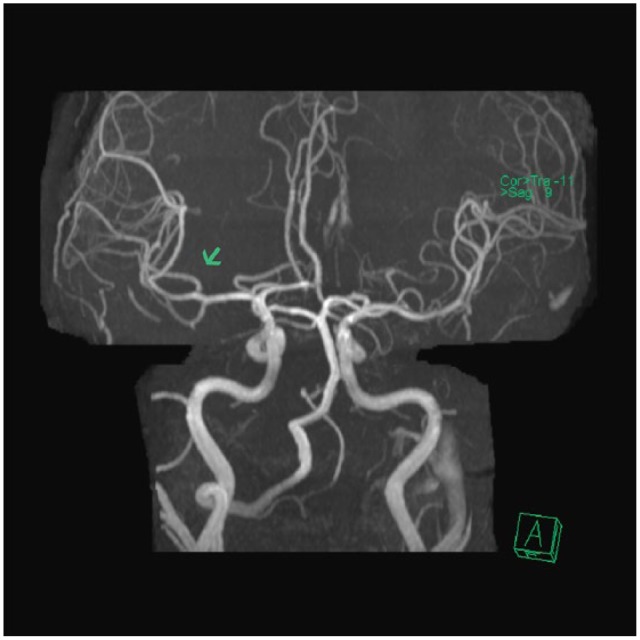
MRI without contrast of the brain completed approximately 5 h after the administration of intravenous tissue plasminogen activator. There is interval recanalization of the right distal most middle cerebral artery (MCA) M1 segment and of the superior division of the right MCA.
Figure 4.

MRI including diffusion weighted imaging (DWI) of the brain completed approximately 5 h after the administration of intravenous tissue plasminogen activator. Only faint hyperintense DWI signal involving the right insula. No large territorial infarct.
Residual stroke symptoms persisted during pregnancy, including weakness of the left arm and increased salivation and drooling, but NIHSS and mRS remained 0 and 1, respectively. The patient required iron therapy for anemia and routine studies revealed gestational thrombocytopenia with nadir of 79 K/µL platelets. Anemia and thrombocytopenia were thought to be unrelated to receipt of IV tPA. The hematologist consultant recommended stopping aspirin and changing enoxaparin to heparin 2 weeks prior to scheduled induction of labor. The pregnancy was otherwise uncomplicated.
The patient presented at 39 weeks and 3 days for induction of labor with a Bishop score of 4. On ultrasound, fetus was found to be in transverse position, and external cephalic version was attempted and successful. Induction with misoprostol 1000 mcg per rectum was started, and she progressed to vaginal delivery of a live female infant with a weight of 3600 g and Apgar score of 9 at 1 and 5 min after birth. Baby required phototherapy for hyperbilirubinemia, but otherwise, baby and patient recovered well. The patient was discharged on prophylactic enoxaparin and aspirin for 6 weeks with hematology follow-up. On discharge, NIHSS and mRS remained 0 and 1, respectively. At 4 months post-partum, the patient reported no new complications and baby was doing well.
Discussion
To our knowledge, this is the first case report describing tPA and the body weight used for dosing during pregnancy and adds to the limited literature reporting outcomes for both mother and fetus when IV tPA is administered during the first trimester for acute ischemic stroke. In a previous case report, a patient at gestational week 12 had good outcomes along with good fetal outcomes, but experienced a hemorrhagic infarction post IV tPA.3 In addition, Murugappan et al.4 published a case series with three patients who received IV tPA and were in their first trimester of pregnancy. The first patient had a minor complication (intrauterine hematoma), the second patient had no complications, and the third patient died from dissection during angioplasty. In the first two cases, pregnancies were medically terminated, and the latter resulted in fetal death. Hirano5 completed a review and reported five additional patients with good outcomes for mother and fetus; however, all patients were past their first trimester of pregnancy. More recently, a large study analyzed the outcomes of thrombolytic therapy from the “Get with the Guidelines-Stroke” registry from 2008 to 2013.2 Of the 338 pregnant or postpartum patients with ischemic stroke, 7 patients received tPA monotherapy during pregnancy. Details regarding pregnancy term and fetal outcomes were not provided. In contrast to previous literature, our case resulted in successful outcomes for mother and fetus after receiving IV tPA during the first trimester of pregnancy without bleeding complications.
The use of pre-pregnancy versus actual body weight presented a challenge when calculating IV tPA for our patient. Previously published literature acknowledges IV tPA dosed as 0.9 mg/kg in pregnancy; however, previous literature is silent on the body weight used (e.g. pre-pregnancy versus pregnancy) for IV tPA dosing despite significant weight changes throughout pregnancy.2–5 Women are expected to gain 5–18 kg of body weight throughout pregnancy.6 This leads to uncertainty regarding the correct weight to use for dosing calculations. Furthermore, plasma volume increases by 42% during pregnancy and increases the volume of distribution of drugs.7 The volume of distribution of tPA is similar to that of plasma.8 Therefore, theoretically the volume of distribution of tPA will also increase during pregnancy.
In the patient presented, the body weight was 6.9 kg higher at the time of event than the last documented pre-pregnancy weight. The actual body weight was utilized for dosing calculations which equated to a 6.2% increase in dose compared to the pre-pregnancy weight. Actual body weight was utilized in this case to reflect the increase in plasma volume. In patients with more drastic weight differences during pregnancy, it is unknown if actual, adjusted, or pre-pregnancy body weight should be utilized for dosing calculations of IV tPA. The theoretical risk of bleeding with higher doses should be taken into consideration.
Conclusion
Without randomized controlled trials regarding the use of IV tPA for the treatment of ischemic stroke during pregnancy, the acute management must be individualized. The benefits and risks should be carefully discussed with all parties on the medical team as well as the patient and caregivers. We present positive outcomes of a mother and fetus after receipt of IV tPA using actual body weight during the first trimester of pregnancy for an acute ischemic stroke. Additional information is necessary to provide recommendations for the application to future patients in early pregnancy.
Acknowledgments
We would like to acknowledge Benjamin Bienia and Victor D. Nguyen for assistance with radiological imaging selection.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Gary D Peksa  https://orcid.org/0000-0003-3625-8137
https://orcid.org/0000-0003-3625-8137
References
- 1. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 2. Leffert LR, Clancy CR, Bateman BT, et al. Treatment patterns and short-term outcomes in ischemic stroke in pregnancy or postpartum period. Am J Obstet Gynecol 2016; 214: 723.e1–723.e11. [DOI] [PubMed] [Google Scholar]
- 3. Dapprich M, Boessenecker W. Fibrinolysis with alteplase in a pregnant woman with stroke. Cerebrovasc Dis 2002; 13(4): 290. [DOI] [PubMed] [Google Scholar]
- 4. Murugappan A, Coplin WM, Al-Sadat AN, et al. Thrombolytic therapy of acute ischemic stroke during pregnancy. Neurology 2006; 66(5): 768–770. [DOI] [PubMed] [Google Scholar]
- 5. Hirano T. Acute revascularization therapy in pregnant patients. Neurol Med Chir 2013; 53(8): 531–536. [DOI] [PubMed] [Google Scholar]
- 6. IOM (Institute of Medicine) and NRC (National Research Council). 2009. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 7. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7): 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alteplase (package insert). San Francisco, CA: Genentech, 1987. [Google Scholar]


