Abstract
In this open-label study, we evaluated the effect of upfront macitentan and riociguat combination in newly diagnosed pulmonary arterial hypertension (PAH) patients. In 15 consecutive PAH patients, we collected clinical and hemodynamic data at baseline, visit 1 (median 4 months) and visit 2 (median 12 months). Survival and transplantation status were analyzed over 36 months. Statistical analysis included student t-test and 95% confidence interval (CI) (t-statistic or Clopper-Pearson). Kaplan-Meier was used to estimate survival rate. There were 11/15 women (mean age 56 years), in World Health Organization (WHO) functional class (FC) III (n = 14) or IV (n = 1). The 6 min walk distance increased from 281.6 m (baseline) to 315.7 m (visit 1) and visit 2 (313.9 m), representing a 34- and 32-m change (P < 0.05), respectively, associated with Borg score improvements. Brain natriuretic peptide decreased: 318.2 pg/mL (baseline) to 122.0 pg/mL (visit 1) and 98.6 pg/mL (visit 2) (P < 0.05). WHO FC improved in eight patients (53%, 95% CI 27%–79%). Pulmonary vascular resistance (9.2 to 5.7 Wood Units) and mean pulmonary artery pressure (47.3 to 38.9 mmHg) decreased; cardiac index increased (2.3 to 3.0 L/min/m2) (baseline to visit 2, all P < 0.05). All patients had intermediate and high risk score (baseline); at 1-year follow-up, dual therapy led to reduction to low risk score in 7/15 (47%) patients. There were no unexpected or serious side effects. Three patients died due to unrelated causes; one patient received a lung transplant. Transplant-free survival rate (36 months) was 85%. Preliminary evidence is provided for effectiveness of initial macitentan and riociguat combination therapy in PAH.
Keywords: pulmonary arterial hypertension, macitentan, riociguat, upfront combination
Introduction
Pulmonary arterial hypertension (PAH) is a rare and progressive disease characterized by vascular proliferation and vasoconstriction of the pulmonary arterial bed, associated with increased pulmonary vascular resistance, which, over time, may lead to progressive clinical deterioration resulting in right ventricular failure and death.1,2 PAH is diagnosed by right heart catheterization (RHC) and, at the time of study enrollment, was defined as a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg at rest, pulmonary artery occlusion pressure (PAOP) ≤ 15 mmHg, and pulmonary vascular resistance (PVR) > 3 Wood Units.3–5 The broader term “pulmonary hypertension” is categorized into five World Health Organization (WHO) groups according to clinical presentation, pathological findings and hemodynamic characteristics.5 WHO Group 1 includes patients with PAH.
Dysfunction in three pathways (endothelin, nitric oxide (NO), and prostacyclin) involved in PAH pathophysiology has now been targeted with five classes of medication (endothelin receptor antagonists (ERAs), phosphodiesterase type 5 (PDE-5) inhibitors, soluble guanylate cyclase (sGC) stimulators, prostacyclin analogues, and prostacyclin receptor agonists).6 Fourteen formulations of medications from these pathways are approved by the Food and Drug Administration (FDA) for treatment of PAH. Despite treatment, mortality remains high in patients with PAH, with the lack of curative therapy.7 Treatment goals in PAH include improvement in symptoms and functional capacity, slowing of disease progression, and increased survival.8
Given the complex pathogenesis of PAH, there is a strong rationale to use combinations of drugs that target multiple pathways simultaneously. Historically, combination therapy in PAH has been used in a sequential manner in patients with inadequate response to monotherapy. There is a growing body of evidence that dual, or even triple, upfront combination therapy in PAH leads to improved clinical outcomes.9,10 The Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension (AMBITION) trial is the first randomized controlled trial of initial dual combination therapy, and showed a highly significant reduction in the risk of clinical failure for patients on upfront dual therapy as compared with the pooled monotherapy arm.11 This clinical trial did not, however, include hemodynamic data, and it is currently unknown if this is a class effect, and if similar results might be obtained by substituting the respective agents with other compounds from the respective class.
In this analysis, we sought to evaluate the effect of initial combination of macitentan and riociguat in patients newly diagnosed with PAH. In our practice, we have successfully employed macitentan and riociguat combination either as de novo therapy, or as transition from other NO-pathway-ERA combinations.12 Macitentan is an ERA that was developed by modifying the structure of bosentan to increase efficacy and safety.13 Macitentan has been shown to reduce morbidity and mortality among patients with PAH.14 Riociguat—a sGC stimulator—has a dual mode of action: synergy with endogenous NO and stimulation of sGC independent of NO availability.15,16 Riociguat has shown improved hemodynamic variables, symptoms, and exercise capacity in patients with PAH, and recent data showed potential benefit in patients who do not reach treatment goals with PDE-5 inhibitors.12,15,17–19
Methods
Objectives
Our goal was to evaluate the clinical and hemodynamic effect of the combination of macitentan and riociguat as the first-line treatment in PAH patients from our practice. We defined as initial or upfront combination the use of the two medications in the following manner: the decision to start both medications in combination was made upon the diagnostic RHC without a waiting period to assess the effect of the medication started first, and if logistical considerations (insurance approval) allowed medication initiation within less than 3 months from each other.
Patients
Between 2014 and 2016, we evaluated 15 consecutive newly diagnosed PAH patients (incident cases) from the Mount Sinai Beth Israel Pulmonary Hypertension Program who received upfront dual macitentan and riociguat combination therapy. The diagnosis of PAH was established at RHC and defined by mPAP ≥ 25 mmHg at rest, mean PAOP ≤ 15 mmHg, and PVR > 3 Wood units.4 We included only patients diagnosed with idiopathic PAH (IPAH) or associated PAH (APAH) as determined by the recommended diagnostic algorithm. We excluded patients with high risk PAH in need of parenteral PAH therapy.
Data collection and study design
The study was a retrospective chart review of consecutive PAH patients in our program who received upfront macitentan-riociguat combination in an open-label fashion. The choice of this particular combination was made at the discretion of the treating physician. At baseline, we collected demographic, clinical (WHO functional class (WHO FC), 6-min walk distance [6MWD], Borg score, and brain natriuretic peptide (BNP)), and hemodynamic data. We collected follow-up data at two time points. The first follow-up, visit 1, was planned in accordance to current guidelines at 3–4 months after medication combination initiation. It was practically performed at a median time interval of 4 months (range 3–10 months), and mean of 4.9 months (SD 3.8 months). Visit 1 included evaluation of WHO FC, 6MWD, Borg score, and BNP levels. The second follow-up, visit 2, was performed at a median of 12.0 months (range 6–12 months), mean 13.7 months (SD 3.6 months), as dictated by the time of the first follow-up RHC on combination therapy. Throughout the study, patients could receive prostacyclin therapy in case of clinical or hemodynamic deterioration, or in clinical scenarios that required additional PAH treatment (for example, one patient had parenteral prostacyclin started after 6 months of dual macitentan-riociguat therapy for hemodynamic optimization for planned abdominal surgery). In these cases, both clinical and hemodynamic data presented as the second follow-up was collected prior to starting the third PAH medication. Clinical outcome data (survival and transplantation status) was collected by 12 September 2018, with a median time of 41.3 months (mean 41.5 months, SD = 10.4 months), with a total follow-up time from the moment of the last patient’s enrollment of 56.8 months. Throughout the study, we collected information on adverse events, including medication side effects and clinical deterioration events (death, transplantation, hospital admission for right heart failure, and the need to escalate PAH therapy due to clinical and/or hemodynamic deterioration or lack of improvement). The local institutional review board approved the protocol and data collection.
Statistical analysis
Statistical analysis, mainly descriptive, also included student t-test, 95% CI from t statistic and Clopper-Pearson method as appropriate. Survival time was calculated from the initiation of the first PAH therapy. Patients who were alive as of 12 September 2018 were censored on that date. Survival rate was estimated by Kaplan-Meier method. Patients were classified as low, intermediate, and high risk at baseline and follow-up visit 2, where RHC were measured. Average risk from variables WHO FC, 6MWD, BNP, right atrial pressure, cardiac index, and mixed venous oxygen saturation were calculated per European Society of Cardiology (ESC)/ European Respiratory Society (ERS) 2015 guidelines to determine patient’s risk group.
Results
Baseline characteristics were as follows; our patients had a female predominance, with 11/15 (73.3%) women, and a mean age of 55.8 years (range from 27 to 82 years). At the time of treatment initiation (baseline), all patients belonged to WHO FC III, with the exception of one patient with systemic lupus erythematosus (SLE)-PAH who was classified as WHO FC IV based on the history of exertional syncope. She had been hospitalized and briefly received subcutaneous treprostinil, but the medication had been discontinued at patient’s request when she achieved a dose of 16 ng/kg/min. We have included her in the analysis since she was switched to dual macitentan-riociguat combination, started upon discharge, subsequent to discontinuation of treprostinil. Six patients (40.0%) had IPAH, and nine patients had APAH (including six patients with connective tissue disease and five patients associated with other risk factors) (Table 1).
Table 1.
Demographics and baseline characteristics.
| Variable | All patients |
|---|---|
| Age (year) at Baseline, 15 patients, mean (SD) | 55.8 (17.1) |
| Median | 55.0 |
| Gender, n (percent) | |
| Female | 11 (73.3%) |
| Male | 4 (26.7%) |
| Baseline WHO Functional Class, n (percent) | |
| III | 14 (93.3%) |
| IV | 1 (6.7%) |
| Time to RHC (months), 14 patients, mean (SD) | 14.2 (4.7) |
| Median | 14.0 |
| Pulmonary Hypertension Risk Factors, n (percent) | |
| ASD, Cirrhosis, HIV | 1 (6.7%) |
| ASD, VSD | 1 (6.7%) |
| CTD-Scleroderma | 5 (33.3%) |
| CTD-RA | 1 (6.7%) |
| HIV | 1 (6.7%) |
| IPAH | 6 (40.0%) |
ASD: atrial septal defect; CTD: connective tissue disease; HIV: human immunodeficiency virus; IPAH: idiopathic pulmonary arterial hypertension; RA: rheumatoid arthritis; RHC: right heart catherization; VSD: ventricular septal defect; WHO: World Health Organization.
Patients were included in the study with follow-up for survival and transplantation status by 12 September 2018, with a median time of 41.3 months (mean 41.5 months, SD = 10.4 months). The mean (SD) time of first follow-up was at 4.9 (3.8) months and 13.7 (3.6) months at time of second follow-up. At the end of study, 12 patients were alive, including 1 patient who had received lung transplantation, and 3 patients had died.
Data on 6MWD was available in 14 out of 15 patients, since 1 patient with multifactorial PAH (atrial septal defect (ASD), portal hypertension and HIV infection) was wheelchair-bound due to a prior stroke and was unable to walk. For the group, the 6MWD increased from a mean of 281.6 m at baseline to 315.7 m at the first follow-up and was maintained at 313.9 m at the second follow-up, representing a 6MWD increase of 34 m (P < 0.05) and 32 m (P < 0.05), respectively. For this patient population, the mean 6MWD at baseline of 281.6 m ranged from 91.4 to 457.2 m. Four patients that had exercise limitation due to musculoskeletal reasons with reduced 6MWD at 91.44, 179.82, 198.12, and 213.36 m. We therefore analyzed the change by using percent change from baseline. The mean percent change was 12.3% (P < 0.05) and 13.5% (P < 0.05) at follow-up visits 1 and 2, respectively (Table 2).
Table 2.
Summary of 6-minute walk distance (6MWD) and Borg at baseline, first follow-up and second follow-up (n = 14).
| Parameter | Baseline mean (SD) | First follow-up mean (SD) | Change from baseline mean (SD) | P-value* for change | %Change from baseline mean (SD) | P-value* for %change | Second follow-up mean (SD) | Change from baseline mean (SD) | P-value* for change | %Change from baseline mean (SD) | P-value* for %change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6MWD (m) | 281.6 (93.4) | 315.7 (108.4) | 34.1 (56.6) | 0.0421 | 12.3 (20.2) | 0.0397 | 313.9 (108.4) | 32.2 (58.8) | 0.0610 | 13.5 (19.9) | 0.0244 |
| Borg | 3.0 (2.0) | 1.7 (1.9) | –1.3 (2.0) | 0.0295 | NA | NA | 2.0 (2.7) | –1.0 (2.7) | 0.2087 | NA | NA |
Note: P-value is calculated from paired t-test to test whether or not the change is equal to zero. MWD: 6-minute walk distance; NA: not applicable; SD: standard deviation.
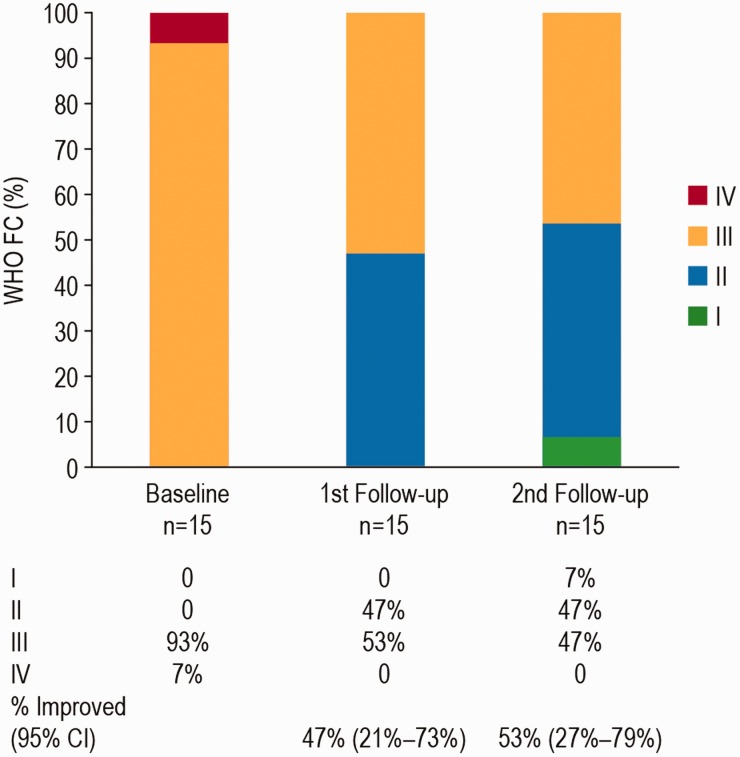
Mean Borg score was 3.0 at baseline, and decreased to 1.7 at the first follow-up and 2.0 at the second follow-up (Table 2). Mean BNP decreased from 318.2 pg/mL at baseline to 122.0 pg/mL at first follow-up and 98.6 pg/mL at second follow-up (P < 0.05 for the second follow-up compared with Baseline (Table 3). There was an improvement in FC in 8 (53%, 95% CI 27%–79%) patients, and no patient had FC deterioration (Fig. 1).
Table 3.
Biomarker (brain natriuretic peptide (BNP), pg/mL) at baseline, first follow-up, and second follow-up (n = 15).
| Baseline mean (SD) | First follow-up mean (SD) | Change from baseline mean (SD) | P-value* for change | Second follow-up mean (SD) | Change from baseline mean (SD) | P-value* for change |
|---|---|---|---|---|---|---|
| 318.2 (369.7) | 122.0 (156.2) | –196.2 (365.7) | 0.0566 | 98.6 (62.6) | –219.7 (325.4) | 0.0204 |
Note: P-value is calculated from paired t-test to test whether or not the change is equal to zero.
Fig. 1.
Functional class status at baseline, first follow-up, and second follow-up. First follow-up: median time of 4 months (range 3–10 months) and a mean of 4.9 months (SD 3.8 months). Second follow-up was performed at a median of 12 months (range 6–20 months), and a mean of 13.7 months (SD 3.6 months). From baseline to second follow-up survival was 100% (long-term survival data is presented separately, see Fig. 2). CI: confidence interval; FC: functional class; WHO: World Health Organization.
One patient refused repeat RHC, and complete hemodynamic assessment was available in only 14 patients. Significant improvements in mean hemodynamic measurements included PVR decrease from 9.2 to 5.7 Wood Units, mPAP reduction from 47.3 to 38.9 mmHg, CI increase from 2.3 to 3.0 L/min/m2, and cardiac output (CO) increase from 4.1 to 5.2 L/min from baseline (Table 4).
Table 4.
Summary of right heart catherization data at baseline and follow-up.
| Parameters | Baseline (n = 15); mean (SD) | Follow-up (n = 14); mean (SD) | Change from baseline mean (SD) | P-value* for change |
|---|---|---|---|---|
| Mean blood pressure (mmHg) | 100.4 (11.7) | 87.1 (12.0) | –13.3 (12.8) | 0.0019 |
| Heart rate (bpm) | 85.1 (15.1) | 79.1 (11.3) | –7.9 (18.9) | 0.1412 |
| O2 saturation (%) | 93.9 (2.8) | 93.2 (3.5) | –0.9 (3.2) | 0.3038 |
| RAP (mmHg) | 11.2 (4.4) | 8.0 (3.4) | –2.8 (5.1) | 0.0632 |
| SPAP (mmHg) | 77.8 (17.9) | 65.2 (12.3) | –11.1 (15.0) | 0.0158 |
| DPAP (mmHg) | 33.1 (8.5) | 28.4 (6.3) | –4.9 (6.6) | 0.0157 |
| mPAP (mmHg) | 47.3 (10.0) | 38.9 (6.9) | –8.1 (8.6) | 0.0039 |
| PA saturation (%) | 59.3 (7.5) | 68.5 (3.4) | 9.7 (7.6) | 0.0004 |
| CO (L/min) | 4.1 (0.8) | 5.2 (1.0) | 1.2 (0.6) | <.0001 |
| CI (L/min/m2) | 2.3 (0.4) | 3.0 (0.5) | 0.7 (0.4) | <.0001 |
| PVR (Wood units) | 9.2 (3.0) | 5.7 (1.8) | –3.6 (2.5) | 0.0001 |
| PVRI (Wood units/m2) | 16.9 (5.6) | 10.0 (3.5) | –6.9 (4.6) | <.0001 |
Note: p-value is calculated from paired t-test to test whether or not the change is equal to zero. CI: cardiac index; CO: cardiac output; DPAP: diastolic pulmonary arterial pressure; mPAP: mean pulmonary arterial pressure; O2: oxygen; PAS: pulmonary artery systolic pressure; PVR: pulmonary vascular resistance; PVRI: pulmonary vascular resistance index; RAP: right arterial pressure; SPAP: Systolic pulmonary artery pressure.
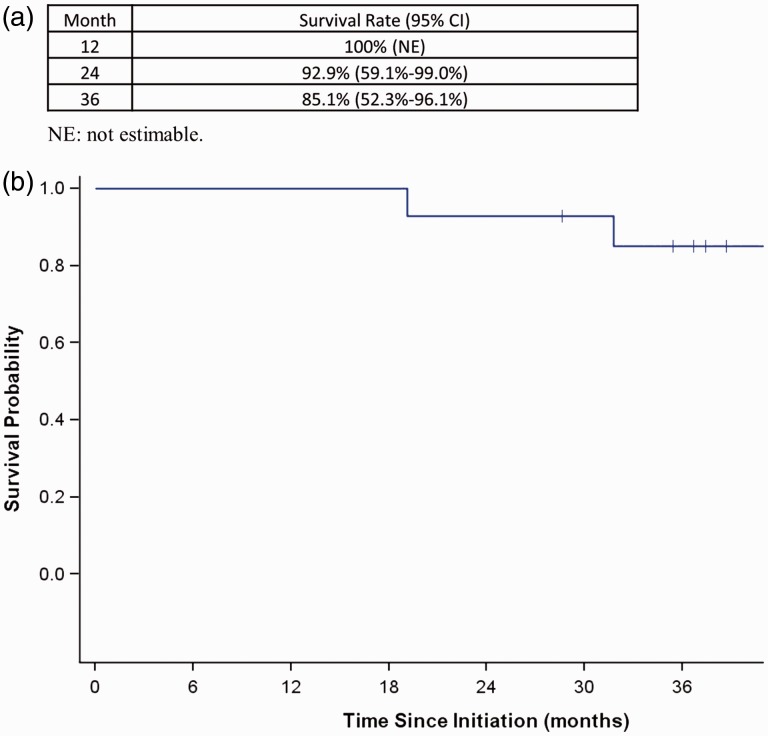
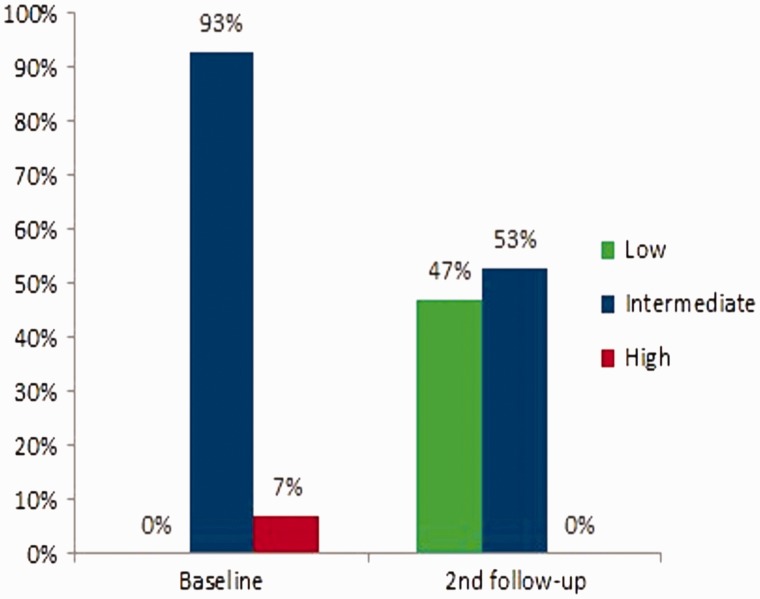
Kaplan Meier analysis showed a survival of 100% at 1 year, 92.9% at 2 years, and 85.1% at 3 years (Fig. 2). When the risk of progression and adverse outcomes defined by the 2015 European guidelines at baseline and follow-up were examined for the group, treatment effect led to achievement of a low status in approximately 50% of the patients at the first follow-up, which had been maintained at the second follow-up. In Fig. 3 we present data at baseline and at follow-up visit 2, the two time points where the largest number of parameters to calculate the risk score were available.
Fig. 2.
Kaplan-Meier curve.
At Baseline, 14 patients (93%) were at intermediate risk and 1 patient (7%) was at high risk (Fig. 3). At follow-up visit 2, seven patients (47%) had a risk reduction; six patients (40% had a decrease in their risk from intermediate to low, and one patient (7%) from high to low); eight patients (53%) had no change and maintained intermediate risk status (Fig. 3).
Fig. 3.
Risk score at baseline and second follow-up. Risk Score was calculated average score from individual assessments collected in the study including WHO FC, 6MWD, BNP, and RHC. Second follow-up was performed at a median of 12 months (range 6–20 months), and a mean of 13.7 months (SD 3.6 months).
Mild medication-related side effects were recorded in five patients: two patients had increased nasal congestion, two patients had headaches, and one patient had increased lower extremity edema, all resolved with supportive therapy. Riociguat up-titration was stopped in three patients due to hypotension, and in one patient due to headache, but the medication had been continued at a lower dose, with 73% of the patients achieving and maintaining maximum FDA-approved dose of 2.5 mg po tid.
By the end of the second follow-up visit, one patient required hospital admission for right heart failure/fluid overload, which resolved with intravenous diuretics. By the end of the observation period, this patient was still receiving treatment with dual therapy only, with stable 6MWD, and improved RV function by echocardiogram.
Four patients required addition of a third medication. One patient has been started on continuous prostacyclin at 6 months for hemodynamic optimization due to impending cholecystectomy, while three other patients required a third class of drugs, as a measure of insufficient therapeutic response, two patients due to lack of improvement, and one patient due to clinical and hemodynamic deterioration.
The two patients who received additional prostacyclin therapy due to lack of clinical and/or hemodynamic improvement at the second follow-up, were both started on inhaled treprostinil, but one of them switched subsequently to selexipag, for reasons of convenience. By the end of the observation period, one more patient had been started on additional selexipag due to clinical deterioration (increased exertional symptoms and decreasing 6MWD). One patient with scleroderma received bilateral lung transplant, and there were three deaths at 19, 32, and 51 months since initiation of the dual therapy, all from causes unrelated to pulmonary hypertension: one intra-abdominal sepsis in a patient with cirrhosis, one hypoxic respiratory failure due to aspiration pneumonia in a patient with scleroderma, and one with Acute Respiratory Distress Syndrome (ARDS) from bacterial pneumonia in an 84-year-old patient with rheumatoid arthritis. At month 36, overall survival rate was 85.1% (Fig. 2). Of the 11 non-transplanted patients alive at 36 months, 7 were still on dual macitentan-riociguat therapy.
Discussion
Currently, there is ample evidence that supports the use of dual upfront combination therapy as the standard of care in most patients with low and intermediate risk PAH.11 Most of the available data demonstrates that the initial combination of an ERA with a PDE-5 inhibitor leads to significant clinical, hemodynamic, and functional improvement in PAH patients.20–25
In this retrospective analysis of real-world data, we provide the first evidence in the literature of the use of upfront combination of macitentan and riociguat in incident patients with PAH. Results of this study demonstrate improvement in the exercise capacity and functional class, degree of dyspnea on exertion, right ventricular function biomarkers, and hemodynamic variables, which represent guideline-recommended treatment targets, known to be associated with outcome and survival benefit.
The 6MWD in our study showed a significant increase at first follow-up and it was maintained at the second follow-up, and was comparable with the minimally important difference in PAH trials of 33 m.26 It did not, however, reach the target distance on therapy of 380 m or 440 m associated with improved outcomes.27,28,29 One explanation for this observation is the fact that a large number of patients in our cohort had a low initial 6MWD, not because of the PAH and right ventricular impairment, but due to musculoskeletal limitation, associated with co-morbidities, arthritis in rheumatologic disorders, fatigue in cirrhosis, or HIV neuropathy. This explanation is substantiated by the low 6MWD at baseline of 281.6 m, which is substantially shorter than the 324 m or 353 m reported in comparable retrospective case series of upfront dual ERA-PDE-5 inhibitor combination, such as the one from France or Italy.20,23 This lower baseline distance is encountered in PAH patients with co-morbidities, as it has been described in the scleroderma PAH series in the French registry or in contemporary PAH registries that include older patients.30 In the COMPERA registry, the average age was 64 years, and the baseline 6MWD 298 m. The improvement in exercise capacity had been associated with a significant decrease in the Borg index of dyspnea on exertion, which was significantly lower by 1.3 and 1 units at first and second follow-up, and was of a similar magnitude to that described in the French and Italian case series.
There was a significant improvement in FC; from a majority of FC III and IV on presentation, more than half of the patients achieved the goal of FC I and II at the second follow-up, again with similar findings to the Sitbon and D’Alto cohorts.20,23
BNP, a marker of right ventricular function, had normalized at the first follow-up and decreased even further by the time of the second follow-up. Hemodynamic follow-up demonstrated a mean mPAP decrease by 8.1 mmHg and below 40 mmHg, while PVR had decreased by 40%, which is the expected change in studies of dual upfront combination therapy (Table 4).20 Mean hemodynamic parameters of right ventricular function all showed significant improvement; RAP was 8 mmHg, CI 3 L/min/m2, and PA saturation 68.5%—all consistent with the prognostic status of low risk (Table 4).29
Compared with individual results from both PATENT and SERAPHIN trials, improved hemodynamic parameters in our study showed a more substantial change as effect of therapy, with a mean decrease in the mPAP of 8.1 mmHg and a mean increase in the CI of 0.7 L/min/m2. For the treatment naïve riociguat-treated patients in PATENT mean reduction in mPAP was 4.4 mmHg.31 Similarly, for patients treated with 10 mg of macitentan without PAH background therapy in the SERAPHIN trial, the mean decrease at 6 months in mPAP was 7 mmHg.32 The mean increase of 0.7 L/min/m2 in CI in our series also compares favorably with the mean increase in CI of 0.6 L/min/m2 in PATENT and 0.32 L/min/m2 in SERAPHIN.
Survival data shows results comparable with other studies of upfront combination therapy;20 in our study, three recorded deaths were unrelated to pulmonary hypertension, but were secondary to intraabdominal sepsis, aspiration pneumonia with acute respiratory failure, and bacterial pneumonia with ARDS. This is the first study of upfront dual combination therapy to evaluate the effect of treatment on the risk score, and we have shown that, in a cohort of patients at intermediate and high risk, a status of low risk can be achieved in approximately half of these patients as early as 4 months, and can be maintained at 12 months, without the addition of a third drug. It was also noted that we have observed only one episode of worsening of right ventricular function throughout the duration of the study.
Side effect profile of the combination was favorable, with no unexpected adverse events that were treated with supportive measures. Even though for the group the mean blood pressure had decreased (Table 4), the change was mostly not clinically significant. In four patients, riociguat uptitration had to be stopped due to blood pressure decrease, with 73% of patients achieving the maximal dose of 2.5 mg tid. This compares favorably with data from PATENT study, in which 75% of patients received the maximal dose, but 50% of the patients had been on riociguat monotherapy.
The main limitations to our study include the single center and retrospective design, and the relatively small number of patients with heterogeneous etiologies for PAH. The concept of upfront combination therapy in PAH is gaining momentum, and our study offers preliminary evidence for the use of this novel dual combination in patients with advanced PAH.
Conclusion
Current clinical guidelines recommend upfront dual therapy in most patients with PAH and functional class II–III symptoms. This small retrospective cohort of patients with group I PAH is the first report to show that other dual combinations besides ambrisentan and tadalafil may be used. Further investigations, with a more definitive design are required to delineate the role of this upfront combination in PAH.
Contributorship
Roxana Sulica contributed to the research design and writing of the manuscript. All authors revised critically for important intellectual content, approved and agree to be accountable for all aspects of the manuscript.
Declaration of Conflicting Interests
Roxana Sulica: Ad board Actelion, Arena, Bayer, United Therapeutics; research Reata, Bellerophon, United Therapeutics Swathi Sangli: The author has no conflict of interest. Aloke Chakravarti: The author has no conflict of interest. David Steiger: The author has no conflict of interest.
Ethical approval
The local institutional review board approved the protocol and data collection.
Funding/Acknowledgments
Actelion Pharmaceuticals US, Inc provided funding for this manuscript produced by Donna Simcoe of Simcoe Consultants, Inc., and did not contribute to the content nor provide any review or editorial support. The manuscript was written independently by the authors with writing support provided by Donna Simcoe, MS, MS, MBA, CMPP, of Simcoe Consultants, Inc and statistical support provided by Carol Zhao, Actelion Pharmaceuticals US, Inc. All statements and opinions expressed in the manuscript are those of the authors and do not reflect those of Actelion Pharmaceuticals US, Inc or its representatives.
Guarantor
Roxana Sulica accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish the work.
References
- 1.Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: From the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J 2010; 31: 2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009; 119: 2250–2294. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Lau EMT, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014; 130: 2189–2208. [DOI] [PubMed] [Google Scholar]
- 7.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D73–D81. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon O, Jaïs X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43(6): 1691–1697. [DOI] [PubMed] [Google Scholar]
- 10.Sitbon O, Canuet M, Picard F, et al. Initial combination therapy with macitentan and tadalafil in newly diagnosed patients with pulmonary arterial hypertension: Results from the OPTIMA Trial. InA68. WOW: PHARMACOLOGICAL TREATMENT OF PULMONARY HYPERTENSION 2017 May, pp. A2297–A2297. New York: American Thoracic Society.
- 11.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373(9): 834–844. [DOI] [PubMed] [Google Scholar]
- 12.Sulica R, Fenton R, Cefali F. Early observations on the use of riociguat in a large, metropolitan pulmonary arterial hypertension/chronic thromboembolic pulmonary hypertension treatment center. Cardiol Ther 2015; 4(2): 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolli MH, Boss C, Binkert C, et al. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl) oxy] ethoxy]-4-pyrimidinyl]-N′-propylsulfamide (macitentan), an orally active, potent dual endothelin receptor antagonist. J Med Chem 2012; 55(17): 7849–7861. [DOI] [PubMed] [Google Scholar]
- 14.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369(9): 809–818. [DOI] [PubMed] [Google Scholar]
- 15.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009; 33(4): 785–792. [DOI] [PubMed] [Google Scholar]
- 16.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011; 123(20): 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 2010; 36(4): 792–799. [DOI] [PubMed] [Google Scholar]
- 18.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369(4): 330–340. [DOI] [PubMed] [Google Scholar]
- 19.Hoeper MM, Simonneau G, Corris PA, et al. RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J 2017; 50(3): 1602425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitbon O, Sattler C, Bertoletti L, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 2016; 47(6): 1727–1736. [DOI] [PubMed] [Google Scholar]
- 21.Hassoun PM, Zamanian RT, Damico R, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192(9): 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Veerdonk MC, Marcus JT, Westerhof N, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J 2017; 49(6): 1700007. [DOI] [PubMed] [Google Scholar]
- 23.D’Alto M, Romeo E, Argiento P, et al. Initial tadalafil and ambrisentan combination therapy in pulmonary arterial hypertension: cLinical and haemodYnamic long-term efficacy (ITALY study). J Cardiovasc Med (Hagerstown) 2018; 19(1): 12–17. [DOI] [PubMed] [Google Scholar]
- 24.Badagliacca R, Raina A, Ghio S, et al. Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37(3): 365–375. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, Ambale-Venkatesh B, Lima JA, et al. The impact of ambrisentan and tadalafil upfront combination therapy on cardiac function in scleroderma associated pulmonary arterial hypertension patients: cardiac magnetic resonance feature tracking study. Pulm Circ 2018; 8(1): 2045893217748307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathai SC, Puhan MA, Lam D, et al. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Critical Care Med 2012; 186(5): 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40(4): 780–788. [DOI] [PubMed] [Google Scholar]
- 28.Provencher S, Chemla D, Herve P, et al. Heart rate responses during the 6-minute walk test in pulmonary arterial hypertension. Eur Respir J 2006; 27(1): 114–120. [DOI] [PubMed] [Google Scholar]
- 29.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010; 122(2): 164–172. [DOI] [PubMed] [Google Scholar]
- 30.Weatherald JC, Boucly A, Taniguchi Y, et al. Hemodynamics and prognosis in scleroderma-associated PAH. InA27. You got another thing coming: diagnosis and prognostication in pulmonary hypertension. May 2018, pp. A1179–A1179). New York: American Thoracic Society.
- 31.Galiè N, Grimminger F, Grünig E, et al. Comparison of hemodynamic parameters in treatment-naïve and pre-treated patients with pulmonary arterial hypertension in the randomized phase III PATENT-1 study. J Heart Lung Transplant 2017; 36(5): 509–519. [DOI] [PubMed] [Google Scholar]
- 32.Galiè N, Jansa P, Pulido T, et al. SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur Heart J 2017; 38(15): 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]