Short abstract
Objective
Brain atrophy has been correlated with objective cognitive dysfunction in multiple sclerosis but few studies have explored self-reported subjective cognitive concerns and their relationship to brain volume changes. This study explores the relationship between subjective cognitive concerns in multiple sclerosis and reduced brain volume in regions of interest implicated in cognitive dysfunction.
Methods
A total of 158 patients with multiple sclerosis completed the Quality of Life in Neurologic Disorders Measures (Neuro-QoL) short forms to assess subjective cognitive concerns and underwent brain magnetic resonance imaging. Regional brain volumes from regions of interest implicated in cognitive dysfunction were measured using NeuroQuant automated volumetric quantitation. Linear regression was used to analyze the relationship between subjective cognitive concerns and brain volume.
Results
Controlling for age, disease duration, gender, depression and fatigue, increased subjective cognitive concerns were associated with reduced thalamic volume (standardized β = 0.223, t150 =2.406, P = 0.017) and reduced cortical gray matter volume (standardized β = 0.240, t150 = 2.777, P = 0.006). Increased subjective cognitive concerns were not associated with any other regions of interest that were analyzed.
Conclusions
Subjective cognitive concern in MS is associated with reduced thalamic and cortical gray matter volumes, areas of the brain that have been implicated in objective cognitive impairment. These findings may lend neuroanatomical significance to subjective cognitive concerns and patient-reported outcomes as measured by Neuro-QoL.
Keywords: Subjective cognitive concern, multiple sclerosis, gray matter, metacognition, quantitative MRI, atrophy
Introduction
Cognitive dysfunction has long been recognized as one of the prominent disabling sequelae of multiple sclerosis (MS). The prevalence of cognitive dysfunction ranges from 43% to 70% and can be present even in the early stages of the disease.1 Patients with MS experience cognitive dysfunction in a number of domains, most prominently in attention,2 visual and verbal memory and processing speed.3 While the exact mechanism of cognitive dysfunction in MS is not yet known, brain atrophy is increasingly recognized as a marker of MS disease progression and severity, likely reflecting ongoing degeneration in both white and gray matter.4 Brain volume loss in patients with MS has been shown to occur at a faster rate than in healthy controls. Estimates of average brain volume loss in a normal adult range from 0.1% to 0.3% annually while brain volume loss in an untreated patient with MS is estimated at 0.7% annually.5
Brain volume loss in MS has been shown to correlate with worsening of disability as assessed by a number of clinical scales.6 Whole brain volume and regional brain volume loss have also been shown to correlate with cognitive decline in MS as assessed by the cognitive elements of a number of disability scales and neuropsychological tests.7 Various brain regions have been identified as regions of interest (ROIs) related to cognitive dysfunction in MS including cortical gray8 and white matter,7 thalami,9 basal ganglia,10 amygdalae and hippocampi.11
Subjective cognitive concerns (SCCs),12 previously called subjective cognitive complaints or memory complaints, are a self-reported perception of dysfunction in memory or thinking with or without impairment on objective cognitive testing.13 SCC in the absence of objective impairment on neuropsychological testing has been termed subjective cognitive decline (SCD) and is recognized as a preclinical stage of mild cognitive impairment and dementia.14 SCD has been explored most extensively in Alzheimer dementia in which it has been shown to be a predictor of progression to dementia14 and to correlate with neuroanatomical changes including reduced hippocampal volumes,15 but little is known about the clinical and pathological relevance of SCC and SCD in MS. Some studies have shown a correlation between SCC in MS and objective cognitive impairment, especially in cases of mild impairment of immediate recall and processing speed,16 while other studies have shown a gap between subjective reports and objective testing and a stronger correlation of SCC with depression17 and fatigue.18
Our aim is to explore whether SCCs can relate to reduced brain volumes in MS, in particular, if the patient’s experience of dysfunction is associated with pathological changes in the brain. A number of different measurement tools have been used to assess self-reported SCC in MS including the cognitive elements of the Multiple Sclerosis Quality of Life-54,17 the Cognitive Failures Questionnaire,18 the Perceived Deficits Questionnaire,16 the Multiple Sclerosis Neuropsychological Screening Questionnaire (MSNQ)19 and the Cognitive Function Scale.20 Given the prevalence of standardized patient-reported outcomes (PROs) for assessing the patient’s subjective experience of illness as well as their use in clinical trials in MS,21 we relied on PROs reflecting cognition from the Quality of Life in Neurological Disorders (Neuro-QoL) measures in our study. Neuro-QoL is a widely used, National Institute of Neurological Disorders and Stroke (NINDS)-funded, self-report battery of short form questionnaires that is completed by patients to help assess various aspects of quality of life as related to neurological disease,22 has been validated in MS23 and employed to assess self-report SCCs in other research protocols.24 We hypothesized that PROs assessed by Neuro-QoL reflecting perceived cognitive dysfunction in patients with MS would be associated with regional brain volume loss in ROIs, such as the thalami, basal ganglia, amygdalae and hippocampi, as measured by automated volumetric quantitation from standard of care magnetic resonance imaging (MRI).
Methods
Participants
De-identified PROs and NeuroQuant volumetric data were gathered by retrospective chart review. All patients were seen at the Rocky Mountain MS Center between May 2014 and October 2016 and had a diagnosis of MS made by neuro-immunology trained faculty following McDonald criteria. As part of standard of care, all patients completed a battery of PROs, including those related to upper and lower mobility, mood, cognitive concerns, and other disease-related symptoms. Standard of care quantitative MRIs were also performed in each patient, and automated volumetric quantitation was performed using NeuroQuant software. For purposes of the current analysis, we reviewed records of patients who underwent quantitative brain MRIs within 90 days of completing the Neuro-QoL short forms. Research studies using the clinical database of de-identified PROs and NeuroQuant volumetric data were approved under Colorado Multiple Institutional Review Board #14-0394. All patients who were seen at the Rocky Mountain MS Center between May 2014 and October 2016, met criteria for diagnosis of MS, completed PROs and had NeuroQuant MRI within 90 days of completing PROs were included in the study.
Assessing SCCs
SCCs were assessed using Neuro-QoL, which includes short forms on anxiety, depression, fatigue, upper and lower extremity functions, applied cognition including executive function and general concerns, emotional and behavioral dyscontrol, positive affect and wellbeing, sleep disturbance, social participation and satisfaction and disease stigma. Specific questions per domain are rated by patients on a five-point scale (e.g. ‘never’ to ‘very often’). Raw scores from the ‘Applied Cognition: General Cognitive Concerns’ (GCC) short form were used as a marker of SCC. The GCC short form includes eight questions related to applied cognition such as ‘my thinking was slow’, or ‘I had trouble thinking clearly’ with a total raw score ranging from 8 to 40. This section has been shown in a previous analysis to be a strong factor that accounts for much of the variance in Neuro-QoL responses for our samples,25 and assesses for subjective concerns in processing speed and working memory. As a comparator, we also analyzed scores from the ‘Lower Extremity Function (Mobility)’ (LEF) short form. Despite overlap in ROIs related to physical disability and cognitive impairment in MS, we used this domain for comparison to allow added specificity in testing our primary hypothesis. This short form also includes eight questions answered with a five-point scale for lower extremity functions, a 1 denoting ‘unable to do’ and 5 able to do ‘without any difficulty’ with a total raw score also ranging from 8 to 40. Neuro-QoL also provides the opportunity to convert raw scores into normalized T-scores, which help compare the respondent to either a clinical population or healthy normative population. In order to help simplify interpretation of findings without requiring comparison with a normative sample, raw scores were used for all analyses.
MRI acquisition and analysis
All MRI was obtained as standard of care at our institution, which includes a standardized sagittal three-dimensional (3D) T1 acquisition for brain volumetric analysis generally following recommended NeuroQuant parameters with few modifications. MRI parameters for each scanner are as follows: Siemens Symphony Tims 1.5 T scanner: sagittal 3D T1 magnetization-prepared rapid gradient echo, TR = 1890 ms, TE = minimum, TI = 1100 ms, flip angle = 8, matrix = 192 × 192, FOV = 240 mm2, slice thickness = 1 mm. Phillips Achieva 1.5 T scanner: sagittal 3D T1 fast field echo, TR = shortest, TE = 4 ms, flip angle = 8, matrix = 192 × 192, FOV = 240 mm2, slice thickness = 1 mm. Phillips Achieva 3T scanner: sagittal 3D T1 fast field echo, TR = shortest, TE = shortest, flip angle = 9, matrix = 192 × 192, FOV = 240 mm2, slice thickness = 1 mm. GE Discovery 750W 3.0T scanner: sagittal 3D T1 inversion recovery fast spoiled gradient echo, inversion time = 600 ms, TE:M in full, flip angle = 8, matrix = 192 × 192, FOV = 240 mm2, slice thickness = 1 mm.
All sagittal 3D T1 volumetric images were analyzed using NeuroQuant software, which is a fully automated method for quantifying brain structures shown to have significant statistical agreement in calculating brain volumes in MS to other validated methods such as SIENAX.26 All processing was performed in standard fashion as described in https://www.cortechslabs.com/resources/installed-system/ at the time of image acquisition per clinical protocol at our institution. NeuroQuant analyzes a high resolution non-contrasted T1-weighted 3D sagittal MRI and constructs a segmentation-based measurement of both cortical and subcortical volumes. The software corrects for a number of factors, deletes non-brain tissue using its active contour model and separates various anatomical structures using a probabilistic atlas. NeuroQuant then compares volumes to a normative database adjusting for age, gender and intracranial volume.26,27 Using a customized data retrieval pipeline, calculated volumes for each brain region were automatically extracted from the NeuroQuant processing server for storage in the study database.
Statistical analysis
Linear regression was used to analyze the relationship between SCC and brain ROI volumes (cortical white matter, cortical gray matter, thalami, basal ganglia, amygdalae and hippocampi) after inclusion of relevant covariates as explained below. All brain volumes were standardized to intracranial volume. We also ran a comparison analysis for specificity; in this second set of analyses, we examined self-reported lower extremity mobility and the same brain ROI volumes and covariates. All statistical analyses were performed using SPSS software (IBM Corp, released 2016. IBM SPSS Statistics for Windows, version 24.0. Armonk, NY, USA: IBM Corp.) using a P≤0.05 threshold.
Relevant covariates were chosen for both theoretical and statistical purposes. Age, disease-modifying treatment, disease severity, disease duration6 and gender28 have been associated with differences in brain volume, while fatigue18 and depression17 have been associated with differences on measures of cognition. Theoretically, we considered age (in years), disease severity (patient determined disease steps; PDDS), disease-modifying treatment, disease duration (in years), gender, self-reported depression (as measured by Neuro-QoL depression short form), and self-reported fatigue (as measured by Neuro-QoL fatigue short form) as potential covariates. Statistically, univariable linear regression analyses with a lax significance value (P≤0.10) were used to identify which theoretically determined covariates were predictive of our outcome variables (ROI volumes). Age, PDDS, disease duration, gender and fatigue were found to be significant covariates following this method. However, collinearity analyses revealed a strong relationship between PDDS and disease duration. Given that disease duration was more strongly predictive of ROI volumes than PDDS, PDDS was dropped as a relevant covariate. Depression did not significantly predict any of the ROI volumes, but given its well-established role in SCC in MS,17 it was included as a covariate. Therefore, the following covariates were included in all subsequent analyses: age, disease duration, gender, depression and fatigue.
Results
Patient population
We identified 921 unique patients with a diagnosis of MS who underwent quantitative brain MRIs during the selected study period. Of those 537 had completed PROs and 158 participants had completed PROs within ± 90 days of imaging (see Table 1).
Table 1.
Patient population and demographics.
| Characteristic | Study population (N=158) | Percentage |
|---|---|---|
| Demographics | ||
| Age (years ± SD) | 49.66 ± 11.54 | |
| Sex | ||
| Men | 30 | 19.0 |
| Women | 128 | 81.0 |
| Race | ||
| Caucasian | 127 | 80.4 |
| Hispanic/Latino | 6 | 3.8 |
| Black/African American | 4 | 2.5 |
| Other | 6 | 3.8 |
| Unknown | 19 | 12.0 |
| Disease-modifying therapy | ||
| None | 13 | 8.2 |
| Injectable | 24 | 15.2 |
| Oral | 53 | 33.5 |
| Intravenous | 68 | 43.0 |
| Disease duration (years ± SD) | 12.33 ± 8.26 |
Raw scores on Neuro-QoL assessment of GCC ranged from 8 to 40 points with a mean score of 29.9 ± 9.2 and on LEF scores ranged from 12 to 40 points with a mean score of 35.9 ± 6.0.
Association of SCC with thalamic and cortical gray matter volumes
Linear regression supported a relationship between SCCs and normalized thalamic and cortical gray matter volumes after controlling for disease duration, age, gender, depression and fatigue. Greater self-reported SCCs was associated with smaller thalamic volumes, t150 = 2.406, P = 0.017. Independent of the covariates, SCC accounted for a modest amount of variance on thalamus/intracranial volume, partial r2 = 0.038 (see Figure 1). Similarly, greater SCCs was associated with smaller cortical gray matter after accounting for covariates, t150 = 2.777, P = 0.006, partial r2 = 0.050 (Figure 2).
Figure 1.
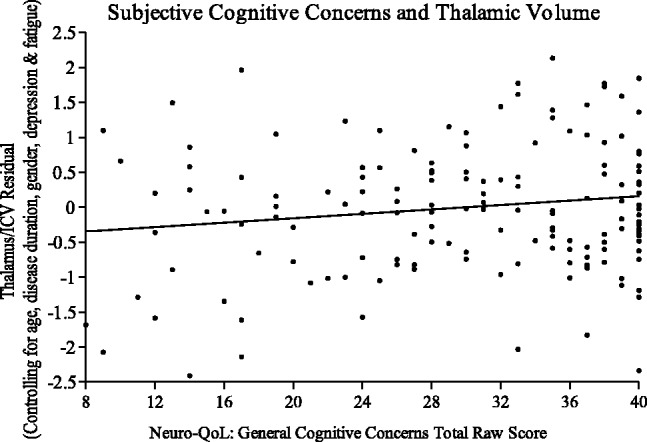
Standardized residuals of thalamic volume, controlling for covariates, regressed on general cognitive concerns score.
Figure 2.
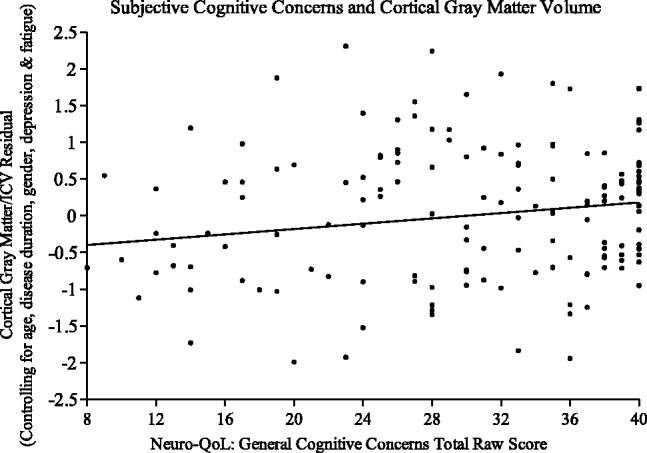
Standardized residuals of cortical gray volume, controlling for covariates, regressed on general cognitive concerns score.
SCC was not significantly associated with any other ROIs after controlling for covariates. By comparison, LEF short form scores were not significantly associated with any ROIs that we examined (all P > 0.05) (see Table 2).
Table 2.
Results of linear regression model relating patient-reported outcomes to standardized brain regions of interest with age, disease duration, gender, depression, and fatigue as covariates.
| Region of interest |
General cognitive concerns |
Lower extremity function |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std β | t | P value | Partial r2 |
95% Confidence interval |
Std β | t | P value | Partial R2 |
95% Confidence interval |
|||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||||||
| Amygdalae and hippocampi | 0.057 | 0.744 | 0.458 | 0.004 | –0.000008 | 0.000018 | 0.055 | 0.803 | 0.423 | 0.004 | –0.000011 | 0.000025 |
| Basal ganglia | 0.125 | 1.591 | 0.114 | 0.017 | –0.000004 | 0.000039 | –0.011 | –0.154 | 0.878 | <0.001 | –0.000032 | 0.000027 |
| Thalami | 0.223 | 2.406 | 0.017 | 0.038 | 0.000005 | 0.000055 | 0.060 | 0.717 | 0.474 | 0.003 | –0.000022 | 0.000047 |
| Cortical white matter | –0.026 | –0.249 | 0.804 | <0.001 | –0.000723 | 0.000561 | 0.003 | 0.028 | 0.977 | <0.001 | –0.000852 | 0.000877 |
| Cortical gray matter | 0.240 | 2.777 | 0.006 | 0.050 | 0.000250 | 0.001482 | 0.135 | 1.732 | 0.085 | 0.020 | –0.000104 | 0.001581 |
Discussion
SCCs have been little studied in MS. Our results suggest a possible association between self-report SCCs and reduced volume of thalamic and cortical gray matter accounting for 3.8% of the variance in thalamic volume and 5.0% of the variance in cortical gray matter volume. Reduced thalamic9 and cortical gray matter8 volumes have been implicated in objective cognitive impairment in MS. Certain cognitive domains feature prominently in the Neuro-QoL GCC short form section used in our study as a marker of SCC, including processing speed, attention and episodic memory. Objective measures of cognitive performance in patients with MS have shown an association of both reduced cortical gray matter and thalamic volumes with slowed cognitive processing speed29 and an association of reduced thalamic volume with reduced episodic memory performance.30 To our knowledge, our study is the first to show an association between increased SCCs and reduced volumes in ROIs that have correlated with objective cognitive dysfunction in MS. Still, not all ROIs implicated in cognitive dysfunction in MS reached significance in our study including amygdalae, hippocampi and basal ganglia.
In MS, as in other neurological disorders that can cause cognitive impairment, SCCs have not clearly correlated with objective cognitive impairment on neuropsychological testing,17,18 and there are conflicting data.16 Our study suggests a relationship between SCCs and changes on volumetric imaging, lending possible neuro-anatomical significance to self-report SCCs and patients’ subjective experiences as well as to the use of Neuro-QoL as an evaluation tool. Further research will be needed to explore differences between patients who report SCCs with or without objective impairment on neuropsychological testing and the volumetric patterns of these groups.
Two previous studies reported on the association between SCC and brain volumes in MS. A 2006 study by Benedict and Zivadinov,19 which used MSNQ to evaluate SCC, showed no association between self-report SCC and MRI outcomes but did show an association between informant-report SCC and increased T1 and T2 lesion volume and reduced whole brain parenchymal fraction. There are likely to be a number of reasons why that study did not show an association between self-report SCCs and brain volumes and ours did. In our study a greater number of participants underwent volumetric imaging (158 versus 27) and we calculated regional volumes for ROIs which yielded our significant results rather than employing whole brain parenchymal fraction and global lesion volume. We also utilized a different tool for evaluating SCC, Neuro-QoL, and employed a continuous raw score for SCC rather than a binary cut-off which may have allowed greater sensitivity for subtle subjective differences. We did not collect informant-report SCC which could have yielded additional results. Another study20 reported an association between increased hippocampal volumes and increased SCCs; that study also had fewer participants and employed a scale for measuring SCC that has not been validated in MS.
The relationship between cortical gray matter atrophy and cognitive dysfunction in MS is well established.8 An earlier study identified more extensive reductions in cortical gray matter volume in bilateral frontal, temporal and parietal cortices in MS patients with lower performance on neuropsychological testing,31 while other studies that used different criteria for cognitive impairment showed preferential reduced volume of temporo-occipital gray matter.32 While our study did not distinguish between different cortical gray matter regions, it is consistent with the increasing evidence for the role of cortical gray matter atrophy in cognitive dysfunction in MS.
Why thalamic atrophy is associated with cognitive impairment in MS is a field of ongoing investigation. A recent study found that decreased cognitive processing speed was related to localized atrophy of the anterior and superior surface of the left thalamus,33 indicating the likely involvement of anterior thalamic nuclei that help make up the Papez circuit, which is known to be involved in episodic memory. Functional studies have shown increased activation of the thalamus by functional MRI in patients with MS on accurate encoding of visuospatial tasks.30 Diffusion tensor imaging failed to show a relationship between changes in white matter tracts of the Papez circuit and cognitive impairment,34 possibly indicating direct involvement of the gray matter structures. Alternatively, thalamic volume may simply serve as a marker of white matter disease throughout the brain due to its diffuse networks and rich reciprocal connectivity also accounting for its association with non-cognitive disability.35
The most prominent limitation of our study is the lack of objective cognitive testing, which limits our ability to understand the relationship of SCCs to objective cognitive impairment which is planned in future work. While SCCs may therefore capture a subset of patients with objective impairment, the lack of clear correlation of SCCs to objective cognitive impairment on neuropsychological testing17,18 may lend support to SCCs existing separate from or prior to objective impairment. Other limitations of our study include not differentiating between different MS subtypes and lack of other imaging analyses such as brain parenchymal fraction and T2 lesion volume load. Our effect size was also modest probably for a number of reasons. While most research studies that investigate brain volume with MRI use more time-consuming volumetric analysis such as SIENAX and FIRST, we employed the fully automated NeuroQuant image processing pipeline that did not require significant human post-processing.
It is challenging to study subjective concerns, which are based entirely on patients’ subjective report adding another layer of variability; subjective human experience in its uniquely individual nature is beyond standardization. Self-report SCCs may also be confounded by a host of other factors including depression, anxiety and fatigue. Deficits of insight into cognitive impairment, or in a person’s ability to think about their own thinking, metacognition, may have different patterns of injury or be related to more advanced impairment. This may account for the greater variability at the extremes whereby a highly functioning patient with nearly intact cognition will report subjective impairment due to decline from a previous baseline that may not be detectable in any structural changes, while another patient with advanced pathological impairment may not be aware of or accurately report their subjective deficits;16 this may explain the variability of thalamic volumes at the extremes of patient reported scoring of cognitive concerns (see Figure 1).
We expected our comparator, LEF, to relate to some motor ROIs such as cortical white matter volume but did not find our comparator to relate to any ROIs. The NeuroQuant software may not be as accurate at detecting cortical white matter volume as it is at calculating certain gray structures such as the thalamus given their more clearly delineated borders and centralized location. Also, the spinal cord, which contributes significantly to lower extremity disability in MS, was not included in our analysis. Alternatively, in our sample, patients had higher scores on LEF than on GCC indicating a lesser degree of subjective lower extremity dysfunction perhaps making it more challenging to associate this subjective dysfunction with neuroanatomical changes.
Despite these limitations, our study may lend neuroanatomical significance to patient-reported SCCs by associating them with reduced thalamic and cortical gray matter volumes, regions that have been shown to correlate with objective measures of cognitive dysfunction in MS.
Acknowledgements
The authors would like to extend their appreciation to the patients, staff and clinicians at the Rocky Mountain MS Center and the COPIC Medical Foundation.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Enrique Alvarez has consulted for the following companies: Biogen, EMD Serono, Genzyme, Genentech, TG Pharmaceuticals, and Novartis. He received research funding from the Rocky Mountain MS Center, Biogen, Novartis, Acorda, and TG pharmaceuticals.
Justin M Honce has consulted for and/or received research support from Genentech, Novartis and Biogen.
Timothy Vollmer has received compensation for activities such as advisory boards, lectures and consultancy with the following companies and organizations: Academic CME; Alcimed; Anthem Blue Cross; Genentech/Roche; Biogen IDEC; Novartis; CellGene; Epigene; Rocky Mountain MS Center; GLG Consulting; Ohio Health; TG Therapeutics; Topaz Therapeutics; Dleara Lawyers; Teva Neuroscience. He has received research support from the following: Teva Neuroscience; NIH/NINDS; Rocky Mountain MS Center; Actelion; Biogen; Novartis, Roche/Genentech, UT South Western and TG Therapeutics, Inc.
Luis D Medina has received research support from the Alzheimer’s Association.
The other authors declare no relevant disclosures or conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by the COPIC Medical Foundation.
References
- 1.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 2006; 245: 41–46. 2006/04/29. DOI: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Filley CM, Heaton RK, Nelson LM, et al. A comparison of dementia in Alzheimer’s disease and multiple sclerosis. Arch Neurol 1989; 46: 157–161. DOI: 10.1001/archneur.1989.00520380061013. [DOI] [PubMed] [Google Scholar]
- 3.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7: 1139–1151. DOI: 10.1016/s1474-4422(08)70259-x. [DOI] [PubMed] [Google Scholar]
- 4.Siffrin V, Vogt J, Radbruch H, et al. Multiple sclerosis – candidate mechanisms underlying CNS atrophy. Trends Neurosci 2010; 33: 202–210. 2010/02/16. DOI: 10.1016/j.tins.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Vollmer T, Signorovitch J, Huynh L, et al. The natural history of brain volume loss among patients with multiple sclerosis: a systematic literature review and meta-analysis. J Neurol Sci 2015; 357: 8–18. 2015/08/05. DOI: 10.1016/j.jns.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84: 784–793. 2015/01/30. DOI: 10.1212/WNL.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanfilipo MP, Benedict RH, Weinstock-Guttman B, et al. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 2006; 66: 685–692. 2006/03/15. DOI: 10.1212/01.wnl.0000201238.93586.d9. [DOI] [PubMed] [Google Scholar]
- 8.Amato MP, Bartolozzi ML, Zipoli V, et al. Neocortical volume decrease in relapsing–remitting MS patients with mild cognitive impairment. Neurology 2004; 63: 89–93. DOI: 10.1212/01.Wnl.0000129544.79539.D5. [DOI] [PubMed] [Google Scholar]
- 9.Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007; 69: 1213–1223. 2007/09/19. DOI: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- 10.Brass SD, Benedict RH, Weinstock-Guttman B, et al. Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult Scler 2006; 12: 437–444. 2006/08/12. DOI: 10.1191/135248506ms1301oa. [DOI] [PubMed] [Google Scholar]
- 11.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain 2008; 131: 1134–1141. 2008/04/01. DOI: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 12.Amariglio RE, Mormino EC, Pietras AC, et al. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology 2015; 85: 56–62. 2015/06/07. DOI: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell AJ. Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age Ageing 2008; 37: 497–499. 2008/08/07. DOI: 10.1093/ageing/afn147. [DOI] [PubMed] [Google Scholar]
- 14.Geerlings MI, Jonker C, Bouter LM, et al. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 1999; 156: 531–537. 1999/04/14. DOI: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 15.van Norden AG, Fick WF, de Laat KF, et al. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology 2008; 71: 1152–1159. 2008/10/08. DOI: 10.1212/01.wnl.0000327564.44819.49. [DOI] [PubMed] [Google Scholar]
- 16.Marrie RA, Chelune GJ, Miller DM, et al. Subjective cognitive complaints relate to mild impairment of cognition in multiple sclerosis. Mult Scler 2005; 11: 69–75. 2005/03/01. DOI: 10.1191/1352458505ms1110oa. [DOI] [PubMed] [Google Scholar]
- 17.Maor Y, Olmer L, Mozes B. The relation between objective and subjective impairment in cognitive function among multiple sclerosis patients – the role of depression. Mult Scler 2001; 7: 131–135. 2001/06/27. DOI: 10.1177/135245850100700209. [DOI] [PubMed] [Google Scholar]
- 18.Middleton LS, Denney DR, Lynch SG, et al. The relationship between perceived and objective cognitive functioning in multiple sclerosis. Arch Clin Neuropsychol 2006; 21: 487–494. 2006/08/02. DOI: 10.1016/j.acn.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Benedict RH, Zivadinov R. Predicting neuropsychological abnormalities in multiple sclerosis. J Neurol Sci 2006; 245: 67–72. 2006/04/22. DOI: 10.1016/j.jns.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Hulst HE, Gehring K, Uitdehaag BM, et al. Indicators for cognitive performance and subjective cognitive complaints in multiple sclerosis: a role for advanced MRI? Mult Scler 2014; 20: 1131–1134. 2013/11/28. DOI: 10.1177/1352458513513969. [DOI] [PubMed] [Google Scholar]
- 21.Tur C, Moccia M, Barkhof F, et al. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat Rev Neurol 2018; 14: 75–93. 2018/01/13. DOI: 10.1038/nrneurol.2017.171. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012; 78: 1860–1867. 2012/05/11. DOI: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller DM, Bethoux F, Victorson D, et al. Validating Neuro-QoL short forms and targeted scales with people who have multiple sclerosis. Mult Scler 2016; 22: 830–841. 2015/08/05. DOI: 10.1177/1352458515599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apple AC, Schroeder MP, Ryals AJ, et al. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin 2018; 20: 110–118. 2018/08/11. DOI: 10.1016/j.nicl.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina L, Valdez B, Alvarez E, et al. Exploratory factor analysis of a patient-reported outcome measure in a multiple sclerosis population cohort at a large academic center (P3.365). Neurology 2017; 88: 16 Suppl. [Google Scholar]
- 26.Wang C, Beadnall HN, Hatton SN, et al. Automated brain volumetrics in multiple sclerosis: a step closer to clinical application. J Neurol Neurosurg Psychiatry 2016; 87: 754–757. 2016/04/14. DOI: 10.1136/jnnp-2015-312304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer JB, Magda S, Airriess C, et al. Fully automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR Am J Neuroradiol 2009; 30: 578–580. 2008/12/30. DOI: 10.3174/ajnr.A1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci U S A 1991; 88: 2845–2849. 1991/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista S, Zivadinov R, Hoogs M, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 2012; 259: 139–146. 2011/07/02. DOI: 10.1007/s00415-011-6147-1. [DOI] [PubMed] [Google Scholar]
- 30.Koenig KA, Rao SM, Lowe MJ, et al. The role of the thalamus and hippocampus in episodic memory performance in patients with multiple sclerosis. Mult Scler 2018; 1352458518760716. 2018/03/08. DOI: 10.1177/1352458518760716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing–remitting MS. Neuroimage 2006; 30: 891–898. 2005/12/20. DOI: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 32.Riccitelli G, Rocca MA, Pagani E, et al. Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum Brain Mapp 2011; 32: 1535–1543. 2010/08/27. DOI: 10.1002/hbm.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergsland N, Zivadinov R, Dwyer MG, et al. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler 2016; 22: 1327–1336. 2015/11/07. DOI: 10.1177/1352458515616204. [DOI] [PubMed] [Google Scholar]
- 34.Kern KC, Gold SM, Lee B, et al. Thalamic-hippocampal-prefrontal disruption in relapsing–remitting multiple sclerosis. Neuroimage Clin 2015; 8: 440–447. 2015/06/25. DOI: 10.1016/j.nicl.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zivadinov R, Bergsland N, Dolezal O, et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing–remitting MS during 5 years. AJNR Am J Neuroradiol 2013; 34: 1931–1939. 2013/04/13. DOI: 10.3174/ajnr.A3503. [DOI] [PMC free article] [PubMed] [Google Scholar]


