Abstract
Women with polycystic ovary syndrome (PCOS) have an adverse metabolic profile with an increased risk of prediabetes and type 2 diabetes (T2DM); however, it is unclear if PCOS is associated with increased cardiovascular events in later years independent of the presence of T2DM. Many therapies have been used to treat the differing facets of PCOS, including those for menstrual irregularity, hirsutism, acne and anovulatory infertility. The aim of this review was to evaluate the cardiovascular profiles associated with the medications used in the management of PCOS and evaluate whether they have cardiovascular benefit, detriment or are neutral. The medications reviewed include oral contraceptive pills, antiandrogens, clomiphene and drugs specifically used in diabetes therapy; metformin, glitazones, dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 receptor agonists. This review concludes that therapies that are used to treat these patients appear not to add to the cardiovascular risk and that there is no evidence that any interventional medical therapy may prevent the onset of diabetes in patients with PCOS, though in the case of metformin, this agent may be beneficial in preventing development of gestational diabetes.
Keywords: androgen, cardiovascular risk, clomiphene, oral contraceptives, PCOS, Pharmacotherapy
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among reproductive-aged women, and a recent systematic analysis suggests that the prevalence is between 6% (National Institutes of Health criteria) and 10% (Rotterdam and Androgen Excess Society guidelines).1 PCOS leads to irregular periods, infertility and increased androgen levels causing hirsutism and acne.2,3 Women with PCOS show increased cardiovascular risk through a higher incidence of hypertension, an adverse lipid profile, and insulin resistance (IR).4,5 Obesity affects the majority of women with PCOS, and they have a higher prevalence of impaired glucose tolerance (IGT), reported as 4.7%, and a higher prevalence of type 2 diabetes (T2D), reported as 2.4–5.1% in cross-sectional studies, compared with a baseline prevalence of diabetes in non-PCOS women of 1%.6–8 A diagnosis of PCOS is said to confer a 5–10-fold increased risk of developing T2D and recent guidelines report IGT in obese and nonobese women with PCOS in the United States of America in 30–35% and 10–15%, respectively, with an additional 3–10% and 1–2%, respectively, having T2DM.9 A recent Finnish study suggested that obese but not nonobese PCOS women were at an increased risk of T2D;10 however, up to 60% of women with PCOS have insulin resistance, up to 40% have impaired glucose tolerance and 10% may develop T2D by the age of 40 years.11
Prediabetes is associated with the combination of IR and beta cell dysfunction, and it is reported that 5–10% of people with prediabetes convert to T2D, though this may differ by population.12
Metabolic syndrome is comprised of obesity, dyslipidemia, hypertension and IR that is associated with increased cardiovascular risk, particularly if diabetes supervenes.13 PCOS patients have a higher prevalence of metabolic syndrome, while women with metabolic syndrome have features more commonly of PCOS;14 however, when weight matched with controls, obesity and IR are independently associated with metabolic syndrome in PCOS.15 It has been suggested that metformin may be beneficial for those women with PCOS with metabolic syndrome or IGT to be combined with lifestyle advice and weight loss.9,13
There is a 2.7-fold increased risk in endometrial cancer development that is related to unopposed estrogen exposure through chronic anovulation, which is addressed by ensuring endometrial protection and that regular withdrawal bleeds occur.16 There is no increase in breast cancer, though there is the suggestion that ovarian cancer may be increased but that oral contraceptive use is protective; however, the medications used in the treatment of PCOS do not appear to have an excess cancer risk associated with them.16
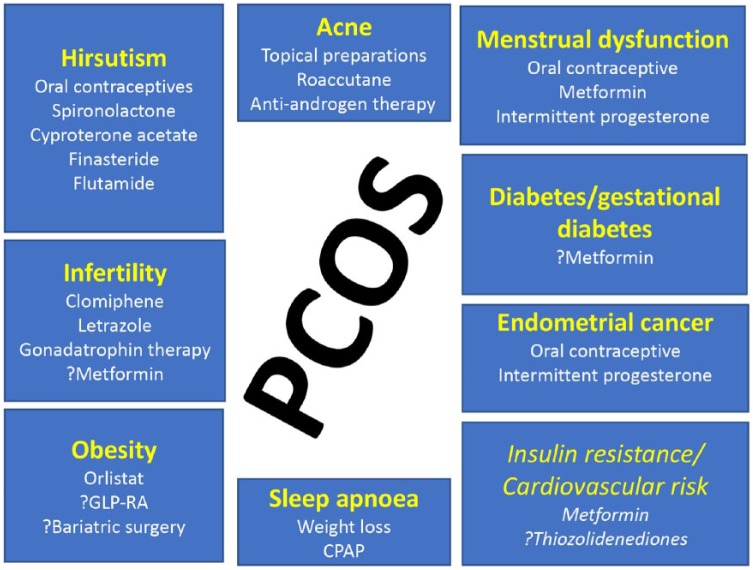
It is clear that those women with PCOS have an increased cardiovascular risk, with many studies reporting surrogate measures of atherosclerosis, including increased carotid intima media thickness, more angiographic coronary artery disease, increased endothelial dysfunction and a multitude of serum risk marker changes.17,18 However, contradictory results from observational population studies make it difficult to assess the potential for PCOS to enhance cardiovascular morbidity and mortality. Initial studies in patients with PCOS undergoing wedge resection suggested that there was no increase in cardiovascular mortality.19 Conversely, the Nurses’ Health Study has shown that women with a history of menstrual irregularity have an increased risk of both nonfatal and fatal coronary heart disease,20 although PCOS was not diagnosed specifically in that study. A large United Kingdom (UK) general practice dataset of women with PCOS (over 21,000 participants) reported that there was no evidence of an increase in cardiovascular disease.7 Conversely, a retrospective study on a database of 2301 PCOS patients over an 11-year period with a total follow-up time of over 12,000 person-years reported that there was an increased prevalence rate for both myocardial infarction and angina from the age of 45–54 years of 1.9% and 2.6% respectively and from 55 years to 64 years of 6.0% for both. The clinical aspects that need to be addressed in PCOS are shown in Figure 1.
Figure 1.
Therapeutic targets in polycystic ovary syndrome and pharmacological treatment (not all may be licensed in different countries). Targets in italics are those not specifically addressed clinically. GLP-RA: Glucagon like peptide receptor agonist; CPAP: Continous positive airway pressure.
It is therefore unclear whether PCOS confers an increased cardiovascular morbidity and mortality per se or whether this may be due to the development of diabetes and contributed to by obesity related parameters. One facet that has not been well described is the positive and negative cardiovascular risk conferred by the medications that are used as therapies in PCOS and are summarized in Table 1. The aim of this review was to evaluate the cardiovascular profiles associated with the medications used in the management of PCOS and evaluate whether they have cardiovascular benefit, detriment or are neutral.
Table 1.
Therapy used in PCOS and possible cardiovascular impact.
| Classes of antidiabetic agents | Possible cardiovascular impact | References |
|---|---|---|
| Hormone contraception | Estrogen-containing OCPs may potentially have an adverse cardiovascular risk, increased risk of hypertension and dyslipidemia, elevated inflammatory markers, decreased insulin sensitivity | Sathyapalan and Atkin;17 Randeva and colleagues;18 Solomon and colleagues;20 de Bastos and colleagues21 |
| Spironolactone | Decrease in triglycerides, increase in HDL and decreased insulin resistance. Cardiovascular benefits in patients with established cardiovascular disease | Christakou and colleagues;22 Lidegaard and colleagues;23 Okoroh and colleagues24 |
| Finasteride | No data on the benefits or detriments of this treatment on cardiovascular risk, only studies in men | Anand and colleagues25 |
| Flutamide | Decreases in the LDL/HDL ratio, total cholesterol and triglycerides | Zulian and colleagues26 |
| Metformin | Decrease in oxidative damage, inflammation and improvement in endothelial dysfunction. Cardiovascular benefit shown in diabetes; prevention of diabetes | Velazquez and colleagues;27 Legro and colleagues;28 Insel and colleagues29 |
| Thiazolidinediones (glitazones): | Reduction in insulin resistance, alteration in visceral to subcutaneous fat ratio, improved endothelial function | Day;30 Ferwana and colleagues;31 Cho and colleagues32 |
| DPP IV inhibitors/ GLP-1 receptor agonists | Very few studies. Weight loss with GLP-1RAs and potential cardiovascular benefit in diabetes | Ahren;33 Gautier and colleagues;34 Jensterle and colleagues35 |
| SGLT2 inhibitor | Studies awaited in PCOS to determine if the cardiovascular benefit seen in diabetes translates to PCOS | Pi-Sunyer and colleagues36 |
| Clomiphene | ECG QT interval decrease that may be protective for cardiovascular events | Dickey and colleagues37 |
DPP, dipeptidyl peptidase; ECG, electrocardiograph; GLP, glucagon-like peptide; GLP-1RA, GLP-1 receptor agonist; HDL, high density lipoprotein; LDL, low density lipoprotein; OCP, oral contraceptive; PCOS, polycystic ovary syndrome; SGLT2, sodium–glucose co-transporter type 2.
Hormonal contraception
Combined oral contraceptives (OCPs) are often used as first-line therapy in women with PCOS to address menstrual regularity, acne and hirsutism. It has been well recognized that the long-term use of estrogen-containing OCPs may have an adverse cardiovascular risk, but whether this translates into additional cardiovascular risk in these patients is unclear.38
Oral contraceptives are associated with hypertension, dyslipidemia and an elevated C-reactive protein (hs-CRP), a marker of inflammation.21 In addition, there is an increased risk of developing metabolic syndrome.21 The decreased insulin sensitivity associated with their use depends on the progestin used, with fourth generation progestins being less impactful,39 and those containing cyproterone being associated with increased insulin secretion.40 All of these features are associated with obese patients with PCOS and with the increased risk of developing prediabetes and T2D;6,7 however, a Cochrane meta-analysis suggested that OCPs did not affect glucose tolerance, though the evidence reviewed was not comprehensive.41 The effect of OCPs on lipid parameters depends on the progestin component, with estrogen increasing high density lipoprotein (HDL) and triglycerides while decreasing low density lipoprotein (LDL) and total cholesterol. Those progestins with an androgenic profile are associated with a lower HDL and increased LDL cholesterol, the converse occurring with less androgenic preparations.22
Studies on the use of OCPs and alternate cardiovascular risk indices have shown that they are associated with increased hs-CRP, as a marker of inflammation, as well as elevated advanced glycation end products that are associated with increased cardiovascular risk, though whether these effects are offset or exacerbated by the metabolic effects of OCPs remains unclear.22 However, no studies have addressed long-term OCP use in patients with PCOS to determine if there is an accelerated development of diabetes or other cardiovascular risk indices.
The risk of developing vein thromboembolism (VTE) with OCP use has been well established and increases with both obesity and age.21 Oral contraceptives containing desogestrel, gestodene, or drospirenone were associated with a significantly higher risk of venous thrombosis than oral contraceptives with levonorgestrel.23 A higher rate of VTE is reported in those women with PCOS treated with OCPs,24 and in another study the risk of VTE was increased two-fold in PCOS with OCPs but was 1.5 fold higher without OCP use.42 From a clinical perspective, many women use OCPs for short periods of time and, given the youth of these patients, the additional cardiovascular risk is likely to be clinically irrelevant. However, these studies suggest that long-term use in older obese patients with PCOS may not be beneficial for cardiovascular risk.
Antiandrogens
Spironolactone
Spironolactone is an aldosterone antagonist that binds to the testosterone receptor and is used in PCOS for the reduction of hirsutism. In patients with established cardiovascular disease and heart failure, spironolactone has been shown to be beneficial.25 In a number of small studies, spironolactone therapy was shown to decrease triglycerides, increase HDL and decrease IR in patients with PCOS26 and normalize endothelial dysfunction,43 though in combination with an OCP there were no cardiovascular risk advantages.44 Spironolactone may therefore have some cardioprotective benefit, but there are no medium to long-term clinical trials to advise on this in PCOS, though it may be of clear benefit in heart failure patients.
Finasteride
Finasteride is a 5-α-reductase inhibitor that inhibits the type 2 isoform, specifically leading to a reduction in the production of dihydrotestosterone, thereby leading to a decrease in hirsutism. Primarily used in men for the treatment of benign prostatic hypertrophy and prostatic cancer, a large cohort population concluded that there was no adverse cardiovascular risk in men.45 This is likely to also be the case in women, but there are no data to confirm this.
Flutamide
Flutamide is a nonsteroidal antiandrogen that competes for the binding of testosterone to its receptor and is primarily used in men to treat prostate cancer. It has been used in PCOS for the treatment of hirsutism and studies report significant decreases in the LDL/HDL ratio, total cholesterol and triglycerides that would appear to confer cardiovascular benefit.46 However, a serious limitation of therapy with flutamide is the rare occurrence of fatal hepatic necrosis, perhaps mediated by mitochondrial dysfunction.
Metformin
This guanidine-derived biochemical compound exerts anti-hyperglycemic effects via an increase in cellular insulin sensitivity and peripheral glucose uptake, a decrease in hepatic gluconeogenesis, and a reduction in the amount of glucose reabsorbed by the intestine and up-regulation of GLUT-4.47 However, its exact mechanism of action remains unclear, but adenosine monophosphate-activated protein kinase activation can explain many aspects of its effects.48 Metformin is a first-line treatment for glycemic control in patients with T2D, its major mechanism being a reduction in IR49 via its action on the liver. Given the increased insulin found in PCOS, metformin is used in PCOS where it improves menstrual regularity, though fertility may not be enhanced.27,28 Metformin’s potential to reduce diabetes incidence was shown in the diabetes prevention study29 though this was not as effective as lifestyle intervention. Currently, the early use of metformin in PCOS to prevent diabetes remains unclear, though its role in gestational diabetes has been advocated.50 Metformin’s cardiovascular benefits in T2D were shown in the UK Prospective Diabetes study.49 This retrospective study involving 645,710 participants concluded that a decreased risk of atrial fibrillation in T2D patients resulted in those who used metformin51 and this was attributed to metformin’s protective role against oxidative damage. However, this decreased risk was diminished after prolonged use of metformin for more than 3 years according to the same study.
Metformin has been shown to improve additional cardiovascular indices of inflammation with the reduction of CRP, advanced glycation end products52, soluble markers of inflammation and endothelial dysfunction.53 Unfortunately, there are no long-term studies looking at the cardiovascular benefit of metformin in PCOS, compounded by the issue that this is an unlicensed indication for metformin use and often used for short periods of time; however, the impact of metformin on cardiovascular disease is uncertain.54
Thiazolidinediones (glitazones)
Thiazolidinediones (TZDs) or glitazones are a group of oral antidiabetic agents that primarily act as selective agonists for PPAR-γ peroxisome proliferator-activated receptor gamma (PPAR-γ) and then modify the expression of various enzymes and proteins involved in metabolic pathways.30 TZDs have pleiotropic therapeutic effects in many metabolic disorders and improve insulin sensitivity and glucose absorption in peripheral tissues leading to their hypoglycemic effects.55 Pioglitazone is the only drug in this class available since the withdrawal of rosiglitazone (due to an increase in cardiovascular events) and troglitazone (due to liver toxicity). However, pioglitazone has been associated with bladder cancer.31
Both pioglitazone and rosiglitazone have been shown to reduce IR in PCOS32,56 and a meta-analysis confirmed the improvement in IR even though body weight increased.57 Treatment with glitazones have shown improvements in cardiovascular risk indices in PCOS with a decrease in visceral adiposity, lower triglyceride levels and higher adiponectin levels,56,58 and with improvement in endothelial function.59 While it is clear that a beneficial cardiovascular risk may result from pioglitazone therapy, all of the studies to date have been small, short term or focused on an improvement in menstrual regularity and ovulation. It is therefore unknown whether long-term treatment of patients with PCOS would prevent the onset of T2D or improve cardiovascular events, though the PROActive and IRIS trials suggested a reduced risk of myocardial infarction and stroke.60
Incretin mimetics
Glucagon-like peptide-1 (GLP-1) is a hormone secreted from the L-cells of the small intestine in response to oral intake that stimulates a glucose-dependent insulin response.33 GLP-1 is rapidly degraded in the circulation to its inactive form by the enzyme dipeptidyl peptidase (DPP) IV34 that contributes to a short half-life and limits its clinical use.61,62 Circulating GLP-1 may be prolonged by use of oral DPP IV inhibitors that result in physiological levels of GLP-1 or by use of the injectable GLP-1 receptor agonists (GLP-1RAs), resulting in pharmacological levels.
GLP-1 receptor agonists
Studies using Glucagon like peptide-1 receptor agonists (GLP-1 RA) have been undertaken with exenatide63 and with liraglutide,35,64,65 and both therapies showing a decrease in weight, together with the suggestion of a decrease in testosterone levels. Large clinical trials on their cardiovascular safety are currently underway and the results of the exenatide EXSCEL trial confirmed cardiovascular safety.66 However, the LEADER study on liraglutide reported that the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke among patients with T2D was lower with liraglutide than with placebo.67 This suggests that this group of drugs may have promise in the treatment of women with PCOS, leading to a decrease in weight and potentially offering cardiovascular protection. However, the cost of this therapy is likely to be too great and so will be restricted to aiding weight loss alone.36
DPP IV
DPP IV inhibitors inhibit DPP IV enzyme activity resulting in higher endogenous GLP-1 levels. There have been very few studies on the use of DPP IV inhibitors in women with PCOS, although alogliptin has been shown to improve IR in patients with PCOS.68 Since this class of drugs is weight neutral and their mechanism of action does not address any of the parameters directly in PCOS, few trials have been undertaken. However, they are largely well tolerated from a cardiovascular viewpoint, with the larger studies using sitagliptin (TECOS), saxagliptin (SAVOR-TIMI) and alogliptin (EXAMINE) showing no additional cardiovascular events, although there is still an unanswered question on hospitalization for heart failure.69
Sodium–glucose co-transporter 2 (SGLT2) inhibitors
Sodium–glucose co-transporter type 2 (SGLT2) inhibitors are a group of newly introduced antidiabetic drugs that inhibit glucose reuptake in the renal proximal tubule and lower blood glucose by inducing glycosuria,70 and decreasing weight. The cardiovascular safety profiles of two SGLT2 inhibitors, empagliflozin and canagliflozin, were studied in the EMPA-REG and CANVAS trials respectively.71 Both studies showed benefit of treatment with SGLT2 inhibitors, with a decrease in cardiovascular deaths, as well as a decrease in the incidence of myocardial infarction and stroke.72,73 These drugs may have promise in the treatment of PCOS but there are no trials to date and it is unclear if these agents have a direct cardiovascular effect.
Clomiphene
Clomiphene is commonly used in women with PCOS to address consequent anovulatory infertility. Clomiphene is a selective estrogen receptor modulator that competitively binds to the estrogen receptor. The main action of clomiphene is at the level of the hypothalamus, where it binds to the estrogen receptor resulting in its depletion, consequently blocking the negative feedback effect of circulating endogenous estradiol.37
While clomiphene is only usually used for 5 days continuously in any one menstrual cycle, it has been reported that those patients on clomiphene as therapy for infertility showed a negative correlation between the length of the QT interval and the increased levels of estradiol in these patients. This decrease in QT interval may be protective, as it would decrease the risk of developing cardiac arrhythmias in patients with long QT intervals, which can have fatal consequences.74 This observation can help explain the different outcomes in the two studies assessing the length of QT interval in PCOS patients noted above, as it is unclear if patients were on clomiphene therapy, which may have influenced the results.
PCOS and QT dispersion
The QT interval is the time during the cardiac cycle when ventricles are depolarizing and repolarizing. Prolongation of the QT interval arises from a dysfunction in the potassium channel responsible for the repolarization phase, therefore prolonging the duration of depolarization of the myocyte. This increase leads to the development of arrhythmias and syncope and may lead to sudden cardiac death.75
Studies have reported the length of the QT interval in PCOS patients, though with contradictory results. One study compared 25 patients with PCOS to 22 control participants based on their testosterone and estradiol levels, as well as insulin levels, and correlated those levels with QT length and QT dispersion. There was an increased QT in patients with PCOS associated with increased testosterone levels.76 Conversely, others have reported that in 119 PCOS patients with a median age of 32 years compared with 64 control group participants, there was a decrease in the QT interval despite the increased levels of testosterone in patients with PCOS.77 This controversy has not been resolved by further studies on the length of the QT interval in patients with PCOS; however, animal studies have correlated a decrease in the QT interval with increased levels of testosterone.77 It remains to be seen whether the therapeutic reduction of testosterone may therefore impact on the QT interval. In addition, spironolactone could potentially cause hyperkalemia which, in turn, could cause QT shortening.
Conclusion
While PCOS is associated with a plethora of cardiovascular risk factors, it is still unclear whether there are inherently increased cardiovascular events. The therapies that have been described in this review that are used to treat these patients, appear not to add to that cardiovascular risk and, indeed, in the case of metformin, may be beneficial. To date, the new therapies in diabetes, DPP IV and GLP-1RAs, appear not to have a place in the direct treatment of PCOS features, other than as treatments to effect weight loss using liraglutide, and SGLT2 inhibitors have yet to be evaluated.
Acknowledgments
HA drafted the initial version of this article. TS and SLA contributed to the writing to the review.
Footnotes
Ethics Approval: Ethics approval was not required for this review.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Huda Alalami, Weill Cornell Medicine Qatar, Research Department, Doha, Qatar.
Thozhukat Sathyapalan, Department of Diabetes and Endocrinology, University of Hull, Hull, UK.
Stephen L. Atkin, Weill Cornell Medicine Qatar, Research Department, PO Box 24144, Doha, Qatar.
References
- 1. Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016; 31: 2841–2855. [DOI] [PubMed] [Google Scholar]
- 2. Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet 2007; 370: 685–697. [DOI] [PubMed] [Google Scholar]
- 3. Ehrmann DA. Polycystic ovary syndrome. New Engl J Med 2005; 352: 1223–1236. [DOI] [PubMed] [Google Scholar]
- 4. Sathyapalan T, Atkin S. Review topic on mechanisms in endocrinology recent advances in the cardiovascular aspects of polycystic ovary syndrome. Eur J Endocrinol 2012; 166: 575–583. [DOI] [PubMed] [Google Scholar]
- 5. Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update 2009; 15: 477–488. [DOI] [PubMed] [Google Scholar]
- 6. March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25: 544–551. [DOI] [PubMed] [Google Scholar]
- 7. Morgan CL, Jenkins-Jones S, Currie CJ, et al. Evaluation of adverse outcome in young women with polycystic ovary syndrome versus matched, reference controls: a retrospective, observational study. J Clin Endocrinol Metab 2012; 97: 3251–3260. [DOI] [PubMed] [Google Scholar]
- 8. Joham AE, Ranasinha S, Zoungas S, et al. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab 2014; 99: E447–E452. [DOI] [PubMed] [Google Scholar]
- 9. Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013; 98: 4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ollila ME, West S, Keinanen-Kiukaanniemi S, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective, population-based cohort study. Hum Reprod 2017; 32: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumesic DA, Akopians AL, Madrigal VK, et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab 2016; 101: 4178–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tabak AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet 2012; 379: 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahalingaiah S, Diamanti-Kandarakis E. Targets to treat metabolic syndrome in polycystic ovary syndrome. Expert Opin Ther Targets 2015; 19: 1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caserta D, Adducchio G, Picchia S, et al. Metabolic syndrome and polycystic ovary syndrome: an intriguing overlapping. Gynecol Endocrinol 2014; 30: 397–402. [DOI] [PubMed] [Google Scholar]
- 15. Ranasinha S, Joham AE, Norman RJ, et al. The association between Polycystic Ovary Syndrome (PCOS) and metabolic syndrome: a statistical modelling approach. Clin Endocrinol (Oxf) 2015; 83: 879–887. [DOI] [PubMed] [Google Scholar]
- 16. Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids 2013; 78: 782–785. [DOI] [PubMed] [Google Scholar]
- 17. Sathyapalan T, Atkin SL. Recent advances in cardiovascular aspects of polycystic ovary syndrome. Eur J Endocrinol 2012; 166: 575–583. [DOI] [PubMed] [Google Scholar]
- 18. Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev 2012; 33: 812–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010; 95: 2038–2049. [DOI] [PubMed] [Google Scholar]
- 20. Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 2002; 87: 2013–2017. [DOI] [PubMed] [Google Scholar]
- 21. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev 2014; 3: Cd010813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christakou C, Kollias A, Piperi C, et al. The benefit-to-risk ratio of common treatments in PCOS: effect of oral contraceptives versus metformin on atherogenic markers. Hormones 2014; 13: 488–497. [DOI] [PubMed] [Google Scholar]
- 23. Lidegaard O, Lokkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ 2009; 339: b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okoroh EM, Hooper WC, Atrash HK, et al. Is polycystic ovary syndrome another risk factor for venous thromboembolism? United States, 2003–2008. Am J Obstet Gynecol 2012; 207: 377.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anand IS, Claggett B, Liu J, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart failure 2017; 5: 241–252. [DOI] [PubMed] [Google Scholar]
- 26. Zulian E, Sartorato P, Benedini S, et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Invest 2005; 28: 49–53. [DOI] [PubMed] [Google Scholar]
- 27. Velazquez EM, Mendoza S, Hamer T, et al. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994; 43: 647–654. [DOI] [PubMed] [Google Scholar]
- 28. Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356: 551–566. [DOI] [PubMed] [Google Scholar]
- 29. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care 2015; 38: 1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Day C. Thiazolidinediones: a new class of antidiabetic drugs. Diabet Med 1999; 16: 179–192. [DOI] [PubMed] [Google Scholar]
- 31. Ferwana M, Firwana B, Hasan R, et al. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med 2013; 30: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 32. Cho LW, Kilpatrick ES, Keevil BG, et al. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol 2009; 70: 233–237. [DOI] [PubMed] [Google Scholar]
- 33. Ahren B. Glucagon-like peptide-1 (GLP-1): a gut hormone of potential interest in the treatment of diabetes. Bioessays 1998; 20: 642–651. [DOI] [PubMed] [Google Scholar]
- 34. Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab 2008; 34 (Suppl. 2): S65–S72. [DOI] [PubMed] [Google Scholar]
- 35. Jensterle M, Kravos NA, Goricar K, et al. Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: randomized trial. BMC Endocr Disord 2017; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 37. Dickey RP, Holtkamp DE. Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update 1996; 2: 483–506. [DOI] [PubMed] [Google Scholar]
- 38. Dokras A. Noncontraceptive use of oral combined hormonal contraceptives in polycystic ovary syndrome-risks versus benefits. Fertil Steril 2016; 106: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 39. Cagnacci A, Ferrari S, Tirelli A, et al. Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: a randomized trial. Contraception 2009; 79: 111–116. [DOI] [PubMed] [Google Scholar]
- 40. Diamanti-Kandarakis E, Baillargeon JP, Iuorno MJ, et al. A modern medical quandary: polycystic ovary syndrome, insulin resistance, and oral contraceptive pills. J Clin Endocrinol Metab 2003; 88: 1927–1932. [DOI] [PubMed] [Google Scholar]
- 41. Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev 2014; 4: Cd006133. [DOI] [PubMed] [Google Scholar]
- 42. Bird ST, Hartzema AG, Brophy JM, et al. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. CMAJ 2013; 185: E115–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Studen KB, Sebestjen M, Pfeifer M, et al. Influence of spironolactone treatment on endothelial function in non-obese women with polycystic ovary syndrome. Eur J Endocrinol 2011; 164: 389–395. [DOI] [PubMed] [Google Scholar]
- 44. Vieira CS, Martins WP, Fernandes JB, et al. The effects of 2 mg chlormadinone acetate/30 mcg ethinylestradiol, alone or combined with spironolactone, on cardiovascular risk markers in women with polycystic ovary syndrome. Contraception 2012; 86: 268–275. [DOI] [PubMed] [Google Scholar]
- 45. Skeldon SC, Macdonald EM, Law MR, et al. The cardiovascular safety of dutasteride. J Urol 2017; 197: 1309–1314. [DOI] [PubMed] [Google Scholar]
- 46. Diamanti-Kandarakis E, Mitrakou A, Raptis S, et al. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary synd. J Clin Endocrinol Metab 1998; 83: 2699–2705. [DOI] [PubMed] [Google Scholar]
- 47. Bailey CJ. Biguanides and NIDDM. Diabetes Care 1992; 15: 755–772. [DOI] [PubMed] [Google Scholar]
- 48. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes study (UKPDS) group. Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 50. Zeng XL, Zhang YF, Tian Q, et al. Effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis. Medicine (Baltimore) 2016; 95: e4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang SH, Wu LS, Chiou MJ, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014; 13: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Diamanti-Kandarakis E, Alexandraki K, Piperi C, et al. Effect of metformin administration on plasma advanced glycation end product levels in women with polycystic ovary syndrome. Metabolism 2007; 56: 129–134. [DOI] [PubMed] [Google Scholar]
- 53. Diamanti-Kandarakis E, Paterakis T, Alexandraki K, et al. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod 2006; 21: 1426–1431. [DOI] [PubMed] [Google Scholar]
- 54. Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017; 60: 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev 2002; 18: S10–S15. [DOI] [PubMed] [Google Scholar]
- 56. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab 2011; 96: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Du Q, Wang YJ, Yang S, et al. A systematic review and meta-analysis of randomized controlled trials comparing pioglitazone versus metformin in the treatment of polycystic ovary syndrome. Curr Med Res Opin 2012; 28: 723–730. [DOI] [PubMed] [Google Scholar]
- 58. Glintborg D, Andersen M. Thiazolinedione treatment in PCOS–an update. Gynecol Endocrinol 2010; 26: 791–803. [DOI] [PubMed] [Google Scholar]
- 59. Naka KK, Kalantaridou SN, Kravariti M, et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: a prospective randomized study. Fertil Steril 2011; 95: 203–209. [DOI] [PubMed] [Google Scholar]
- 60. Paneni F, Luscher TF. Cardiovascular protection in the treatment of type 2 diabetes: a review of clinical trial results across drug classes. Am J Cardiol 2017; 120: S17–S27. [DOI] [PubMed] [Google Scholar]
- 61. Hansen L, Deacon CF, Orskov C, et al. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140: 5356–5363. [DOI] [PubMed] [Google Scholar]
- 62. Mentlein R. Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul Pept 1999; 85: 9–24. [DOI] [PubMed] [Google Scholar]
- 63. Elkind-Hirsch K, Marrioneaux O, Bhushan M, et al. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008; 93: 2670–2678. [DOI] [PubMed] [Google Scholar]
- 64. Jensterle M, Kravos NA, Pfeifer M, et al. A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones (Athens) 2015; 14: 81–90. [DOI] [PubMed] [Google Scholar]
- 65. Kahal H, Aburima A, Ungvari T, et al. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr Disord 2015; 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mentz RJ, Bethel MA, Gustavson S, et al. Baseline characteristics of patients enrolled in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL). Am Heart J 2017; 187: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jensterle M, Goricar K, Janez A. Add on DPP-4 inhibitor alogliptin alone or in combination with pioglitazone improved beta-cell function and insulin sensitivity in metformin treated PCOS. Endocr Res 2017: 1–8. [DOI] [PubMed] [Google Scholar]
- 69. Schernthaner G, Cahn A, Raz I. Is the use of DPP-4 inhibitors associated with an increased risk for heart failure? Lessons from EXAMINE, SAVOR-TIMI 53, and TECOS. Diabetes Care 2016; 39 (Suppl. 2): S210–S218. [DOI] [PubMed] [Google Scholar]
- 70. Storgaard H, Bagger JI, Knop FK, et al. Diabetic ketoacidosis in a patient with type 2 diabetes after initiation of sodium–glucose cotransporter 2 inhibitor treatment. Basic Clin Pharmacol Toxicol 2016; 118: 168–170. [DOI] [PubMed] [Google Scholar]
- 71. Rastogi A, Bhansali A. SGLT2 inhibitors through the windows of EMPA-REG and CANVAS trials: a review. Diabetes Ther 2017; 8: 1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 73. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 74. Anneken L, Baumann S, Vigneault P, et al. Estradiol regulates human QT-interval: acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking. Eur Heart J 2016; 37: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chugh SS, Reinier K, Singh T, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation 2009; 119: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ozkececi G, Unlu BS, Dursun H, et al. Heart rate variability and heart rate turbulence in patients with polycystic ovary syndrome. Anatol J Cardiol 2016; 16: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vrtovec B, Meden-Vrtovec H, Jensterle M, et al. Testosterone-related shortening of QTc interval in women with polycystic ovary syndrome. J Endocrinol Invest 2008; 31: 653–655. [DOI] [PubMed] [Google Scholar]