Short abstract
Little is known about the mechanisms involved in the regulation of nociceptin and its receptor (nociceptin opioid peptide receptor, NOP) in response to inflammation and pain in humans. In this study, specific signaling pathways contributing to the regulation of nociceptin and NOP in human peripheral blood leukocytes were investigated. After approval by the ethics committee, peripheral blood obtained from healthy donors was cultured with or without phorbol-12-myristate-13-acetate (PMA). Prepronociceptin (ppNOC) and NOP mRNA were analyzed by real-time quantitative polymerase chain reaction, and nociceptin concentrations in culture supernatants by fluorescent enzyme immunoassay. Nociceptin and NOP protein levels in blood leukocyte subsets were determined using flow cytometry. To examine the contribution of signaling pathways to ppNOC and NOP regulation, blood was pre-treated with kinase inhibitors specific for ERK, JNK, p38, and NFκB pathways prior to culturing with or without PMA. PMA dose-dependently upregulated ppNOC mRNA but downregulated NOP mRNA in human peripheral blood leukocytes. PMA 10 ng/ml increased ppNOC after 6 h and suppressed NOP after 3 h compared to controls (both P <0.005). Nociceptin concentrations were increased in supernatants of PMA-induced blood samples after 24 h (P <0.005), whereas expression of cell-membrane NOP was decreased by PMA in blood leukocyte subsets (all P <0.05). Blockade of ERK or p38 pathways partially prevented PMA effects on ppNOC and NOP mRNA (all P <0.05). The combination of ERK and p38 inhibitors completely reversed the effects of PMA (P <0.05). ERK and p38 are two major signaling pathways regulating nociceptin and its receptor in human peripheral blood leukocytes under inflammatory conditions.
Keywords: Leukocytes, nociceptin, nociceptin receptor, phorbol-12-myristate, signal transduction
Introduction
Opioids are powerful analgesics, but their widespread use has been questioned of late due to their related side effects, including respiratory depression, the risk of addiction, and lack of evidence supporting long-term effectiveness in the management of chronic pain.1,2 Thus, alternative analgesics with a more favorable side effect profile would be welcome treatment options.
Preclinical and clinical studies have indicated that compounds targeting the nociceptin opioid peptide receptor (NOP) are safe and effective alternatives to opioids in the treatment of pain or inflammatory disorders.3–5 Meanwhile, the role of NOP and its endogenous ligand nociceptin in pain processing and inflammation has been confirmed.3,6,7 Despite the close homology to opioids and classical opioid receptors, NOP and nociceptin are distinct from the opioid family.8–10 Classical opioid receptors cannot be detected in human peripheral blood cells, whereas both nociceptin and NOP are constitutively expressed.11–13 NOP and nociceptin exert immunomodulatory effects in circulating leukocytes during nociceptive processes and inflammation.6,14 Aberrant expression of NOP and nociceptin in peripheral blood of patients suffering from pain or inflammatory diseases has been described previously.6,7,14,15 Analysis of mechanisms contributing to the regulation of nociceptin and NOP may provide new insight into the treatment of pain and inflammation. Previous experimental approaches focused on signaling pathways triggered by the activation of the nociceptin system or the regulation of NOP and nociceptin mRNA expression in astrocyte or MM6 cell cultures.16,17 In contrast, information on signaling pathways involved in human blood leukocytes is still scarce.
Ex vivo whole blood culture systems are suitable models to investigate mechanisms of gene regulation in different leukocyte subsets. Stimulation of blood cells with phorbol-12-myristate-13-acetate (PMA) or lipopolysaccharide (LPS) is a common method used to activate leukocytes. Whereas LPS binds to a cell surface receptor complex, PMA directly diffuses through the cell membrane and activates protein kinase C in cytoplasm, triggering downstream intracellular signaling pathways (ERK, JNK, p38, and NFκB).18,19 PMA has frequently been used to stimulate cells by mimicking human monocyte-to-macrophage differentiation and has proven to be an optimal stimulus in immune cells.20 In our previous studies, PMA was the only substance with an upregulating effect on nociceptin in comparison to other inflammatory stimuli.13,17
The aim of our study was to investigate signaling pathways contributing to the regulation of NOP and nociceptin mRNA expression in human blood leukocytes under inflammatory conditions and to verify their protein profiles in blood leukocyte subsets. The hypothesis was that specific signal transduction pathways regulate nociceptin and its receptor in human peripheral blood leukocytes.
Materials and methods
The study was approved by the local ethics committee (Kantonale Ethikkommission: KEK 041/09). Fresh heparinized blood samples were randomly obtained from the Interregionale Blutspende SRK Bern directly before the experiments. Experiments were not conducted in parallel, but consecutively, and for each experiment, blood of different donors was used. In the different phases of the study, we did not have access to the exact same number of volunteers available for blood donation. In total, 50 donors were enrolled after giving written informed consent. The eligibility criteria for donation were age between 18 and 60 years, a body weight of at least 50 kg, no medical or hospital treatment received, no tattoo or piercing during the last four months, and no major surgery or childbirth during the last 12 months.
Whole blood cultures
Whole blood was cultured in 48-well flat-bottom culture plates (BD Bioscience, Allschwil, Switzerland) at a volume of 450 µl per well. All reagents were freshly prepared in RPMI 1640 medium supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich, Buchs, Switzerland). Cultures were incubated at 37°C in a 5% CO2 atmosphere.
Dose-response experiments
In a previous study, PMA increased prepronociceptin (ppNOC) and decreased NOP in MM6, with maximum effects after 24 h and after 6 h, respectively.17 Therefore, ppNOC and NOP mRNA were quantified in blood leukocytes after culturing with or without PMA (0.1–300 ng/ml; Sigma-Aldrich, Buchs, Switzerland) for 24 h or 6 h. Blood samples from four donors were used in order to address possible variation and to investigate dose-dependent effects of PMA on ppNOC and NOP mRNA expression. Based on these dose-response experiments, PMA 10 ng/ml was used in subsequent cultures. To investigate the influence of PMA on nociceptin and NOP, whole blood was treated with or without PMA 10 ng/ml for 0, 3, 6, 9, 12, 24, 48, and 72 h. In each experiment, samples without stimuli served as controls (control group). Blood leukocytes were used for the detection of nociceptin and NOP mRNA and protein levels. Culture supernatants were collected for the measurement of nociceptin concentrations.
Interference with signal transduction pathways
In order to assess the involvement of ERK, JNK, p38, and NFκB signaling pathways in the regulation of ppNOC and NOP mRNA by PMA, intervention experiments employing specific kinase inhibitors were conducted. Blood was pre-treated with PD98059 (PD) 30 μM, SP600125 (SP) 10 μM, SB203580 (SB) 10 μM, Bay 11-7871 (Bay) 3 μM, or the combination of PD and SB (all from Tocris Bioscience, Bristol, UK) for 1 h prior to culturing with or without PMA 10 ng/ml for 6 and 24 h. The concentrations of the inhibitors were based on doses used in previous studies.17,21,22 A culture without any stimulus and one cultured only with PMA 10 ng/ml served as control group and reference group, respectively.
RNA isolation, cDNA synthesis, and relative quantification
Samples were collected at the predefined time points, red blood cells were lysed by the red blood cell lysis buffer, and total RNA was isolated from the leukocytes using the High Pure RNA Isolation Kit following the manufacturer’s protocol (Roche, Rotkreuz, Switzerland). Leukocytes were resuspended in 200 µl PBS and lysed by 400 µl Lysis/Binding Buffer. Cell lysates were then loaded into a High Pure Filter Tube and centrifuged. DNase I solution was applied directly onto the glass fiber fleece and incubated for 15 min at room temperature. Subsequently, the tube was washed with Wash Buffer I and II, and RNA was eluted using 50 µl elution buffer. RNA concentrations and purity were measured by a NanoDrop 2000 (Thermo Scientific, Reinach, Switzerland), and cDNA was subsequently synthesized (Transcriptor First Strand cDNA Synthesis kit, Roche, Rotkreuz, Switzerland).
Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using LightCycler® 480 Probes Master Mix and RealTime ready Assays on a LightCycler® 480 II (Roche, Rotkreuz, Switzerland) as published previously.17 Sequences of the primers used for RT-qPCR are listed in Table 1. Hypoxanthine phosphoribosyl-transferase 1 (HPRT1) and polymerase (RNA) II (DNA directed) polypeptide A (POLR2A) were selected as reference genes. cDNA from SK-N-DZ, a human neuroblastoma cell line, served as a calibrator. Standard curves of ppNOC, NOP, HPRT1, and POLR2A were generated by PCR amplification in a series of diluted cDNA in triplicate. Data of RT-qPCR, including calibrator and samples, were analyzed using the E-Method of the LightCycler® 480 Relative Quantification Software. The calculation of the relative amount of ppNOC, NOP, and the reference genes was based on the crossing point value of a sample and the efficiencies specified by the standard curves. After calculation of the target-to-reference ratios for each sample and for the calibrator, the target/reference ratio of the sample was then divided by the target/reference ratio of the calibrator (normalized ratio).
Table 1.
Real-time quantitative polymerase chain reaction primer sequences.
| Gene | Assay identification no.a | Forward sequence | Reverse sequence | |
|---|---|---|---|---|
| ppNOC | 118250 | GGACAGCTTCGACCTGGAG | TGACCTTGGTGCATGGAGT | |
| NOP | 126571 | CCCAAGGAGGTTGCAGAA | GCCGTAGATAACCTCCCAGA | |
| HPRT1 | 102079 | TGACCTTGATTTATTTTGCATACC | CGAGCAAGACGTTCAGTCCT | |
| POLR2A | 102127 | GCAAATTCACCAAGAGAGACG | CACGTCGACAGGAACATCAG | |
Note: NOP: nociceptin opioid peptide receptor; ppNOC: prepronociceptin; HPRT1: hypoxanthine phosphoribosyl-transferase 1; POLR2A: polymerase (RNA) II (DNA directed) polypeptide A.
aRealTime ready assay from Roche.
Flow cytometry
Intracellular nociceptin and membrane NOP in blood leukocyte subsets were detected by flow cytometry. After 24 h of culturing with or without PMA 10 ng/ml, blood was collected. Samples were incubated with appropriate volumes of fluorochrome-conjugated monoclonal antibodies specific to granulocytes (FITC anti-CD15, clone HI98), monocytes (PerCP/Cy5.5 anti-CD14, clone HIB19), CD4+ T cells (Pacific Blue anti-CD4, clone OKT4), CD8+ T cells (PE/Cy7 anti-CD8, clone HIT8a), B cells (APC anti-CD19, clone HIB19), and NK cells (APC/Cy7 anti-CD56, clone HCD56) for 20 min at room temperature in the dark (reagents from BioLegend, Koblenz, Germany). Blood was then treated with BD FACS lysing solution for 15 min, centrifuged (300 g, 5 min), and washed with staining buffer (BD Biosciences, Allschwil, Switzerland). To block Fc receptors, cells were suspended in 10% human AB serum (Sigma-Aldrich, Buchs, Switzerland) for 10 min. Subsequently, samples were added to the wells in a 96-well U-bottom plate (TPP, Trasadingen, Switzerland) and were incubated with anti-NOP antibody (Neuromics, Edina, MN, USA)23 or an isotype control antibody (Abcam, Cambridge, UK) with a final concentration of 5 µg/ml for 1 h at room temperature. After washing two times, cells were stained with PE-labelled donkey anti-rabbit antibody at a final concentration of 1 µg/ml (BD Biosciences, Allschwil, Switzerland) for 1 h in the dark at room temperature. Washed cells were suspended in staining buffer, and measurements were performed on a CytoFLEX within 6 h (Beckman Coulter, Nyon, Switzerland). Details of intracellular staining of nociceptin were published previously.17 Samples without PMA co-incubation served as controls. To determine the autofluorescence of the cells, samples with the same preparation for flow cytometry but without any staining were used as negative controls.
Fluorescent enzyme immunoassay
Extracellular nociceptin protein levels in culture supernatants were measured by fluorescent enzyme immunoassay (FEIA). After culturing with or without PMA 10 ng/ml for 0, 24, and 48 h, blood samples were collected in the presence of the protease inhibitor aprotinin 600 KIU/ml (Phoenix Pharmaceuticals, Karlsruhe, Germany) and centrifuged (16,000 g, 15 min at 4°C). Supernatants were thoroughly mixed with an equal amount of buffer A (Phoenix Pharmaceuticals, Karlsruhe, Germany) and centrifuged at 12,000 g for 20 min. C-18 SEP cartridges were used to purify small peptides from the supernatant solution, and nociceptin protein levels were determined using the Nociceptin/Orphanin FQ Fluorescent EIA kit (Phoenix Pharmaceuticals, Karlsruhe, Germany) following the manufacturer’s instructions.17
Statistical analysis
The primary endpoint was ppNOC and NOP mRNA expression in blood leukocytes. For comparison of ppNOC and NOP mRNA levels, whole blood was pre-treated with or without specific kinase inhibitors prior to PMA stimulation. Data are presented as medians with interquartile ranges (IQR) or as box-and-whisker plots showing medians, IQR, and 5 to 95 percentiles. The Kruskal–Wallis test and the Wilcoxon signed-rank test were applied for statistical comparisons. P <0.05 was considered statistically significant. Results were corrected for multiple testing.
Results
Dose-dependent effects of PMA
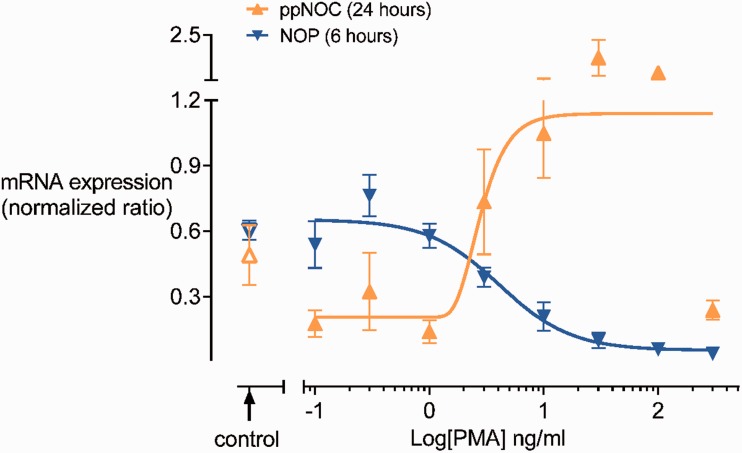
PMA dose-dependently upregulated ppNOC and downregulated NOP mRNA expression in blood leukocytes after 24 and 6 h, respectively (Figure 1). Based on these results, PMA 10 ng/ml was used in the subsequent experiments.
Figure 1.
PMA dose-dependent effects on ppNOC and NOP mRNA expression. Whole blood was cultured with different concentrations of PMA (0.1–300 ng/ml) or without PMA (control) for 24 h (ppNOC) or 6 h (NOP). PMA dose-dependently upregulated ppNOC (pEC50: 0.45 (0.01–0.92)) and downregulated NOP mRNA (pIC50: 0.61 (0.30–0.92)). Medians and interquartile ranges, n=4.
NOP: nociceptin opioid peptide receptor; ppNOC: prepronociceptin.
Time courses of ppNOC and NOP mRNA expression
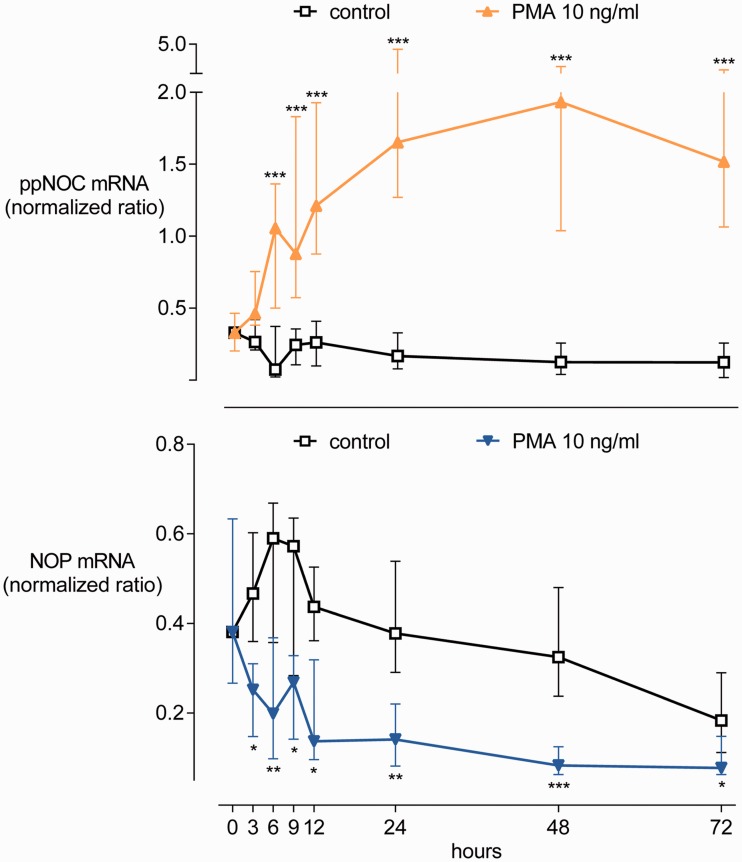
Experiments addressing the time courses of ppNOC and NOP mRNA expression in blood leukocytes cultured with or without PMA 10 ng/ml are presented in Figure 2. RT-qPCR analysis indicated that both ppNOC and NOP mRNA are constitutively expressed in human peripheral blood leukocytes (ppNOC: median normalized ratio with IQR: 0.3 (0.2–0.5); NOP: 0.4 (0.3–0.6)). ppNOC mRNA increased in a time-dependent manner after exposure of the blood to PMA for up to 72 h. Elevated ppNOC mRNA levels were detected in PMA-treated samples after 6 h compared to the control group (1.1 (0.5–1.4) vs. 0.1 (0.0–0.4), P <0.005). In contrast, NOP mRNA was time-dependently suppressed by PMA. A decrease in NOP mRNA was observed in the PMA group after 3 h compared to baseline measures (0.3 (0.1–0.3) vs. 0.5 (0.4–0.6), P <0.02).
Figure 2.
Time courses of PMA-dependent effects on ppNOC and NOP mRNA expression. Whole blood was cultured with PMA or without (control) for up to 72 h. Medians and interquartile ranges, n=14 for each group. *P <0.05, **P <0.01, ***P <0.005.
NOP: nociceptin opioid peptide receptor; ppNOC: prepronociceptin; PMA: phorbol-12-myristate-13-acetate.
PMA increased nociceptin concentrations in culture supernatants
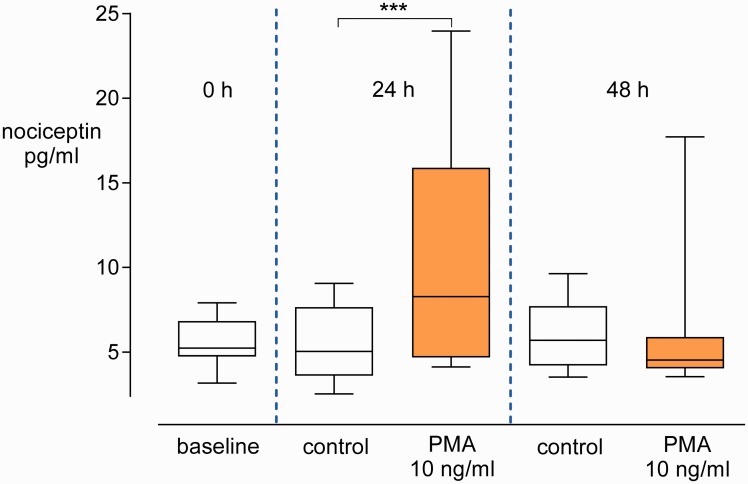
The FEIA revealed low nociceptin protein levels in the supernatants at the start of the cultures (5.2 (4.7–6.8) pg/ml). Nociceptin concentrations were increased in supernatants of the PMA-induced blood after 24 h compared to the controls (8.3 (4.7–15.9) vs. 5.0 (3.6–7.7) pg/ml, P <0.005). In contrast, no difference was observed between the two groups after 48 h (Figure 3).
Figure 3.
Nociceptin protein levels in blood culture supernatants. Whole blood was cultured with PMA or without (control) for 0, 24, or 48 h. Concentrations of nociceptin in supernatants at the start of the experiments were used to set a baseline. Box-and-whisker plots, medians with interquartile ranges, and 5 to 95 percentiles, n=13. ***P <0.005.
PMA: phorbol-12-myristate-13-acetate.
Regulation of nociceptin and NOP proteins in leukocyte subsets by PMA
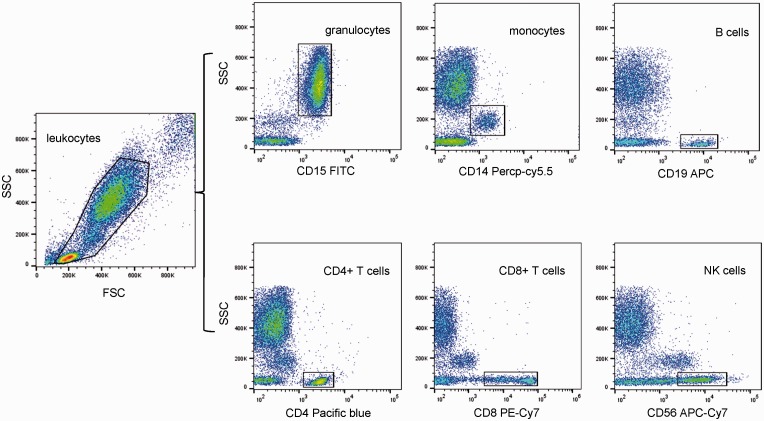
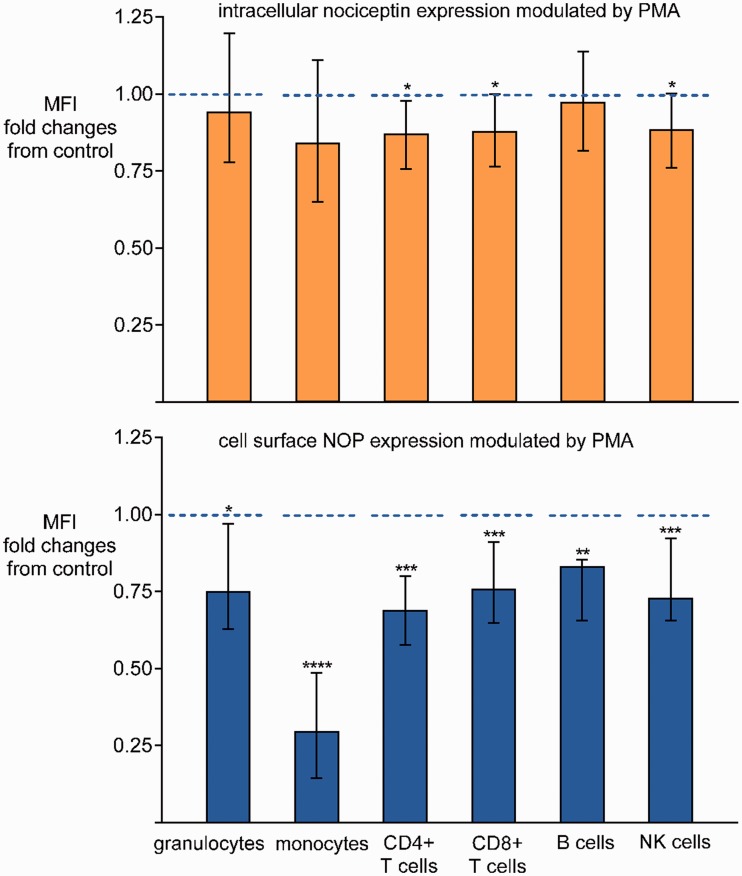
To verify nociceptin and NOP protein profiles in different blood leukocyte subsets, whole blood was cultured with or without PMA 10 ng/ml for 24 h. Intracellular nociceptin and membrane NOP were determined in six blood leukocyte subtypes (granulocytes, monocytes, CD4+ T cells, CD8+ T cells, B cells, and NK cells) identified by specific cell surface markers. Representative gating strategy plots for flow cytometry analysis are presented in Figure 4. Nociceptin and NOP could both be detected at protein levels in all blood leukocyte subsets by flow cytometry, with higher medians of fluorescence intensity for nociceptin and NOP compared to isotype controls (data not shown). PMA significantly downregulated membrane NOP expression in blood leukocyte subsets after 24 h compared to controls (Figure 5). With regard to nociceptin, a decrease in intracellular protein level was detected in CD4+ T cells, CD8+ T cells, and NK cells in the PMA-induced blood after 24 h, whereas no difference was observed in granulocytes, monocytes, or B cells (Figure 5).
Figure 4.
Gating strategy used in flow cytometry analysis to detect different subsets in human peripheral blood leukocytes.
Figure 5.
Flow cytometric analysis of intracellular nociceptin and membrane NOP in blood leukocyte subsets. MFI represents the amount of intracellular nociceptin or membrane NOP in blood leukocytes. Whole blood was cultured with or without PMA 10 ng/ml for 24 h. Nociceptin and NOP protein levels in leukocyte subsets in blood samples without PMA co-incubation served as controls. Data are presented as changes in nociceptin or NOP protein levels in subsets in the PMA group relative to the respective controls . Medians and interquartile ranges, n=12 for each group. *P <0.05, **P <0.01, ***P <0.005, ****P <0.0001. PMA: phorbol-12-myristate-13-acetate; NOP: nociceptin opioid peptide receptor.
. Medians and interquartile ranges, n=12 for each group. *P <0.05, **P <0.01, ***P <0.005, ****P <0.0001. PMA: phorbol-12-myristate-13-acetate; NOP: nociceptin opioid peptide receptor.
Essential signaling pathways for the regulation of ppNOC and NOP mRNA
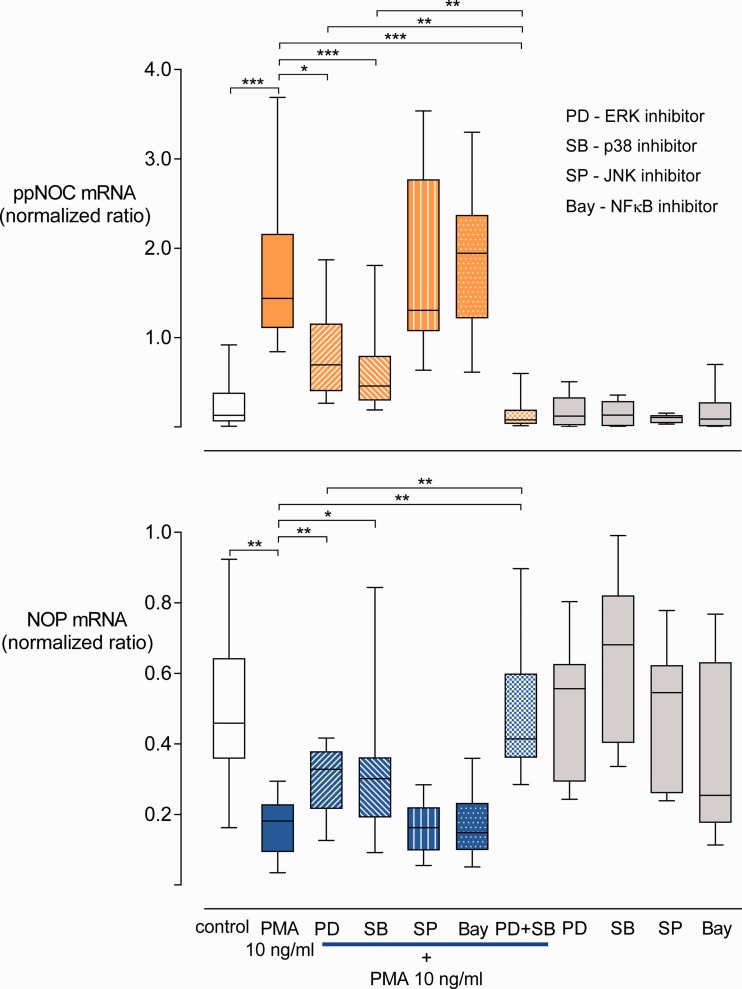
To verify the contributions of ERK, JNK, p38, and NFkB in the modulation of ppNOC and NOP mRNA expression by PMA, specific kinase inhibitors (PD98509, SP600125, SB203580, and Bay 11-7821) were employed. PMA 10 ng/ml significantly increased ppNOC and decreased NOP expression in blood leukocytes after 24 and 6 h, respectively, compared to control groups without stimuli (both P <0.001). Pre-treatment of blood with PD98509 or SB203580 partially prevented PMA effects both on ppNOC as well as on NOP expression with a 1.9-fold (1.5-3.0) and a 2.6-fold (1.2-3.5) increase in NOP mRNA compared to samples treated with PMA only. In contrast, ppNOC mRNA levels decreased to 45.9% (31.6%–66.2%) and 41.1% (19.1%–51.7%) of the PMA-treated samples (all P <0.05). The combination of PD98509 and SB203580 completely blocked PMA-induced regulation of ppNOC (P=0.01) and NOP (P <0.01). In addition, this combination produced enhanced antagonistic effects compared to samples pre-treated with kinase inhibitors PD98509 or SB203580. No inhibiting effects of SP600125 and Bay 11-7821 were observed on PMA-induced modulation of ppNOC and NOP mRNA (Figure 6).
Figure 6.
Influence of kinase inhibitors on ppNOC and NOP mRNA expression. ppNOC and NOP expression in blood leukocytes cultured with PMA or without (control) and with or without pre-treatment with the specific kinase inhibitors PD98059 (PD, ERK inhibitor) 30 μM, SB203580 (SB, p38 inhibitor) 10 μM, SP600125 (SP, JNK inhibitor) 10 μM, Bay 11-7821 (Bay, NFκB inhibitor) 3 μM, or the combination of PD and SB. Box-and-whisker plots, medians with interquartile ranges, and 5 to 95 percentiles, n=10 for the PD+SB+PMA group, n=17 for all other groups. *P <0.05, **P <0.01, ****P <0.0001.
NOP: nociceptin opioid peptide receptor; ppNOC: prepronociceptin; PMA: phorbol-12-myristate-13-acetate.
Discussion
This study focused on specific signal transduction pathways contributing to the modulation of nociceptin and its receptor in human peripheral blood leukocytes. Under inflammatory conditions, MAPK-ERK and p38 were the key signaling pathways regulating the nociceptin system.
Nociceptin and its receptor are constitutively expressed in human peripheral blood leukocytes at mRNA as well as protein levels. Moreover, nociceptin and NOP proteins were detected in all blood leukocyte subsets and were regulated by PMA. These findings underline the results of previous investigations demonstrating that the nociceptin system directly participates in immune response in peripheral blood under inflammatory conditions.3,14,15,24–26
Signaling pathways involved in ppNOC and NOP mRNA expression
It has been well documented that human blood cells respond to PMA exposure by activating a variety of transduction pathways, including three MAPKs (ERK, JNK, and p38) and the NFκB pathway.18,19 In the present study, causal relationships between the ERK and p38 signaling pathways and ppNOC and NOP mRNA expression were shown by blocking respective signaling cascades using specific kinase inhibitors. Furthermore, the combination of PD98509 and SB203580 led to synergistic inhibiting effects and completely prevented PMA effects on ppNOC and NOP expression. Taken together, PMA-activated ERK and p38 MAPKs seem to represent key signaling pathways controlling the regulation of ppNOC and NOP mRNA in human blood leukocytes. Deviation from results obtained in previous investigations might be due to species differences, tissue or cell specificity, and varying inflammatory stimuli used. For instance, in a human monocytic cell line (MM6 cultures), like ERK and p38, MAPK-JNK also contributed to the upregulation of ppNOC mRNA by PMA.17 In contrast, in studies using in vitro culture models of rat astrocytes and LPS or hydrogen peroxide as stimuli, the NFκB pathway was involved in the induction of nociceptin transcription in addition to MAPK-ERK and p38.16,27 Data concerning the regulation of the nociceptin receptor have been scarce up to now. The present study contributes to our knowledge of nociceptin receptor regulation by revealing that ERK and p38 pathways are also involved in its regulation in human blood leukocytes.
Nociceptin and NOP protein profiles
Flow cytometry was employed to determine nociceptin and NOP protein levels in blood leukocyte subsets. The suppression of membrane NOP in all leukocyte subtypes after culturing with PMA is in line with its mRNA profile. For nociceptin, upregulation was most pronounced at the mRNA level, whereas only a slight increase in extracellular nociceptin was detected in the PMA group after 24 h. However, decreased intracellular nociceptin signals were observed in CD4+ T cells, CD8+ T cells, and NK cells in PMA-treated blood. These findings, together with the FEIA results, indicate that nociceptin is secreted by the blood leukocytes following its synthesis under inflammatory conditions. In addition, a short degradation half-life of nociceptin in mouse plasma and brain was previously reported.28,29 Thus, the extracellular secretion of nociceptin under inflammatory conditions30,31 as well as the short half-life of this peptide might be a possible explanation for the extracellular and intracellular nociceptin protein profiles observed in the present study. Alternatively, the detection of nociceptin and NOP proteins in all blood leukocyte subsets and their regulation upon PMA stimulation suggests that the nociceptin system exerts considerable influence on peripheral blood cells during inflammatory processes.
NOP-mediated activation of ERK, JNK, p38, and NFκB signaling has been previously reported.32–38 In the present study, nociceptin mRNA and nociceptin protein secreted into the supernatant increased in whole blood cultures after PMA exposure. Therefore, it is possible that nociceptin synthesized and secreted by blood leukocytes is involved in inflammation.
In contrast to LPS effects observed in a previous study,13 PMA resulted in a significant upregulation of ppNOC mRNA levels in our study. It has been well documented that an LPS-mediated inflammatory response in blood cells is quite different from PMA effects, for example, different inflammatory cytokine profiles are induced and different signal transduction pathways are activated by these stimuli.39,40 In addition, receptors for LPS are expressed on cell surfaces, while phorbol ester receptors for PMA are predominantly localized in the cytoplasm. Binding PMA to its receptor activates protein kinase C and subsequently triggers downstream signaling cascades. Therefore, inflammatory substances participating in the regulation of the nociceptin system and signal transduction pathways activated in PMA- or LPS-induced blood cells may be different. Thus, nociceptin and NOP expression profiles in blood leukocytes under different inflammatory conditions, as well as potential factors involved in their regulation, need to be further investigated in future studies.
In the present study, antithetic regulation of nociceptin and NOP by PMA was observed in different leukocyte subsets. The differences indicate that the response of subsets to PMA might vary. Exploration of the nociceptin system specifically regulated in individual circulating leukocyte subtypes may contribute to further understanding of their biologic function during pain processing and inflammation. In addition, high biological variability of nociceptin and NOP expression in peripheral blood samples was observed in the present setting. Reasons for the high variability might include (1) the fact that blood samples were obtained from different volunteers, (2) the variations in response of blood leukocytes to PMA treatment, and (3) genetic variation in the expression of nociceptin and NOP.
Some limitations of the present investigation need to be considered. Experiments were only performed in PMA-induced whole blood. Although PMA-induced whole blood is an optimal ex vivo culture model mimicking pathological conditions,41 the cultures may not accurately represent pathophysiological changes occurring in vivo. Second, the involvement of signaling pathways in the modulation of the nociceptin system was investigated at mRNA levels only.
Whereas the function of the nociceptin system and related mechanisms in the nervous system have been well characterized, regulation of nociceptin and NOP in human circulating blood has still not been fully elucidated.23 Due to the widespread distribution of nociceptin and NOP in the brain, spinal cord, and peripheral organs and their role in physiological processes, NOP-targeted compounds have entered clinical development for various indications.3 The first published phase II randomized controlled trial investigating patients suffering from low back pain demonstrated the analgesic efficacy of cebranopadol, a novel first-in-class analgesic drug candidate combining agonistic activity at NOP and the µ-opioid receptor.4,5 This underlines the important step from basic research to possible clinical use, as well as the need for further investigations to clarify the role of increased extracellular nociceptin in peripheral blood. The contribution of signaling pathways to nociceptin and NOP protein levels in different leukocyte subsets needs to be addressed. Studies using whole blood cultures and blood samples from patients with pain and/or inflammatory diseases are necessary to fully elucidate mechanisms contributing to the regulation of the nociceptin system.
Conclusions
ERK and p38 are major signaling pathways contributing to the PMA-induced regulation of nociceptin and the nociceptin receptor in human whole blood cultures. Elucidating mechanisms involved in the regulation of nociceptin and its receptor may provide further insight valuable for the development of drugs targeting the nociceptin system.
Acknowledgments
The authors thank Jeannie Wurz for her diligent proofreading of the manuscript.
Author Contributions
Participated in research design: F. S., U. S., and L. Z.; Conducted experiments: L. Z.; Contributed new reagents or analytic tools: L. Z., C. L., and M. S.; Performed data analysis: L. Z., U. S., and F. S.; Wrote or contributed to the writing of the manuscript: L. Z., U. S., F. S., C. L., and M. S.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an institutional grant.
References
- 1.Vadivelu N, Kai AM, Kodumudi V, Sramcik J, Kaye AD. The opioid crisis: a comprehensive overview. Curr Pain Headache Rep 2018; 22: 16. [DOI] [PubMed] [Google Scholar]
- 2.Walker G. The opioid crisis: a 21st century pain. Drugs Today 2018; 54: 283–286. [DOI] [PubMed] [Google Scholar]
- 3.Zaveri NT. Nociceptin Opioid Receptor (NOP) as a therapeutic target: progress in translation from preclinical research to clinical utility. J Med Chem 2016; 59: 7011–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christoph A, Eerdekens MH, Kok M, Volkers G, Freynhagen R. Cebranopadol, a novel first-in-class analgesic drug candidate: first experience in patients with chronic low back pain in a randomized clinical trial. Pain 2017; 158: 1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert DG, Bird MF, Rowbotham DJ. Cebranopadol: a first in-class example of a nociceptin/orphanin FQ receptor and opioid receptor agonist. Br J Anaesth 2015; 114: 364–366. [DOI] [PubMed] [Google Scholar]
- 6.Gavioli EC, de Medeiros IU, Monteiro MC, Calo G, Romao PR. Nociceptin/orphanin FQ-NOP receptor system in inflammatory and immune-mediated diseases. Vitam Horm 2015; 97: 241–266. [DOI] [PubMed] [Google Scholar]
- 7.Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 2008; 7: 694–710. [DOI] [PubMed] [Google Scholar]
- 8.Calo G, Lambert DG. Nociceptin/orphanin FQ receptor ligands and translational challenges: focus on cebranopadol as an innovative analgesic. Br J Anaesth 2018; 121: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S. Targeting multiple opioid receptors—improved analgesics with reduced side effects? Br J Pharmacol 2018; 175: 2857–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 2016; 68: 419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hashimi M, McDonald J, Thompson JP, Lambert DG. Evidence for nociceptin/orphanin FQ (NOP) but not micro (MOP), delta (DOP) or kappa (KOP) opioid receptor mRNA in whole human blood. Br J Anaesth 2016; 116: 423–429. [DOI] [PubMed] [Google Scholar]
- 12.Williams JP, Thompson JP, McDonald J, Barnes TA, Cote T, Rowbotham DJ, Lambert DG. Human peripheral blood mononuclear cells express nociceptin/orphanin FQ, but not mu, delta, or kappa opioid receptors. Anesth Analg 2007; 105: 998–1005. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Stuber F, Stamer UM. Inflammatory mediators influence the expression of nociceptin and its receptor in human whole blood cultures. PLoS One 2013; 8: e74138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano-Gomez A, Thompson JP, Lambert DG. Nociceptin/orphanin FQ in inflammation and sepsis. Br J Anaesth 2011; 106: 6–12. [DOI] [PubMed] [Google Scholar]
- 15.Stamer UM, Book M, Comos C, Zhang L, Nauck F, Stuber F. Expression of the nociceptin precursor and nociceptin receptor is modulated in cancer and septic patients. Br J Anaesth 2011; 106: 566–572. [DOI] [PubMed] [Google Scholar]
- 16.Buzas B, Rosenberger J, Kim KW, Cox BM. Inflammatory mediators increase the expression of nociceptin/orphanin FQ in rat astrocytes in culture. Glia 2002; 39: 237–246. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Stuber F, Lippuner C, Schiff M, Stamer UM. Phorbol-12-myristate-13-acetate induces nociceptin in human Mono Mac 6 cells via multiple transduction signalling pathways. Br J Anaesth 2016; 117: 250–257. [DOI] [PubMed] [Google Scholar]
- 18.Bou G, Villasis-Keever A, Paya CV. Detection of JNK and p38 activation by flow cytometry analysis. Anal Biochem 2003; 317: 147–155. [DOI] [PubMed] [Google Scholar]
- 19.Davies R, Vogelsang P, Jonsson R, Appel S. An optimized multiplex flow cytometry protocol for the analysis of intracellular signaling in peripheral blood mononuclear cells. J Immunol Methods 2016; 436: 58–63. [DOI] [PubMed] [Google Scholar]
- 20.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- 21.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 2003; 371: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 2007; 408: 297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekine Y, Siegel CS, Sekine-Konno T, Cafferty WBJ, Strittmatter SM. The nociceptin receptor inhibits axonal regeneration and recovery from spinal cord injury. Sci Signal 2018; 11: eaao4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaminsky DE, Rogers TJ. Suppression of CCL2/MCP-1 and CCL5/RANTES expression by nociceptin in human monocytes. J Neuroimmune Pharmacol 2008; 3: 75–82. [DOI] [PubMed] [Google Scholar]
- 25.Williams JP, Thompson JP, Young SP, Gold SJ, McDonald J, Rowbotham DJ, Lambert DG. Nociceptin and urotensin-II concentrations in critically ill patients with sepsis. Br J Anaesth 2008; 100: 810–814. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JP, Serrano-Gomez A, McDonald J, Ladak N, Bowrey S, Lambert DG. The nociceptin/orphanin FQ system is modulated in patients admitted to ICU with sepsis and after cardiopulmonary bypass. PLoS One 2013; 8: e76682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J Neurochem 2001; 79: 35–44. [DOI] [PubMed] [Google Scholar]
- 28.Gunduz O, Rizzi A, Baldisserotto A, Guerrini R, Spagnolo B, Gavioli EC, Kocsis L, Magyar A, Benyhe S, Borsodi A, Calo G. In vitro and in vivo pharmacological characterization of the nociceptin/orphanin FQ receptor ligand Ac-RYYRIK-ol. Eur J Pharmacol 2006; 539: 39–48. [DOI] [PubMed] [Google Scholar]
- 29.Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, Calo G. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides 2007; 28: 1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiset ME, Gilbert C, Poubelle PE, Pouliot M. Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochemistry 2003; 42: 10498–10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller TR, Fulford AJ. Regulation of nociceptin/orphanin FQ secretion by immune cells and functional modulation of interleukin-2. Peptides 2007; 28: 2243–2252. [DOI] [PubMed] [Google Scholar]
- 32.Bedini A, Baiula M, Vincelli G, Formaggio F, Lombardi S, Caprini M, Spampinato S. Nociceptin/orphanin FQ antagonizes lipopolysaccharide-stimulated proliferation, migration and inflammatory signaling in human glioblastoma U87 cells. Biochem Pharmacol 2017; 140: 89–104. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata K, Nishimura I, Fujiwara T, Terauchi S, Minami T, Ito S, Okuda-Ashitaka E. Intrathecal administration of low-dose nociceptin/orphanin FQ induces allodynia via c-Jun N-terminal kinase and monocyte chemoattractant protein-1. Eur J Neurosci 2016; 43: 1499–1508. [DOI] [PubMed] [Google Scholar]
- 34.Donica CL, Ramirez VI, Awwad HO, Zaveri NT, Toll L, Standifer KM. Orphanin FQ/nociceptin activates nuclear factor kappa B. J Neuroimmune Pharmacol 2011; 6: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstead WM. Differential activation of ERK, p38, and JNK MAPK by nociceptin/orphanin FQ in the potentiation of prostaglandin cerebrovasoconstriction after brain injury. Eur J Pharmacol 2006; 529: 129–135. [DOI] [PubMed] [Google Scholar]
- 36.Ross J, Armstead WM. NOC/oFQ activates ERK and JNK but not p38 MAPK to impair prostaglandin cerebrovasodilation after brain injury. Brain Res 2005; 1054: 95–102. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Cui Q, Li Y, Li B, Yang X, Cui L, Jin H, Qu L. The role of ERK-1/2 in the N/OFQ-induced inhibition of delayed rectifier potassium currents. Biochem Biophys Res Commun 2010; 394: 1058–1062. [DOI] [PubMed] [Google Scholar]
- 38.Goeldner C, Reiss D, Wichmann J, Meziane H, Kieffer BL, Ouagazzal AM. Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. J Neurosci 2008; 28: 2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ai W, Li H, Song N, Li L, Chen H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int J Environ Res Public Health 2013; 10: 3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeurink PV, Noguera CL, Savelkoul HF, Wichers HJ. Immunomodulatory capacity of fungal proteins on the cytokine production of human peripheral blood mononuclear cells. Int Immunopharmacol 2008; 8: 1124–1133. [DOI] [PubMed] [Google Scholar]
- 41.Godoy-Ramirez K, Franck K, Mahdavifar S, Andersson L, Gaines H. Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry. J Immunol Methods 2004; 292: 1–15. [DOI] [PubMed] [Google Scholar]