Abstract
Background:
Tumor-associated tissue eosinophilia is defined as an inflammatory response with the marked infiltration of eosinophils within tumor tissues. Tumor-associated tissue eosinophilia has been reported in various organs; however, no studies have examined the detailed cytopathological findings of tumor-associated tissue eosinophilia.
Case Presentation:
A 49-year-old woman presented with lower abdominal and back pain that had started 1 month earlier. A cervical biopsy revealed a diagnosis of non-keratinizing squamous cell carcinoma. A mildly increased number of eosinophils was observed in both cervical cytology and a biopsy. On pelvic computed tomography, a tumor mass measuring up to 5.5 cm in the largest diameter was seen in the uterine cervix. After 1 month, endometrial cytology was performed, and non-keratinizing squamous cell carcinoma together with normal endometrial glands was obtained in a background of marked eosinophil numbers. Tumor cells in an irregular-shaped solid nest had variable-sized hyperchromatic nuclei and light-green-stained cytoplasm. The number of eosinophils was obviously increased. Considering the possibility of tumor-associated tissue eosinophilia, we evaluated a peripheral blood sample and confirmed an increased number of eosinophils. Radical hysterectomy was performed, and the final pathological diagnosis was adenosquamous carcinoma. Although the number of eosinophils decreased after surgery, it increased again at the time of recurrence 1 year later. Chemo-irradiation was performed, but the patient died 1 year and 8 months after the operation.
Conclusion:
Cytopathologists should consider the presence of tumor-associated tissue eosinophilia by focusing on not only tumor cells but also the markedly eosinophilic background. The eosinophil count might be a useful marker of the disease activity.
Keywords: Non-keratinizing, squamous cell carcinoma, adenosquamous carcinoma, uterine cervix, eosinophilia, tumor-associated tissue eosinophilia
Background
Increased numbers of blood eosinophils, termed eosinophilia, is defined as a blood eosinophil count exceeding 500 μL−1.1 Causes of eosinophilia are classified into intrinsic and extrinsic eosinophilic disorders.2 Intrinsic causes include leukemias and myeloproliferative neoplasms affecting stem cells, while extrinsic causes include various diseases, such as allergic diseases, autoimmune diseases, infectious diseases, graft-versus-host diseases, immunodeficiencies, clonal T cell diseases, drug-induced diseases, Hodgkin’s lymphomas, cutaneous T cell lymphomas, acute lymphoblastic leukemias, Langerhans cell histiocytosis, and epithelial cancers.
Tumor-associated tissue eosinophilia (TATE) is defined as an inflammatory response with the marked infiltration of eosinophils within tumor tissues.3 This term was first proposed in 1896 by Przewoski in carcinoma of cervix-induced TATE. It is characterized by the marked infiltration of eosinophils within the intra- or para-tumor area. TATE has been reported in various organs, including the oral cavity, esophagus, larynx, bile duct, lung, gastrointestinal tract, genitourinary tract, and other rare sites.3–11 Many reported cases of TATE are often associated with squamous cell carcinoma (SqCC), but there are also reports of adenocarcinoma-, urothelial carcinoma-, and large cell carcinoma-induced TATE. To our knowledge, however, no studies have examined the detailed cytological findings of TATE.
We herein report the first case of primary adenosquamous carcinoma of the uterine cervix in which the existence of TATE was confirmed by cytopathological findings.
Case presentation
A 49-year-old Japanese woman (gravida 2 para 1) was admitted to another hospital due to lower abdominal and back pain that had started 1 month earlier. She had a history of asthma since the age of 22 years, but there was no increase in the number of eosinophils. Liquid-based cytology of the uterine cervix was performed, and she was diagnosed with atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H). A cytological examination of the tumor cells revealed irregular cell distances, irregular-shaped nuclei, nuclear anisocytosis, and dense chromatin. In this specimen, a mildly inflammatory background was pointed out, but no marked increase in the number of eosinophils was noted.
The first biopsy was taken from the uterine cervix, resulting in a diagnosis of non-keratinizing SqCC. Mild eosinophilic infiltration was observed in the tumor stroma. After 1 month, she was admitted to our hospital. Pelvic computed tomography (CT) revealed a tumor mass lesion, measuring up to 5.5 cm in the largest diameter, in the uterine cervix. The wall of the cervix was thickened, and the demarcation between the mass and the intrapelvic organs, including the urinary bladder and rectum, was unclear (Figure 1). A second cervical biopsy was performed, resulting in a diagnosis of non-keratinizing SqCC with marked infiltration of eosinophils. Endometrial cytology revealed pleomorphic non-keratinizing SqCC together with normal endometrial glands in a background of marked eosinophil numbers. A few keratinizing SqCC cells were also seen, but no mucous glands were observed. The number of eosinophils was obviously increased (>100 per high-powered field; Figure 2). Considering the possibility of TATE, we asked a clinician to measure the eosinophil count. The number of eosinophils in the peripheral blood was extremely high (643 μL−1), although the other laboratory data were within normal limits. Radical hysterectomy with bilateral salpingo-oophorectomy was performed, and the number of eosinophils drastically decreased after surgery (6 μL−1,Table 1). Macroscopically, the cervical mucosa revealed a rough surface. The cut surface of the tumor looked light tan to yellowish-white and solid, penetrating the cervical wall. Histologically, the tumor was predominantly composed of non-keratinizing SqCC with stromal invasion (Figure 3(a)). However, obvious gland formation was recognized in some tumor tissues. High-grade squamous intraepithelial lesion (HSIL) was slightly spread around the invasive cancer. Although koilocytosis suggestive of human papilloma virus (HPV) infection was not observed, HPV type 68 was detected. The tumor cells had cohesive or cord-like cell nests, oval to polygonal in shape, with eosinophilic cytoplasm. Individual keratinization and mucous cells were found in a small portion of the tumor cell nests (5%). The cytoplasm of these mucous cells was positive for mucicarmine staining. Marked eosinophilic infiltration was observed not only in the tumor stroma but also in the invasive tumor cell nests or in HSIL (Figure 3(b)). CEA (DAKO, diluted 1:4000), MUC1 (Leica, diluted 1:1000), and p63 (NICHIREI, diluted 1:1) were positive for tumor cells. FOXP3 (Abcam, diluted 1:100)-positive regulatory T cells (Treg) were also recognized in the tumor stroma. Furthermore, both lymphatic and venous permeation of the SqCC was noted, and a micro-metastatic lesion was found in the left external iliac lymph node. CRTC1-MAML2 or CRTC3-MAML2 fusion genes were not detected. We diagnosed this case as adenosquamous carcinoma of the uterine cervix displaying TATE. The clinical stage of this case was evaluated as FIGO IIB.
Figure 1.
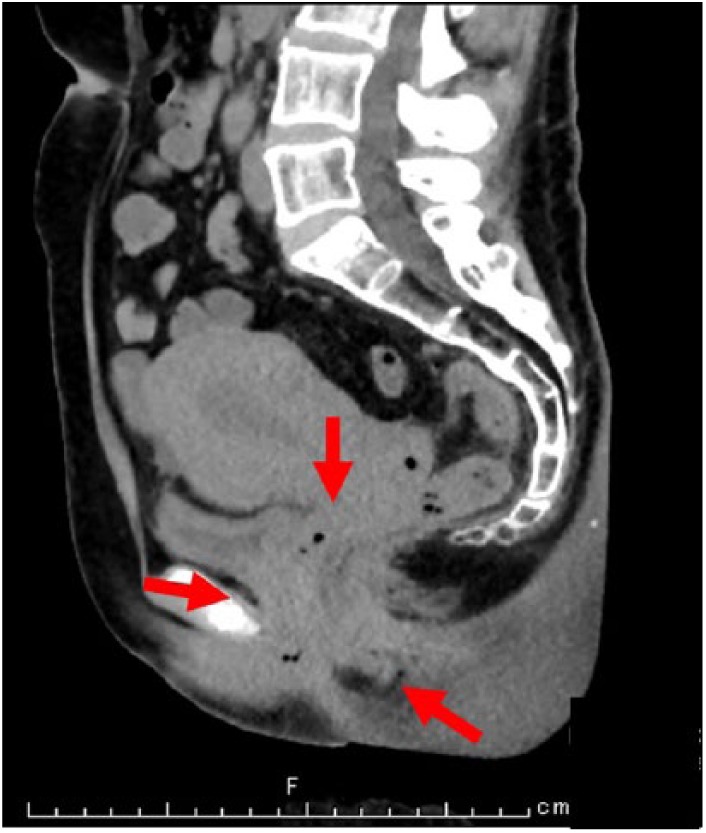
CT in the sagittal plane. The wall of the cervix was thickened, and the demarcation between the mass and the intrapelvic organs, including urinary bladder and rectum, was unclear (arrow).
Figure 2.
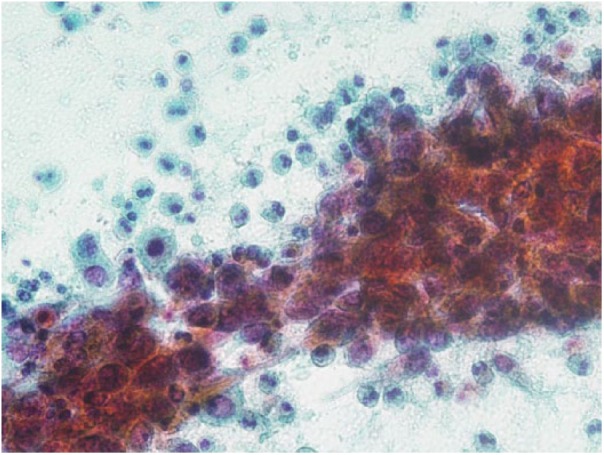
Cytology of the endometrium. Atypical squamous cells revealing round to oval nuclei with coarse chromatin and prominent nucleoli. Many eosinophils are seen in the background (Pap. staining, 400×).
Table 1.
Clinical course of present case.
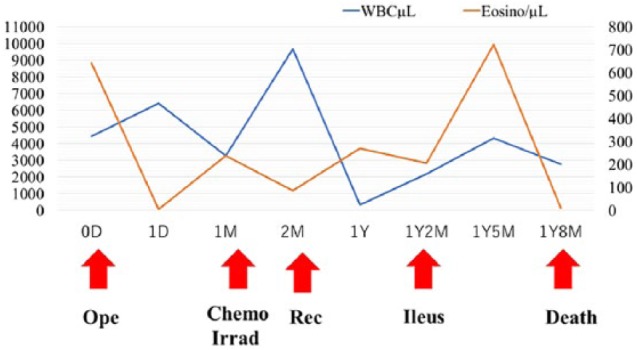
|
WBC: white blood cell; Eosino: eosinophil; Ope: operation; ChemoIrrad: chemotherapy and irradiation; Rec: recurrence; D: day; M: month; Y: year.
Figure 3.
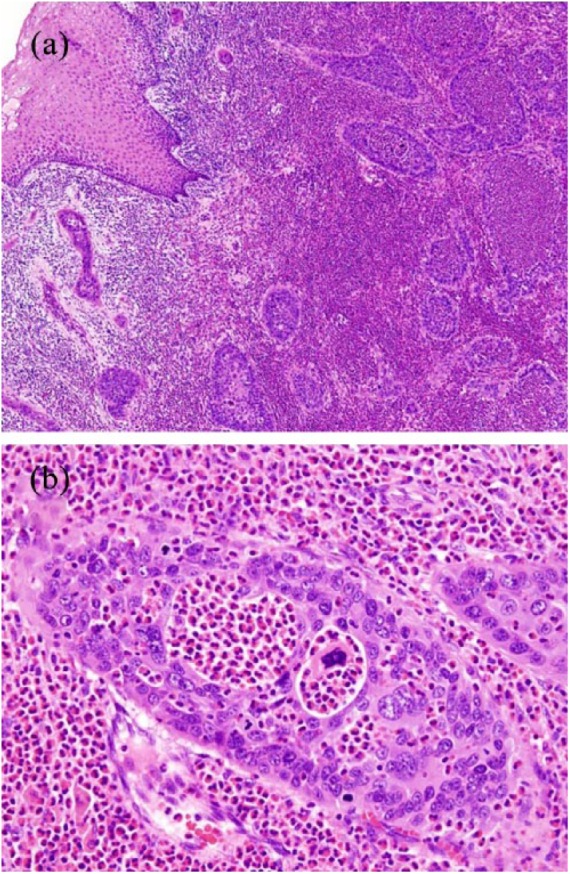
Histological findings of the uterine cervix. (a) Tumor cell nests showing stromal invasion (H&E staining, 20×).
(b) Clusters of adenocarcinomatous cells showing prominent infiltration of eosinophils (H&E staining, 200×).
After 1 month of surgery, the number of eosinophils increased again (240 μL−1, Table 1). Chemo-irradiation was started, and the number decreased (87 μL−1, Table 1). However, it increased again at the time of recurrence 1 year later (270 μL−1, Table 1). Recurrence of adenosquamous carcinoma accompanied by marked eosinophil infiltration was observed at the vaginal stump. After 1 year and 2 months, the patient developed ileus, and ileotransversostomy was performed. The best supportive care was performed, but the patient ultimately died 1 year and 8 months after the operation (Table 1).
Discussion
This case report showed histopathological findings of adenosquamous carcinoma with TATE of the uterine cervix. Furthermore, we were able to detect TATE based on the cytological findings with a remarkable eosinophilic background.
Cytologically, non-keratinizing SqCC conventionally appears as a cluster on a dirty background, such as one of neutrophils, red blood cells, proteinaceous necrotic material, and cytoplasmic debris. However, the cytological findings of non-keratinizing SqCC with TATE in this case were observed not on a dirty background but rather an inflammatory background, with prominent eosinophils. However, our final pathological diagnosis in surgical specimens was adenosquamous carcinoma with marked eosinophil infiltration. The eosinophil count in the peripheral blood was high, and it increased or decreased according to tumor progression, recurrence, and treatment.
The differential diagnoses in this case were mucoepidermoid carcinoma (MEC), adenosquamous carcinoma, and glassy cell carcinoma. In the World Health Organization (WHO) classification, cervical MEC is merely described as a distinctive entity among adenosquamous carcinoma.12 MEC consists of three cell components (epidermoid, mucin-producing, and intermediate), and MEC with eosinophilia has been reported in the thyroid gland, minor salivary gland, and esophagus.13–15 MEC with chromosomal translocation t(11;19)-associated CRTC1-MAML2 gene fusion was found in the salivary gland. It was also identified in cervical MEC, but not in cervical adenosquamous carcinoma.16 Adenosquamous carcinoma is defined as an epithelial malignant tumor comprising both adenocarcinoma and SqCC by WHO classification.12 There should be sufficient differentiation of the adenocarcinomatous component to include histologically recognizable glands.12 Glassy cell carcinoma is defined as poorly differentiated variant of adenosquamous carcinoma. It is characterized by cells with sharp cytoplasmic margins, “ground glass” appearing eosinophilic cytoplasm, and large round to ovoid nuclei with prominent nucleoli.12 In our case, CRTC1-MAML2 fusion genes were not detected. The tumor had obvious glandular formation and non-keratinizing squamous carcinoma components. Mucin-producing cells were sparsely observed, and CEA and MUC1 were positive for tumor cells. In addition, tumor cells had no sharp cytoplasmic margins or ground glass appearing. Therefore, we considered the histological subtype of this case as adenosquamous carcinoma.
The prognostic significance of TATE remains controversial. The increased number of tissue eosinophils has been reported to play an anti-tumoral role, suggesting that a good prognosis can be obtained.17–19 However, TATE is considered a poor prognostic factor, showing a tumor-progressive role.20–22 Yellapurkar et al.3 suggested a possible mechanism of TATE and indicated that the immune response of the tumor stroma differed markedly between early- and advanced-stage cancer. In early-stage cancer, a Type 1 helper cell (Th1)-dominant immune reaction is caused. Th1 cells produce various chemokines, such as interleukin-2 (IL-2) and interferon gamma (IFN-γ). These chemokines induce the release of eotaxin, which shows strong chemotaxis for eosinophils, and promote a tumoricidal effect.23 However, in advanced-stage carcinoma, the tumor cells themselves produce IL-4 and IL-10, which leads to a Th2-dominant immune reaction and immune system inhibition. Th2 cells produce not only IL-4 with an anti-apoptotic effect but also IL-13, which suppresses the activity of IFN-γ and CD8+ cytotoxic T lymphocyte. IL-4 and IL-13 are also inducers of eotaxin, which is considered to cause eosinophilia. Furthermore, transforming growth factor-β (TGF-β), basic fibroblast growth factor and angiogenic factor are supplied to carcinoma cells by eosinophils, and the proliferation of carcinoma cells is promoted.24 Therefore, TATE in early cancer is expected to have a good prognosis, whereas that in advanced cancer has a poor prognosis. In our case, the tumor showed progressive growth with the patient’s unfortunate outcome. In addition, FOXP3, a master transcription factor of Tregs, was expressed in the tumor stroma. Based on these findings, we believe that the immune system was suppressed by a Th2-dominant immune reaction.
In conclusion, we herein report the first case of primary adenosquamous carcinoma of the uterine cervix in which the existence of TATE was confirmed by cytopathological findings. Cytopathologists should consider the presence of TATE by focusing on not only tumor cells but also the markedly eosinophilic background. The eosinophil count might be a useful marker of the disease activity.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iDs: Nozomu Kurose  https://orcid.org/0000-0003-3626-4633
https://orcid.org/0000-0003-3626-4633
Sohsuke Yamada  https://orcid.org/0000-0003-2662-0024
https://orcid.org/0000-0003-2662-0024
References
- 1. Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol 2010; 126(1): 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol 2007; 119: 1291–1300. [DOI] [PubMed] [Google Scholar]
- 3. Yellapurkar S, Natarajan S, Boaz K, et al. Tumour-associated tissue eosinophilia in oral squamous cell carcinoma- a boon or a bane? J Clin Diagn Res 2016; 10: ZC65–ZC68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishibashi S, Ohashi Y, Suzuki T, et al. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res 2006; 26(2B): 1419–1424. [PubMed] [Google Scholar]
- 5. Etit D, Yardim BG, Arslanoglu S, et al. Tumor-associated tissue eosinophilia as a prognostic factor in squamous cell carcinoma of the larynx. Indian J Otolaryngol Head Neck Surg 2014; 66(Suppl. 1): 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tirotta D, Poli L, Durante V. Severe eosinophilia associated with cholangiocarcinoma. J Community Support Oncol 2016; 14(4): 173–177. [DOI] [PubMed] [Google Scholar]
- 7. Caruso RA, Fedele F, Parisi A, et al. Chronic allergic-like inflammation in the tumor stroma of human gastric carcinomas: an ultrastructural study. Ultrastruct Pathol 2012; 36(3): 139–144. [DOI] [PubMed] [Google Scholar]
- 8. Kodama T, Takada K, Kameya T, et al. Large cell carcinoma of the lung associated with marked eosinophilia. Cancer 1984; 54(10): 2313–2317. [DOI] [PubMed] [Google Scholar]
- 9. Marusic Z, Cupic H, Kruslin B, et al. Tumor-associated tissue eosinophilia and inflammation in pTa and pT1 papillary urothelial carcinoma of the bladder. Anal Quant Cytol Histol 2009; 31(4): 239–241. [PubMed] [Google Scholar]
- 10. Lowe DG. Carcinoma of the cervix with massive eosinophilia. Br J Obstet Gynaecol 1988; 95(4): 393–401. [DOI] [PubMed] [Google Scholar]
- 11. Lowe DG, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol 1981; 34(12): 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO classification of tumours of female reproductive organs. 4th ed Lyon: IARC Press, 2014, p. 194. [Google Scholar]
- 13. Quiroga-Garza G, Lee JH, El-Naggar A, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: more aggressive than previously reported. Hum Pathol 2015; 46(5): 725–731. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi Y, Satoh K, Aizawa T, et al. Local recurrence of sclerosing mucoepidermoid carcinoma with eosinophilia in the upper lip: a case report. J Med Case Rep 2015; 9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MewaKinoo S, Maharaj K, Singh B, et al. Primary esophageal sclerosing mucoepidermoid carcinoma with “tissue eosinophilia.” World J Gastroenterol 2014; 20(22): 7055–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lennerz JK, Perry A, Mills JC, et al. Mucoepidermoid carcinoma of the cervix: another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol 2009; 33(6): 835–843. [DOI] [PubMed] [Google Scholar]
- 17. Debta P, Debta FM, Chaudhary M, et al. Evaluation of prognostic significance of immunological cells (Tissue eosinophil and mast cell) infiltration in oral squamous cell carcinoma. J Cancer Sci Ther 2011; 3: 201–204. [Google Scholar]
- 18. Dorta RG, Landman G, Kowalski LP, et al. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology 2002; 41(2): 152–157. [DOI] [PubMed] [Google Scholar]
- 19. Horiuchi K, Mishima K, Ohsawa M, et al. Prognostic factors for well-differentiated squamous cell carcinoma in the oral cavity with emphasis on immunohistochemical evaluation. J Surg Oncol 1993; 53(2): 92–96. [DOI] [PubMed] [Google Scholar]
- 20. Van Driel WJ, Hogendoorn PC, Jansen FW, et al. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol 1996; 27(9): 904–911. [DOI] [PubMed] [Google Scholar]
- 21. Wong DT, Bowen SM, Elovic A, et al. Eosinophil ablation and tumor development. Oral Oncol 1999; 35(5): 496–501. [DOI] [PubMed] [Google Scholar]
- 22. Agarwal A, Rani M, Saha GK, et al. Disregulated expression of the Th2 cytokine gene in patients with intraoral squamous cell carcinoma. Immunol Invest 2003; 32(1–2): 17–30. [DOI] [PubMed] [Google Scholar]
- 23. Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother 2004; 53(2): 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobsen EA, Helmers RA, Lee JJ, et al. The expanding role(s) of eosinophils in health and disease. Blood 2012; 120(19): 3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]


