Short abstract
Electrospinning is one of the techniques to produce structured polymeric fibers in the micro or nano scale and to generate novel materials for biomedical proposes. Electrospinning versatility provides fibers that could support different surgical and rehabilitation treatments. However, its diversity in equipment assembly, polymeric materials, and functional molecules to be incorporated in fibers result in profusion of recent biomaterials that are not fully explored, even though the recognized relevance of the technique. The present article describes the main electrospun polymeric materials used in oral applications, and the main aspects and parameters of the technique. Natural and synthetic polymers, blends, and composites were identified from the available literature and recent developments. Main applications of electrospun fibers were focused on drug delivery systems, tissue regeneration, and material reinforcement or modification, although studies require further investigation in order to enable direct use in human. Current and potential usages as biomaterials for oral applications must motivate the development in the use of electrospinning as an efficient method to produce highly innovative biomaterials, over the next few years.
Impact statement
Nanotechnology is a challenge for many researchers that look for obtaining different materials behaviors by modifying characteristics at a very low scale. Thus, the production of nanostructured materials represents a very important field in bioengineering, in which the electrospinning technique appears as a suitable alternative. This review discusses and provides further explanation on this versatile technique to produce novel polymeric biomaterials for oral applications. The use of electrospun fibers is incipient in oral areas, mainly because of the unfamiliarity with the technique. Provided disclosure, possibilities and state of the art are aimed at supporting interested researchers to better choose proper materials, understand, and design new experiments. This work seeks to encourage many other researchers–Dentists, Biologists, Engineers, Pharmacists–to develop innovative materials from different polymers. We highlight synthetic and natural polymers as trends in treatments to motivate an advance in the worldwide discussion and exploration of this interdisciplinary field.
Keywords: Nano and microfibers, biopolymers, nanotechnology, dental treatments, dentistry
Introduction
The development of new materials and oral treatments led to shorter healing periods and rehabilitation procedures with minor pain. Furthermore, materials to be used in oral conditions are currently challenging concerning durability, resistance, degradability, and biocompatibility. Thus, researches that support studies on production of novel materials are very important to improve current treatments and to provide more efficient procedures.
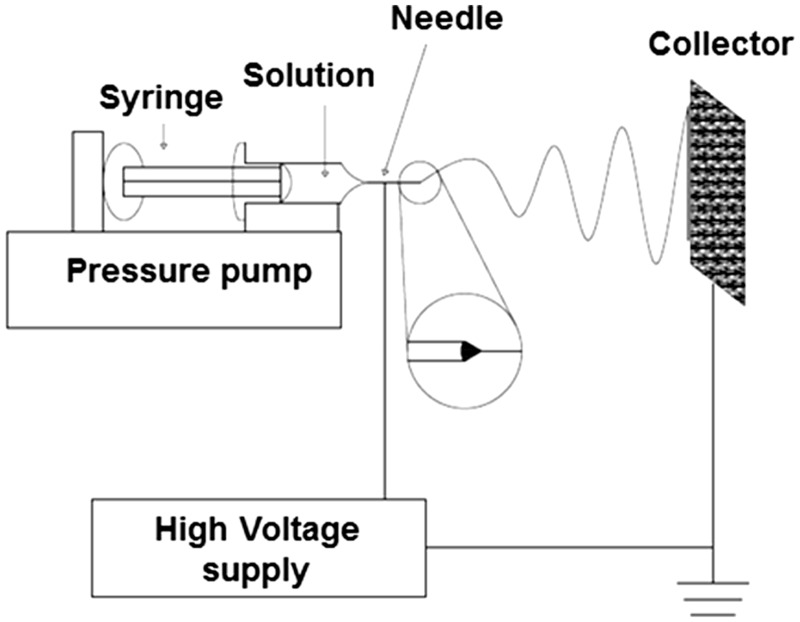
The physical and mechanical properties of structured biomaterials in micro or nano scale could generate superior structures with potential for therapeutic use. Electrospinning is one of the technologies for producing such structured materials. It has been reported as an efficient technique for polymeric fibers fabrication with diameter range from 3 to 5000 nm,1,2 from which several polymers have been successfully electrospun into micro and nanofibers in recent years.3 Electrospinning devices are generally formed by a syringe, a needle, a source of high voltage, a collector field, and a precision controlled pump. The technique consists in creating micro or nanoscale fibers by high voltage application to a syringe loaded with a polymer solution. The fibers are deposited on a metallic collector charged with a voltage, as schematized in Figure 1.4 The process of electrospinning is relatively simple for implementation and presents low cost when compared to other fiber fabrication processes, such as melt fibrillation,5 island-in-sea,6 gas jet,7 nanolithography,8 and self-assembly.9–12 Fibers assemblies from synthetic and natural polymers could be achieved and applications range from sensors, filtration, protective material, electrical or optical areas to biomedical field.13 Tissue engineering (TE),14,15 drug delivery,16 wound dressing, and nanofiber reinforced composites17,18 are potential uses in dentistry. Figure 2 shows a simplified representation how electrospun fibers are employed in dental TE.
Figure 1.
Schematic representation of a typical electrospinning apparatus. A polymeric solution is ejected from a needle controlled by a syringe pump. The needle is connected to a high voltage DC supply that provides charge into the polymer solution. A fiber jet is produced by the solvent evaporation and then deposited on the collector.
Figure 2.
Dental cells regeneration using polymeric electrospun fibers. The fibers can be loaded with different growth factors that can improve cell proliferation, or with the presence of antimicrobial agents to inhibit bacterial contamination.
This review summarizes most frequently used materials in biomedical areas, including natural and artificial polymers exploring possibilities or recent use in researches on oral biomaterials. It is also shortly described the electrospinning process, including important production parameters and introducing recent areas of development.
Electrospinning process and parameters
The fibers morphology (Figure 3) and the process of the electrospinning are affected by different factors: the molecular weight of the polymer, source of voltage, concentration of the polymer solution, needle gauge type, and the distance between the needle and the pitch collector.19 Spinnability, fiber diameters, fiber uniformity, fiber alignment, defects control (e.g. beads, junctions, and pores), and other properties are tunable by adjusting these factors and also including substrate-related parameters (polymer concentration, viscosity, molecular weight, surface tension) and apparatus-related parameters (flow rate and electric field). All these parameters are important to obtain flawless fibers and the choice of a polymer and its production must consider these factors and their modulation.
Figure 3.
SEM images from electrospun PCL fibers. In (a) is presented beads formation (arrows). In (b) and (c) are presented nanofibers with regular aspect. The upper left image presents a wider diameter size distribution compared with the image at the upper right, due to the lack of control of the process.
Spinning voltage is related to some defects as bead formation in fibers (Figure 3(a)).20 However, a low voltage can increase the time of solvent evaporation and high voltages cause the rapid evaporation of solvent, producing a fiber with less pores and defects.17 Spinning voltage influences also the shape of electrospun fibers in a macroscopical level and in the homogeneity of the generated structures (Figure 3(b) and (c)).17
The distance between the needle and the collector field should be carefully adjusted. The fiber morphology is influenced by the distance, which in turn influences in deposition time and evaporation rate of the solvent.21 The fiber may break before reaching the collector field and create thick fibers with greater distances between them; consequently, the solvent cannot fully evaporate resulting in inaccurate fiber.22 The diameter of the needle directly affects the diameter of the fiber, and when it is reduced, pore formation decreases.
Environmental conditions such as: humidity, atmospheric pressure, and temperature differently influence on all process parameters. The humidity directly affects the polymer solution resulting in changes in the morphology, like small holes, increased roughness, etc. The effects of relative humidity on electrospun fiber morphology are dependent on polymer hydrophobicity, solvent miscibility with water, and solvent volatility.23Too low atmospheric pressure does not allow the process to occur. On the other hand, when working temperature increases, surface tension and viscosity of the fluid decrease and solvent evaporation could be accelerated, terminating the process prematurely.24 In other cases, when the environment is very cold, the spinning process is hindered.
The viscosity and conductivity of the polymer solution are the main characteristics that influence electrospinning process. The viscosity is related to the polymer molecular weight and the amount of dissolved solids in the solution. Solution concentration is reported to strongly affect fiber size, with fiber diameter increasing with the solution concentration in a power law relationship.20 In addition, electrospinning from solutions of high concentration is reported to produce fiber sizes with bimodal distribution, reminiscent of distributions observed in the similar droplet generation process of electrospraying. High viscosity can difficult the ejection of the solution from the needle and can also dry before it reaches the metallic collector. The conductivity of the polymer solution influences the diameter of the fiber with direct relation: as the conductivity increases, it produces denser fibers.17
Polymers used in oral applications
Examples and most used polymers are presented in Table 1, indicating the main oral applications and some common composites.
Table 1.
Relation of electrospun polymers for oral applications.
| Electrospun polymer | Application | Year | References |
|---|---|---|---|
| PCL | Drug delivery | 2018 | 25 |
| PCL | Tissue regeneration | 2017 | 26 |
| PCL | Drug delivery | 2016 | 27 |
| PCL | Tissue regeneration | 2016 | 28 |
| PCL | Tissue regeneration | 2016 | 29 |
| PCL-GEL | Tissue regeneration | 2016 | 30 |
| PCL, PCL-GEL | Wound dressing | 2015 | 31 |
| PCL | Tissue regeneration | 2015 | 32 |
| PCL | Modification of materials | 2014 | 33 |
| PCL | Drug delivery | 2013 | 22 |
| PCL | Tissue regeneration | 2013 | 34 |
| PCL | Drug delivery | 2013 | 35 |
| PLA, PCL | Tissue regeneration | 2018 | 36 |
| PLA | Drug delivery | 2016 | 37 |
| PLA, PCL-GEL | Tissue regeneration | 2016 | 38 |
| PLA | Tissue regeneration | 2013 | 39 |
| PLA | Drug delivery | 2012 | 40 |
| PLA | Tissue regeneration | 2011 | 41 |
| PLLA | Drug delivery | 2017 | 42 |
| PLLA, PCL | Tissue regeneration | 2016 | 43 |
| PLLA, PCL | Tissue regeneration | 2014 | 44 |
| PLLA, PLGA, PGA, PCL, Chitosan, PLA | Tissue regeneration | 2011 | 45 |
| PLLA, PLGA | Tissue regeneration | 2011 | 4 |
| PLLA | Modification of materials | 2008 | 46 |
| PLGA, PCL | Tissue regeneration | 2017 | 47 |
| PDS | Drug delivery | 2018 | 48 |
| PDS | Drug delivery | 2016 | 49 |
| PDS | Tissue regeneration | 2015 | 50 |
| PDS | Drug delivery | 2013 | 51 |
| Chitosan | Tissue regeneration | 2015 | 52 |
| Chitosan | Membrane dressing | 2015 | 53 |
| Nylon-6/chitosan | Modification of materials | 2015 | 54 |
| Nylon-6 | Modification of materials | 2015 | 55 |
| Collagen | Tissue regeneration | 2012 | 56 |
| PLGA | Tissue regeneration | 2016 | 57 |
| PVA | Modification of materials | 2016 | 58 |
| PU + GEL | Wound dressing | 2016 | 59 |
| PAN | Modification of materials | 2015 | 60 |
PAN: poliacrylonitrile; PCL: poly(ɛ-caprolactone); PLLA: poly(L-lactide); PDS: polydioxanone; PLGA: poly(lactic-co-glycolic acid); PVA: polyvinyl alcohol.
The poly(ɛ-caprolactone) (PCL), poly(lactic acid) (PLA), poly(L-lactide) (PLLA), and poly(lactic-co-glycolic acid) (PLGA) are polymers commonly used in biomedical and dental applications. Natural polymers such as chitosan, collagen, and cellulose were cited and are also currently used.27,45,52,53,56,60 Other polymers such as poly(ethylene oxide) (PEO),61 polydioxanone (PDS),59 nylon,55 poly(vinylidene fluoride) (PVDF),60 polyurethane (PU),59 and poliacrylonitrile (PAN)60 present low use at present. Next, main polymers or group of polymers listed in Table 1 are described including some characteristics and applications.
PCL
PLC is a biodegradable thermoplastic polymer that present relatively low cost. Its physicochemical properties enable it for applications in the nanometric scale processing and prototyping.45
It has been widely used in drug delivery systems for long-term dental implants area. PCL was used in association with alginate for drug release during dental treatments to prevent bacterial accumulation at the site of implantation.22 PCL has been also used in drug delivery for creation of membranes incorporating nano-hydroxyapatite and amoxicillin,27 obtaining good biocompatibility and reducing bacterial contamination of periodontal defects. Further applications were described for TE. PCL scaffolds mineralized with apatite improve cell proliferation in dental pulp. PCL when combined with bioactive glass gradually improves cell proliferation and the mechanical properties of scaffolds obtained by materials blending.34 In periodontal regeneration, PCL has been effectively used in combination with gelatin, with engineered nanotopology of structures and mechanical stimulation.30
PLA
PLA is a versatile polymer that can be adapted to different morphologies.40 It is degradable in the human tissues; however, its degradation produces acids that difficult the regeneration process requiring blending with other materials.39
PLA is used mostly for controlled drug delivery and tissue regeneration. To reduce tissue damage caused for delivered acids, PLA nanofibers were loaded with beta-tricalcium phosphate (β-TCP) which proved to be a viable option in PCL membranes.39 PLA nanofibers associated with metronidazole (MNZ) were used to control microbiological proliferation during periodontitis treatment, inhibiting bacteria growth during the treatment.40 Ampicilin and MNZ were incorporated in PLA matrix for drug delivery, finding potential applications in periodontal and endodontic infections.37
PLA in combination with PCL and gelatine was produced incorporating tetracycline exhibiting good characteristics to be used as antibacterial dental implant coating.38 PLA nanofibers together with poly(DL-lactide-co-e-caprolactone), nano-hydroxyapatite, n-HAp, have been also produced.41 Resulting nanofibers formed layers that improved mechanical and biological properties resulting in the raise of periodontal membrane regeneration.
PLLA
PLLA is a biodegradable and easily applicable polymer, with a porous morphology when polymerized with other polymers. PLLA has good physical and mechanical properties and it is commonly used in association with other materials.45
PLLA presents advantages for oral applications, because of its degradation rate and its potential for cell adhesion and proliferation. The disadvantages observed are the possibility to release of acids during degradation and its high hydrophobicity.4,45 These aspects can be reduced with the combination of PLLA with other materials. A recent study shows that HA-loaded PLLA/PCL can lead to osteoblast/odontoblast differentiation.43 Scaffolds of PLLA have been also point out as an excellent environment for proliferation and adhesion of cells of different types.62 The mechanical properties such as compressive strength, diametral tensile strength, flexural strength, and linear shrinkage of PLLA fibers have been improved by mixing other materials as bisphenyl glycidylmethacrylate (Bis-GMA) and triethylenglycoldimethacrylate (TEGDMA).41
Polyethylene oxide and polyvinyl alcohol
PEO is described as a relatively high molecular weight, biocompatible material that effectively improves the mechanical and biological properties of composite hydrogels. PEO has presented a significant impact on the mechanical and rheological properties associated with a glucose-sensitive antibacterial chitosan.61 It has been verified that a chitosan fraction contributed to an antimicrobial effect, decreased tendency to spherulitic crystallization of PEO, and enhanced puncture and tensile strength. PEO is used as an additive in tooth paste because it reduces biofilm formation.63 Inhibition of bacterial adhesion on enamel was observed suggesting that the PEO could be utilized in caries prevention.64
Polyvinylalcohol (PVA) presents biocompatibility, being prepared easily and having non-toxic solvent. It is a water-soluble synthetic polymer and the back bone chains are highly interconnected by hydrogen bonding due the presence of abundant hydroxyl groups. These characteristics contribute to chemical resistance and mechanical properties, including high tensile and impact strength. The hydroxyl groups can be used to incorporate molecules of biological origin, such as collagens, hyaluron and deoxyribonucleic acid. PVA is biodegradable, decomposing non toxically into water and carbon dioxide.65 Electrospinning is one of the micro and nano-size production methods for PVA for the reinforcement of dental materials.58
Poly DL-lactide-co-glycolide and PDS
Poly DL-lactide-co-glycolic (PLGA) is a biodegradable and biocompatible polymer that closely resembles natural proteins that may be metabolically hydrolyzed to monomers of lactic acid and glycolic acid. It is used for reinforcement of other materials without changing characteristics such as cell adhesion. Furthermore, the PLGA can be used in the controlled release of drugs during bone growth66,67 and in membranes that simultaneously release drugs, support bone growth, and possess barrier functions.57
The PDS is a polymer frequently used as suture material. In recent studies, investigation for tissue regeneration and drug delivery system was performed. Bioactive scaffolds were produced using PDS for regenerative endodontics and their results demonstrated efficiency of the material for adhesion and proliferation of fibroblasts exhibiting good biocompatibility. It also evidenced its potential for encapsulation of distinct bioactive molecules.68
PVDF and nylon
Polyvinylidene fluoride (PVDF) is a biocompatible, flexible material with high mechanical strength. It has been electrospun with drugs to form membranes, exhibiting potential for antibiotics release in wound healing. In addition, membranes produced with PVDF presented good anti-bacterial properties.69
Nylon-6 (polyamide) is also a biocompatible material with excellent mechanical, chemical, and thermal properties. Nylon composites have been also used for reinforcement of dental materials. Nylon has been produced by electrospinning in dental resins with aligned or random oriented fibers. Best mechanical properties were observed for aligned fibers.55 Silica-nylon reinforcement reported to increase the flexural and fracture strength of interim partial fixed dental protheses.70
Natural polymers
Natural polymers are adequate as biomaterials because they are structurally similar to biological tissues. They sometimes possess similar or identical monomers, as those found in organic matrices, reducing the problems associated to toxicity or inflammatory reactions.71 Nevertheless, there exist some disadvantages as the possible rejection of the material by the organism, strong physiological induced activity, and low mechanical properties.72 Some of the main natural polymers with possible oral applications are presented: chitosan, collagen, and cellulose.
Chitosan
Chitosan is extracted from the crustacean shells and is the second largest natural polysaccharide after cellulose.73 Chitosan possesses good properties, like biodegradability, biocompatibility, it has fungicidal, antimicrobial, and antitumor activity and also has strong healing properties, as it offers an excellent environment for cell adhesion and proliferation. Applications are focus of a range of studies reporting its medical potential as nerve regeneration, bone, cartilage, epidermal healing, gene therapies, drug delivery, semipermeable membranes, and development of biomaterials.74 Another major advantage of chitosan is that its production process is economically and environmentally viable since it comes from by-products of the fishing activity of parts that were discarded in addition to being a renewable source of raw material.75 In oral medicine, chitosan has been produced as membrane for bone regeneration52 and caries prevention.53
Collagen
Collagen is the main organic component of the dentin matrix and represents an alternative to produce scaffolds for dental regeneration. Collagen-hydroxyapatite composite is used due to the enhanced biological and mechanical characteristics obtained by the binary system. Some successful applications on dentin recovery, revascularization in open apex periodontitis treatment, and root caries inhibition were reported.76–78 Collagen-based nanocomposites using bioactive glass are related to indice cell proliferation and osteogenic differentiation in dental pulp.56
Cellulose
Cellulose is the most abundant high molecular weight polysaccharide in nature, and for this reason with relatively low cost. Cellulose is widely used for a large number of applications in the chemical, food, and pharmaceutical industry. The form and order of the crystallite give interesting properties such as tensile strength, rigidity, heat resistance, and also elastic properties which are very important materials aspects for biomedical purposes including reinforce of materials and because of its good biocompatibility, cellulose-based membrane has been recently proposed for medical applications.3,79 It has been reported that electrospun cellulose with nano-hydroxyapatite results in good mechanical properties and biocompatibility with dental cells with possible applications in TE.80 Cellulose derivatives such as methylcellulose and cellulose acetate are alternatives to biomedical applications when cellulose dissolution is complicated, for instance, to generate cellulose acetate membrane for dentistry.81
Oral applications
Next, main applications of fibers produced by electrospinning in oral areas: drug delivery systems, tissue regeneration and modification of materials, are presented. Functional fibers could be achieved by blending polymers mixtures and active molecules or by dispersing nano or microfillers within fibrous matrix. These composites are attractive since novel materials fabricated with electrospinning demonstrated superior mechanical properties, bioactivity and biochemical properties compared to their constituent phases.
Drug delivery systems
The aim of drug delivery systems is to enable the controlled drug releasing towards alleviating medical conditions at a defined rate over a definite period. The promising use of nanofibers in drug delivery systems might result in salient features such as high loading capacity. Current release of diverse drugs ranging from antibiotics and anticancer agents to proteins, aptamers, DNA has been successfully achieved with electrospun fibers. Different drug delivery assemblies are presented in Figure 4.
Figure 4.
Possibilities on drug delivery assemblies using electrospun fibers: (a) impregnation of fibers using an immersion bath in the presence of the drug; (b) nanocarriers attachment to the fiber; (c) polymeric fiber matrix loaded with the drug, produced by electrospinning from a solution containing the drug; (d) coaxial electrospun fiber obtained by the encapsulation of the drug (core) inside a polymeric structure (shell).
Pharmaceutical properties as bioavailability, solubility, and stability could be enhanced with this strategy.82 Electrospun fibers have shown their advantages in the field of drug delivery due to their high surface area and volume ratio with interconnected pores in the fibers.
Strategies to avoid bacterial and fungal diseases are important in the oral-facial field.40 For this reason, scaffolds containing antibiotics have been increasingly used in oral studies22,27,37,40,49,51 and mucoadhesion strategies are also reported.83,84 The presence of anti-inflammatory agents in such composites could activate a signaling cascade and trigger the healing process.82
A combination of ampicillin (AMP), MNZ (20/20%w/w), and PLA single fiber mats were described to suppress periodontopathogenic species A. actinomycetemcomitans within an elution time of 24 h37 and the effect of MNZ (0.1–40% w/w) with electrospun PLA fibers decreased viability of F. nucleatum and P. gingivalis up to 28 days and for A. actinomycetemcomitans up to 2 days.40 In Reise et al.,40 it is described that highly loaded fiber mat with 40% of MNZ (w/w) with a weight of 35 mg contains 14 mg of MNZ and the amount of MNZ when administered systemically adds up to 8400 mg (3 × 400 mg daily over seven days). However, toxic effects are expected if local drug administration provides highly concentrated antibiotics doses. Gradually drug release could be a more biologically friendly approach to be tested such as amounts that promote the elimination of bacteria associated with the reduction of adverse effects.44
Regenerative endodontics requires effective root canal disinfection with no or minimal harm to stem cells and growth factor. Triple antibiotic (ciprofloxacin, MNZ, and minocycline) or double highly concentrated antibiotic (minocycline-free) pastes are typically accomplished for root canal disinfection.51 However, potentially toxic effects of highly concentrated antibiotic pastes on dental pulp stem cells and dental pulp fibroblasts should be concerned. Controlled amounts of antibiotics could be achieved with electrospun antibiotic-containing scaffolds. For this purpose, PDS fibers were fabricated with MNZ, minocycline, and ciprofloxacin via electrospinning and tested in vitro against a dual-species biofilm (A. naeslundii and Enterococcus faecalis, bacteria prevalent in immature infected root canals and responsible for secondary infections in necrotic teeth after treatment, respectively) with a significant bacterial death, whereas they did not affect dental pulp stem cells attachment and proliferation on dentin.44
Controlled localized delivery of antibacterial agents to dental implants site using a biodegradable electrospun material could be designed in order to prevent bacterial infection, one of the causes of dental implant fail. PCL/alginate associated with MNZ rings were custom designed and inserted around dental implant prior to their placement procedure. This strategy minimized burst release effects with MNZ release over 30 days.22
Reported studies suggest the use of these materials for periodontal and endodontical infections and for dental implants. Drug release from nanofibers could be due to desorption of drug from the surface, diffusion from pores and/or matrix degradation, all processes likely to get affected by polymer type, porosity, morphology, and also by geometry of nanofibers.85 Other antibiotics have been used in drug delivery inside electrospun materials: amoxiciline,27 AMP,37 minocycline, and ciprofloxacin.49,51
Antifungal and mucoadhesives properties are also reported in this area. Poly-ε-caprolactone nanofibers functionalized with ketoconazole were produced by electrospinning and tested against filamentous fungi in Veras et al.86 and the functionalized nanofibers showed antifungal activity against Aspergillus flavus, A. carbonarius, A. niger, Aspergillus sp. A29, Fusarium oxysporum and Penicillium citrinum by agar diffusion test with inhibitory zones ranging from 6 to 44 mm.
Mucoadhesivity of polymers is sometimes a desired characteristic in drug delivery systems because it could enhance drug retention time at the application site and increase bioavailability.83 The chitosan-ethylenediaminetetraacetic acid/polyvinyl alcohol (CS-EDTA/PVA) was reported as mucoadhesive and electrospinnable composite that associated with clotrimazole (CZ)-loaded microemulsion could be used for antifungal purposes,84 demonstrating sustained release with antifungal activity in vitro.
Tissue regeneration
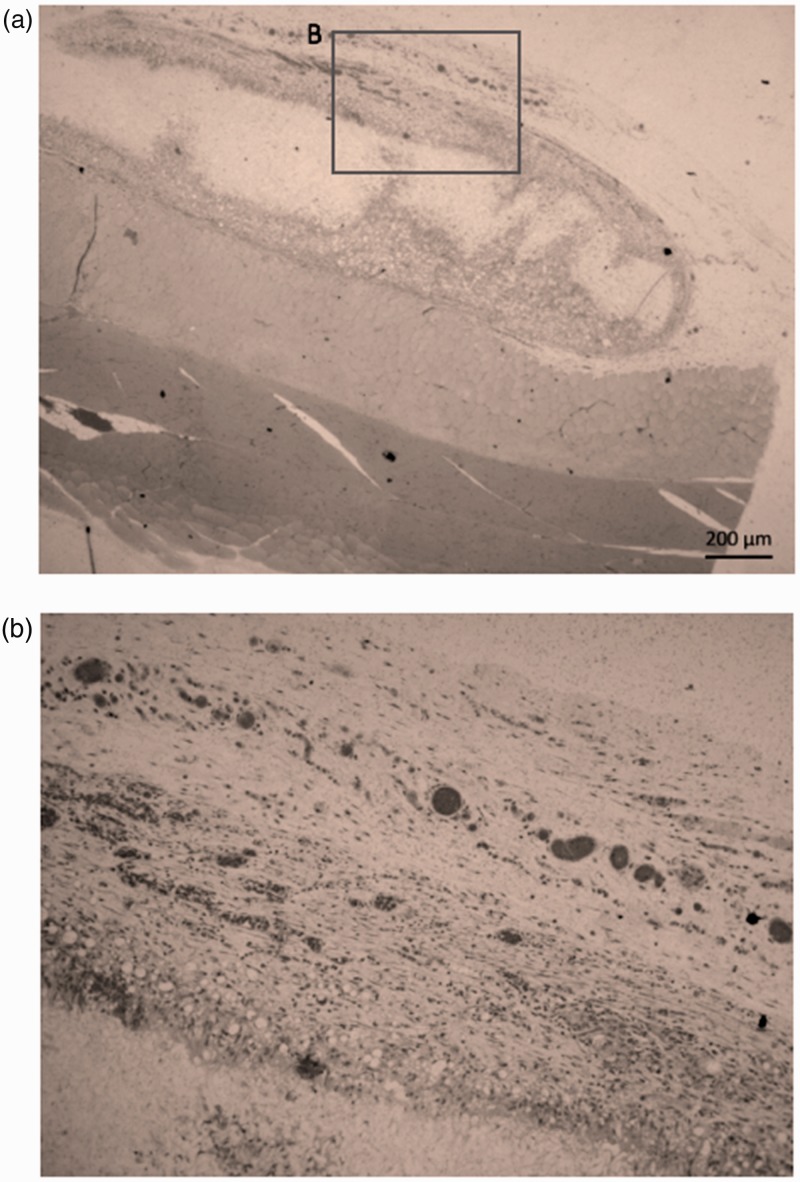
The synthesis of unique materials to support the regeneration of tissues and lost organs due to trauma and/or diseases and TE remains pivotal to the development and translational impact in regenerative dentistry.44 TE uses three basic components (cells, scaffolds and biomolecules) to develop biofunctional substitutes for restore and maintenance of tissue function. The regeneration process can be assisted by scaffolds produced by electrospinning method resulting in biocompatible and sometimes biodegradable polymers.30,87 However, natural tissue integration with functional neovessels can be also obtained (Figure 5). Scaffolds can convey growth factors as well as cells to the target site to assist the injury regeneration88 and generate a support to the increase of cell adhesion, growth, migration rate, and differentiation. Biodegradable polymers withdraw surgical implant removal and can improve patient recovery process.51 Thus, nanostructured materials have been electrospun as mono and multilayered membranes and scaffolds in order to improve tissue regeneration.
Figure 5.
In vivo assay via intramuscular scaffolds implantation in rat. Histological aspect 28 days after implantation of PLGA scaffolds, showing (a) the natural tissue integration capacity and (b) the neovascularization (dark regions) in the implanted region.
Regenerative endodontics includes approaches to treat pulpal and periapical pathologies, for instance, in procedures that could be used for apical closure induction or for teeth that were previously endodontically treated.83 In this area, nanofibrous scaffolds have been designed to stimulate positive cell–ECM interactions, increase cell proliferation, maintain cell phenotype, support differentiation of stem cells, and activate cell-signaling pathways by providing physical and chemical stimuli.44 In Bae et al.,56 collagen was added into electrospun bioactive glass to improve its poor mechanical properties as high brittleness. Proliferation and odontogenic differentiation of dental pulp cells was tested with enhanced cellular growth and differentiation into odontoblasts and human dental pulp cells grew preferentially on the nanocomposite, rather than on type I collagen during culture for 14 days. Additionally, gene expression of the key integrin α2β1 which specifically binds to collagen molecular sequence was highly stimulated in the collagen/electrospun bioglass composite, suggesting that an integrin-mediated process was associated to the odontogenic stimulation.
In Kim et al.,34 a polycaprolactone nanofibrous scaffold was produced by electrospinning and the surface was mineralized with apatite to test cell adhesion, growth, and odontoblastic differentiation. Their results indicated that such scaffold promoted growth and odontogenic differentiation of human dental pulp cells through the integrin-mediated signaling pathway.
Healing of periodontal tissues (ligament and surrounding bone) could be improved by TE and nanofibers.45 In Sundaramv et al.,28 PCL multiscale (micro/nano) membrane was obtained by simultaneous spinning of PCL micro and nanofibers to recover a calcium sulfate/chitosan scaffold to assist in both ligament and bone regeneration. Osteoblastic differentiation of human dental follicle stem cells (hDFCs) on calcium sulfate/chitosan scaffold showed maximum alkaline phosphatase at seventh day followed by a decline thereafter when compared to chitosan control scaffold and also fibroblastic differentiation of hDFCs.
In Bottino et al.,51 chitosan for guided bone regeneration membranes was electrospun using chitosan (70% deacetylated, 312 kDa, 5.5 w/v%), with or without the addition of a crosslinking agent (genipin) in order to extend biodegradation. Genipin-crosslinked mats presented lower degradation rates based on mass loss compared with uncrosslinked mats. In addition, genipin-crosslinked mats supported the proliferation of SAOS-2 cells in a five-day growth study, similar to uncrosslinked mats.
In summary, electrospun of polymeric nanofibers has been used for regeneration of pulp-dentin complex used in combination with biomechanical and biochemical cues in endodontics.44,56 Nanofibers have been used also for regeneration of periodontal ligament alone,45 considering alveolar regeneration at the same time28 or combining oriented nanotopological and mechanical stresses.34,51 Multilayer membrane has been produced for bone and soft tissue regeneration at the same time,29 and barrier membranes in guided bone regeneration.52 Testing cell viability with increase or decrease of the content of nanofibers while maintaining the required structural and biological properties could be challenging. Although initial in vitro results are promising, limited use should be taken until clinical further long-term data are obtained.83
Modification of materials
Ceramic, polymeric, and biodegradable materials have been increasingly used in dentistry as aesthetic options for oral rehabilitation. The improvement of mechanical and physical properties of dental composite such as tensile strength, flexibility and elasticity at nanoscale has stimulated studies to investigate methods to produce modified restorative materials46 to better support the challenging biological and mechanical oral conditions. The use of electrospinning method for these purposes has been indicated.33,46,55,58,70,89 Nanofiber morphology, size, and anisotropy behavior are important factors for reinforced resin composites.60
Recent researches are progressing to further understand how polymers composition influences the success or failure of dental restorations, especially dental resins. Mechanical properties of commercial dental composites modified with electrospun nanopolymers are approaches commonly studied. In Vidotti et al.,60 the effect of bis[4-(methacryloxypropoxy)-phenyl]-propane/triethylene glycol dimethacrylate (BisGMA/TEGDMA) ratio and resin blend to fiber mass ratio on the flexural properties was investigated for electrospun PAN fibers included into resin blends. Their results demonstrated that addition of different ratios of PAN fibers did not affect flexural strength and flexural modulus of the composite beams as compared to neat resin beams; however, the addition of fibers significantly increased the work of fracture of the composite beams, especially for blends with higher TEGDMA ratios.60 Surface silanization of electrospun fibers could be a method to prevent detachment of fibers and the resin matrix in BisGMA/TEGDMA reinforced blends with further increase of mechanical properties as flexural strength, elastic modulus, and work of fracture.89 The composite performance is a function of matrix chemistry, fiber diameter, fiber dispersion, and fiber matrix interaction. The performance of simple polymer systems, like acrylates, can be effected by the introduction of small weight percentage (<1 wt.%) of nanoscale reinforcements.46 Resins for indirect oral application as used in the fabrication of interim restorations70 or acrylic denture material58 could be also benefited with electrospun method. In Almeida et al.,70 bisacrylic partial fixed dental prostheses with and without electrospun silica-nylon reinforcement were tested for different orientations (horizontal or vertical) before and after thermocycling. Results indicated enhanced the flexural strength values of bisacrylic resin bars and that horizontal orientation provided the highest values of fracture strength for partial fixed dental prostheses.
Electrospun polymers with fiber alignment could be an approach since aligned fibers could present round pattern or crossed lines55,58 and dynamic rotating collector is an approach to produce them from different polymers. Fine and nanotexturized biocompatible layers with bioactive compounds could be deposited on dental implants surfaces to increase surface area, reactivity, and interaction with surrounding tissue.90 Implant–cement or implant–bone interfaces are required for implant fixation and the filling of possible tissue defects.33 In Khandaker et al.,33 PCL was deposited with a elestrospinning device in order to create unidirectional fibers on titanium (Ti) plates cemented with poly(methyl methacrylate) (PMMA). The results from fatigue testing and finite-element analysis showed that the addition of the micron- to nanosize PCL fibers on Ti improved the quality of the Ti–PMMA union.
In De Carvalho et al.,91 cellulose acetate and PEO were electrospun and chlorhexidine (CHX) was incorporated into the mats. The CHX-containing fibers showed antibacterial activity against S. mutans and E. faecalis based on the test of inhibition halos formed around the nanofiber discs after the bacterial incubation period. Additionally, fibers with CHX increased release over 90 days from the fibers treated with CHX after spinning.91 This kind of studies is relevant, because most of the commercial restorative composites do not present bacteriostatic and bactericidal properties and such aspects are desirable since restorative resins facilitate bacterial colonization and biofilm formation.92
In Dodiuk-Kenig et al.,46 polyvinyl alcohol (PVOH), PLLA, and nylon 6 were electrospun and added at different ratios into Bis-GMA/TEGDMA acrylate resin system with increase of cross linking for 0.05 wt.%, 250 nm PVOH nanofiber-reinforced composite with decrease of linear shrinkage by 50%. Such result was attributed to the incorporation of hyperbranched moieties into the matrix resin assembling to form nanophases that retard motions in the acrylate backbone through the hydroxy-rich chemistry; however, the exact details of the chemical mechanisms were not fully addressed.
To summarize, the increase of the longevity and performance of direct or indirect dental restorations for oral rehabilitations could be assisted by eletrospinning processes.46,54,55,58,91,93 The achievement of the desirable benefits of nanofiber reinforcement could be improved with the increase of resin ability to properly wet and penetrate the interfibrillar spaces of the fibrous mesh.
However, not all composites ratios are beneficial and limitations of the bonding between the fibers and the resin matrix are an important problem. The incomplete wetting of the nanofibers by the infiltrating resin could result in air inclusion and voids that ultimately compromises the strength of the material.81 Another problem is that methacrylate monomers undergo shrinkage when they polymerize generating shrinkage stresses and leaving restorations susceptible to debonding of the tooth/composite interface. This phenomenon could result in gap formation prone to bacterial infiltration and attempt to reduce polymerization shrinkage comprise varying monomer structure, modifying type, size, size distribution, and amount of fillers added to the resin matrix.94 Finally, another strategy to decrease bacterial infiltration is the incorporation of bacteriostatic and bactericidal agents in dental materials. All promising strategies could be assisted by electrospinning process although further in vitro, in vivo, and clinical investigations are strongly indicated.
Concluding remarks
Collaboration between professionals from different research areas will be essential to the development of new materials and the optimization of techniques for its production, such as electrospinning. Clinical problems and oral health outcomes can be benefited by advances in materials engineering research involving the electrospinning technique.
From the different polymeric materials and applications presented here, it is possible to conclude that the electrospinning technique is increasingly advantageous in oral applications. The improvement of the mechanical properties, the promotion of cell proliferation and differentiation, the degradation rate of different electrospun materials, and the possibility of controlled drug delivery proved the effectiveness of materials produced by this technique. However, some of the analyzed studies require further investigation in order to enable direct human applications. The interaction of nanofiber with biomolecules in animal models may be different in humans and induce different toxic effects. In vivo studies for testing electrospun nanofiber are very low in number compared with in vitro studies. In addition, both in vitro and in vivo toxicity tests must follow well established regulatory guidelines.95 This will enhance the performance of products made from fibers and develop new trends and possibilities in oral treatments.
Natural polymers such as cellulose, collagen, and chitosan, appear as potential electrospun materials, low explored, but with high potential for oral applications.
Although several strategies are being developed to achieve materials with superior performance compared to those commercially available, advances in this field of research are incremental and should be combined or optimized for the effectively production of such materials on a larger scale. Despite the controlled production in laboratory is easy to achieve, the large scale production by electrospinning is in development. For example, in dental and medical applications, the solvent removal from the mat is very important and represents a problem when higher volumes of material are processed. Even though, emerging companies are concerned with these issues, designing innovative devices for industrial scale production.96 Changes in properties cannot be extrapolated by inverse size analysis of the particular but must be determined through in vitro, in vivo, and clinical studies.
Authors’ contributions
This is a multidisciplinary minireview, for this purpose, the professionals that worked were composed by pharmacist, dentist and engineers. All authors participated in design, selection and data analysis and review. ABM, LAGT and DC wrote the manuscript and provided the required information on aspects related to Oral Medicine and Engineering. JVWS helped to discuss polymers from a chemical perspective. ALGM and EB supplied the images from SEM, GEAMB assisted in pharmaceutical aspects described in the minireview.
Declaration of Conflicting Interests
All of the authors of this manuscript, we declare that this paper has not been submitted to, nor published previously elsewhere. We disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work. There are none conflict of interests.
Funding
The work was supported by CAPES, FAPEMIG and CPNq (project number 461922/2014–2), the PRPPG-UFVJM and the PPGCF-UFVJM graduate program was provided for the development of this work.
References
- 1.Subbiah T, Bhat GS, Tock RS, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. J Appl Polym Sci 2005; 96:557–69 [Google Scholar]
- 2.Li G, Zhang T, Li M, Fu N, Fu Y, Ba K, Deng S, Jiang Y, Hu J, Peng Q, Lin Y. Electrospun fibers for dental and craniofacial applications. Curr Stem Cell Res Ther 2014; 9:187–95 [DOI] [PubMed] [Google Scholar]
- 3.Huang Z, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 2003; 63:2223–53 [Google Scholar]
- 4.Gupte MJ, Ma PX. Nanofibrous scaffolds for dental and craniofacial applications. J Dent Res 2012; 91:227–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez MA, Swan MD, Louks JW, inventors ; 3M Innovative Properties Company, applicant. Microfibers and method of making. United States patent US6110588 A, 2002
- 6.Pike RD, inventor ; Kimberly-Clark Worldwide, Inc., applicant. Superfine microfiber nonwoven web. United States patent US 6,624,100 B1, 2003
- 7.Reneker DH, inventor; The University of Akron, applicant. Process and apparatus for the production of nanofibers. United States patent US6520425 B1, 2003
- 8.Tseng AA, Notargiocomo A, Chen TP. Nanofabrication by scanning probe microscope lithography: a review. J Vac Sci Technol B 2005; 23:877–94 [Google Scholar]
- 9.Huie JC. Guided molecular self-assembly: a review of recent efforts. Smart Mater Struct 2003; 12:264–71 [Google Scholar]
- 10.Faul CFJ, Antonietti M. Ionic self-assembly: facile synthesis of supramolecular materials. Adv Mater 2003; 15:673–83 [Google Scholar]
- 11.Whitesides GM, Boncheva M. Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc Natl Acad Sci U S A 2002; 99:4769–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S. Emerging biological materials through molecular self-assembly. Biotechnol Adv 2002; 20:321–9 [DOI] [PubMed] [Google Scholar]
- 13.Rezaei A, Nasirpour A, Fathi M. Application of cellulosic nanofibers in food science using electrospinning and its potential risk. Compreh Rev Food Sci Food Safe 2015; 14:269–84 [DOI] [PubMed] [Google Scholar]
- 14.Seo SJ, Kim HW, Lee JH. Electrospun nanofibers applications in dentistry. J Nanomater 2016;2016:5931946 [Google Scholar]
- 15.Zafar M, Najeeb S, Khurshid Z, Vazirzadeh M, Zohaib S, Najeeb B, Sefat F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016; 9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbas A, Zahra S, Setareh S. A review on electrospun nanofibers for oral drug delivery. Nanomed J 2017; 4:197–207 [Google Scholar]
- 17.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006; 17:R89. [DOI] [PubMed] [Google Scholar]
- 18.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996; 7:216–23 [Google Scholar]
- 19.Li Y, Lim CT, Kotaki M. Study on structural and mechanical properties of porous PLA nanofibers electrospun by channel-based electrospinning system. Polymer 2015; 56:572–80 [Google Scholar]
- 20.Deitzel JM, Kleinmeyer J, Harris DEA, Tan NB. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001; 42:261–72 [Google Scholar]
- 21.Matabola KP, Moutloali RM. The influence of electrospinning parameters on the morphology and diameter of poly(vinyledene fluoride) nanofibers-effect of sodium chloride. J Mater Sci 2013; 48:5475 [Google Scholar]
- 22.Lan SF, Kehinde T, Zhang X, Khajotia S, Schmidtke DW, Starly B. Controlled release of metronidazole from composite poly-ε-caprolactone/alginate (PCL/alginate) rings for dental implants. Dent Mater 2013; 29:656–65 [DOI] [PubMed] [Google Scholar]
- 23.Nezarati RM, Eifert MB, Cosgriff-Hernandez E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng Part C Meth 2013; 19:810–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang GZ, Li HP, Yang JH, Wan J, Yu DG. Influence of working temperature on the formation of electrospun polymer nanofibers. Nanoscale Res Lett 2017; 12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo YJ, Oh J-H, Zhang Q, Lee W, Woo KM. Dimethyloxalylglycine-embedded poly(ε-caprolactone) fiber meshes promote odontoblastic differentiation of human dental pulp–derived cells. J Endodont 2018; 44:98–103 [DOI] [PubMed] [Google Scholar]
- 26.Yin L, Wang K, Lv X, Sun R, Yang S, Yang Y, Liu Y, Liu J, Zhou J, Yu Z. The fabrication of an ICA-SF/PLCL nanofibrous membrane by coaxial electrospinning and its effect on bone regeneration in vitro and in vivo. Sci Rep 2017; 7:8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furtos G, Rivero G, Rapuntean S, Abraham GA. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J Biomed Mater Res Part B Appl Biomater 2016; 105:966–76 [DOI] [PubMed] [Google Scholar]
- 28.Sundaram MN, Sowmya S, Deepthi S, Bumgardener JD, Jayakumar R. Bilayered construct for simultaneous regeneration of alveolar bone and periodontal ligament. J Biomed Mater Res B Res 2016; 104:761–70 [DOI] [PubMed] [Google Scholar]
- 29.Puwanun S, Bye FJ, Ireland MM, MacNeil S, Reilly GC, Green NH. Production and characterization of a novel, electrospun, tri-layer polycaprolactone membrane for the segregated co-culture of bone and soft tissue. Polymers 2016; 8:221–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Kang MS, Eltohamy M, Kim TH, Kim HW. Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering. PLoS One 2016; 11:e0149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Münchow EA, Albuquerque MT, Zero B, Kamocki K, Piva E, Gregory RL, Bottino MC. Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent Mater 2015; 31:1038–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim GH, Park YD, Lee SY, El-Fiqi A, Kim JJ, Lee EJ, Kim HW, Kim EC. Odontogenic stimulation of human dental pulp cells with bioactive nanocomposite fiber. J Biomater Appl 2015; 29:854–66 [DOI] [PubMed] [Google Scholar]
- 33.Khandaker M, Utsaha KC, Morris T. Fracture toughness of titanium–cement interfaces: effects of fibers and loading angles. Int J Nanomed 2014; 9:1689–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JJ, Bae WJ, Kim JM, Kim JJ, Lee EJ, Kim HW, Kim EC. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J Biomater Appl 2014; 28:1069–78 [DOI] [PubMed] [Google Scholar]
- 35.Chaturvedi TP, Srivastava, Srivastava AK, Gupta V, Verma PK. Doxycycline poly e-caprolactone nanofibers in patients with chronic periodontitis – a clinical evaluation. J Clin Diagn Res 2013; 7:2339–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riccitiello F, De Luise A, Conte R, D'Aniello S, Vittoria V, Di Salle A, Calarco A, Peluso G. Effect of resveratrol release kinetic from electrospun nanofibers on osteoblast and osteoclast differentiation. Eur Polym J 2018; 99:289–97 [Google Scholar]
- 37.Schkarpetkin D, Reise M, Wyrwa R, Völpel A, Berg A, Schweder M, Schnabelrauch M, Watts DC, Sigusch BW. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent Mater 2016; 32:951–60 [DOI] [PubMed] [Google Scholar]
- 38.Shahi RG, Albuquerque MT, Münchow EA, Blanchard SB, Gregory RL, Bottino MC. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2016; 105:354–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu HT, Lee SY, Chen CC, Yang YC, Yang JC. Processing and properties of hydrophilic electrospun polylactic acid/beta‐tricalcium phosphate membrane for dental applications. Polym Eng Sci 2013; 53:833–42 [Google Scholar]
- 40.Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, Berg A, Jandt KD, Watts DC, Sigusch BW. Release of metronidazole from electrospun poly (L-lactide-co-D/L-lactide) fibers for local periodontitis treatment. Dent Mater 2012; 28:179–88 [DOI] [PubMed] [Google Scholar]
- 41.Bottino MC, Thomas V, Janowski GM. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater 2011; 7:216–24 [DOI] [PubMed] [Google Scholar]
- 42.Carnaval TG, Gonçalves F, Romano MM, Catalani LH, Mayer MAP, Arana-Chávez VE, Nishida AC, Lage TC, Francci CE, Adde CA. In vitro analysis of a local polymeric device as an alternative for systemic antibiotics in dentistry. Braz Oral Res 2017; 31:e92. [DOI] [PubMed] [Google Scholar]
- 43.Asghari F, Salehi R, Agazadeh M, Alizadeh E, Adibkia K, Samiei M, Akbarzadeh A, Aval NA, Davaran S. The odontogenic differentiation of human dental pulp stem cells on hydroxyapatite-coated biodegradable nanofibrous scaffolds. Int J Polym Mater 2016; 65:720–8 [Google Scholar]
- 44.Albuquerque MT, Valera MC, Nakashima M, Nör JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 2014; 93:1222–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battistella E, Varoni E, Cochis A, Palazzo B, Rimondini L. Degradable polymers may improve dental practice. J Appl Biomater Biomech 2011; 9:223–31 [DOI] [PubMed] [Google Scholar]
- 46.Dodiuk-Kenig H, Lizenboim K, Roth S, Zalsman B, McHale WA, Jaffe M, Griswold K. Performance enhancement of dental composites using electrospun nanofibers. J Nanomater 2008; 2008:1–6 [Google Scholar]
- 47.Cai X, ten Hoopen S, Zhang W, Yi C, Yang W, Yang F, Jansen JA, Walboomers XF, Yelick PC. Influence of highly porous electrospun PLGA/PCL/nHA fibrous scaffolds on the differentiation of tooth bud cells in vitro. J Biomed Mater Res 2017; 105:2597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karczewski A, Feitosa SA, Hamer EI, Pankajakshan D, Gregory RL, Spolnik KJ, Bottino MC. Clindamycin-modified triple antibiotic nanofibers: a stain-free antimicrobial intracanal drug delivery system. J Endodontics 2018; 44:155–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pankajakshan D, Albuquerque MT, Evans JD, Kamocka MM, Gregory RL, Bottino MC. Triple antibiotic polymer nanofibers for intracanal drug delivery: effects on dual species biofilm and cell function. J Endod 2016; 42:1490–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamocki K, Nör JE, Bottino MC. Dental pulp stem cell responses to novel antibiotic-containing scaffolds for regenerative endodontics. Int Endod J 2015; 48:1147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bottino MC, Kamocki K, Yassen GH, Platt JA, Vail MM, Ehrlich Y, Gregory RL. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res 2013; 92:963–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norowski PA, Jr1, Fujiwara T, Clem WC, Adatrow PC, Eckstein EC, Haggard WO, Bumgardner JD. Novel naturally crosslinked electrospun nanofibrous chitosan mats for guided bone regeneration membranes: material characterization and cytocompatibility. J Tissue Eng Regen Med 2015; 9:577–83 [DOI] [PubMed] [Google Scholar]
- 53.Samprasit W, Kaomongkolgit R, Sukma M, Rojanarata T, Ngawhirunpat T, Opanasopit P. Mucoadhesive electrospun chitosan-based nanofibre mats for dental caries prevention. Carbohydr Polym 2015; 117:933–40 [DOI] [PubMed] [Google Scholar]
- 54.Hamilton MF, Otte AD, Gregory RL, Pinal R, Ferreira-Zandoná A, Bottino MC. Physicomechanical and antibacterial properties of experimental resin-based dental sealants modified with nylon-6 and chitosan nanofibers. J Biomed Mater Res B Res 2015; 103:1560–8 [DOI] [PubMed] [Google Scholar]
- 55.Borges AL, Muenchow EA, Oliveira Souza AC, Yoshida T, Vallittu PK, Bottino MC. Effect of random/aligned nylon-6/MWCNT fibers on dental resin composite reinforcement. J Mech Behav Biomed Mater 2015; 48:134–44 [DOI] [PubMed] [Google Scholar]
- 56.Bae WJ, Min KS, Kim JJ, Kim JJ, Kim HW, Kim EC. Odontogenic responses of human dental pulp cells to collagen/nanobioactive glass nanocomposites. Dent Mater 2012; 28:1271–9 [DOI] [PubMed] [Google Scholar]
- 57.Tang Y, Chena L, Zhaoa K, Wub Z, Wanga Y, Tanb Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos Sci Technol 2016; 125:100–7 [Google Scholar]
- 58.Uyar T, Çökeliler D, Doğan M, Koçum IC, Karatay O, Denkbaş EB. Electrospun nanofiber reinforcement of dental composites with electromagnetic alignment approach. Mater Sci Eng C Mater Biol Appl 2016; 62:762–70 [DOI] [PubMed] [Google Scholar]
- 59.Lee SJ, Heo DN, Lee D, Heo M, Rim H, Zhang LG, Park SA, Do SH, Moon JH, Kwon IK, Il K. One-step fabrication of AgNPs embedded hybrid dual nanofibrous oral wound dressings. J Biomed Nanotechnol 2016; 12:2041–50 [DOI] [PubMed] [Google Scholar]
- 60.Vidotti H, Manso AP, Leung V, do Valle AL, Ko F, Carvalho RM. Flexural properties of experimental nanofiber reinforced composite are affected by resin composition and nanofiber/resin ratio. Dent Mater 2015; 31:1132–41 [DOI] [PubMed] [Google Scholar]
- 61.Xiao Y, Gong T, Jiang Y, Wang Y, Wen ZT, Zhou S, Bao C, Xu X. Fabrication and characterization of a glucose-sensitive antibacterial chitosan-polyethylene oxide hydrogel. Polymer 2016; 82:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Liu X, Jin X, Ma H, Hu J, Ni L, Ma PX. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater 2010; 6:3856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zivanovic S, Li J, Davidson PM, Kit K. Physical, mechanical, and antibacterial properties of chitosan/PEO blend films. Biomacromolecules 2007; 8:1505–10 [DOI] [PubMed] [Google Scholar]
- 64.Mai T, Rakhmatullina E, Bleek K, Boye S, Yuan J, Völkel A, Gräwert M, Cheaib Z, Eick S, Günter C, Lederer A, Lussi A, Taubert A. Poly(ethylene oxide)-b-poly(3-sulfopropyl methacrylate) block copolymers for calcium phosphate mineralization and biofilm inhibition. Biomacromolecules 2014; 10:3901–14 [DOI] [PubMed] [Google Scholar]
- 65.Kim GM, Asran AS, Michler GH, Simon P, Kim JS. Electrospun PVA/HAp nanocomposite nanofibers: biomimetics of mineralized hard tissues at a lower level of complexity. Bioinspir Biomim 2008; 3:046003. [DOI] [PubMed] [Google Scholar]
- 66.Ranjbar-Mohammadi M, Zamani M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater Sci Eng C Mater Biol Appl 2016; 58:521–31 [DOI] [PubMed] [Google Scholar]
- 67.Ghorbani F, Nojehdehian H, Zamanian A. Physicochemical and mechanical properties of freeze cast hydroxyapatite-gelatin scaffolds with dexamethasone loaded PLGA microspheres for hard tissue engineering applications. Mater Sci Eng C Mater Biol Appl 2016; 69:208–20 [DOI] [PubMed] [Google Scholar]
- 68.Bottino MC, Yassen GH, Platt JA, Labban N, Windsor LJ, Spolnik KJ, Bressiani AH. A novel three‐dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med 2015; 9:E116–23 [DOI] [PubMed] [Google Scholar]
- 69.He T, Wang J, Huang P, Zeng B, Li H, Cao Q, Zhang S, Luo Z, Deng DY, Zhang H, Zhou W. Electrospinning polyvinylidene fluoride fibrous membranes containing anti-bacterial drugs used as wound dressing. Colloids Surf B Biointerfaces 2015; 130:278–86 [DOI] [PubMed] [Google Scholar]
- 70.Almeida CS, Amaral M, Gonçalves FDCP, Paes-Junior TJA. Effect of an experimental silica-nylon reinforcement on the fracture load and flexural strength of bisacrylic interim partial fixed dental prostheses. J Prosthet Dent 2016; 115:301–5 [DOI] [PubMed] [Google Scholar]
- 71.Kaplan DL. Introduction to Biopolymers from renewable resources In: Abe A, Monnerie L, Shibaev V, et al. (eds) Biopolymers from renewable resources. 1st ed Berlin: Springer-Verlag, 1998, pp.1–29. [Google Scholar]
- 72.Hayashi T. Biodegradable polymers for biomedical uses. Prog Polym Sci 1994; 19:663–702 [Google Scholar]
- 73.Furuya DC, Costa SA, Oliveira RC, Ferraz HG, Pessoa Junior A, Costa SM. Fibers obtained from alginate, chitosan and hybrid used in the development of scaffolds. Mat Res 2017; 20:377–86 [Google Scholar]
- 74.Khan FI, Rahman S, Queen A, Ahamad S, Ali S, Kim J, Hassan MI. Implications of molecular diversity of chitin and its derivatives. Appl Microbiol Biotechnol 2017; 101:3513–36 [DOI] [PubMed] [Google Scholar]
- 75.Campos MGN, Mei LHI, Santos AR., Jr Sorbitol-plasticized and neutralized chitosan membranes as skin substitutes. Mat Res 2015; 18:781–90 [Google Scholar]
- 76.Nevins AJ, Cymerman JJ. Revitalization of open apex teeth with apical periodontitis using a collagen-hydroxyapatite scaffold. J Endod 2015; 41:966–73 [DOI] [PubMed] [Google Scholar]
- 77.Islam MS, Khunkar SJ, Nakashima S, Sadr A, Nikaido T, Tagami J. Comparative study of demineralized collagen degradation determined by hydroxyproline assay and microscopic depth measurement. J Dent 2016; 47:94–7 [DOI] [PubMed] [Google Scholar]
- 78.Hass V, Paula AM, Parreiras S, Gutiérrez MF, Luque-Martinez I, Matos TP, Bandeca MC, Loguercio AD, Yao X, Wang Y, Reis A. Degradation of dentin-bonded interfaces treated with collagen cross-linking agents in a cariogenic oral environment: an in situ study. J Dent 2016; 49:60–7 [DOI] [PubMed] [Google Scholar]
- 79.Zhang Q, Lin D, Yao S. Review on biomedical and bioengineering applications of cellulose sulfate. Carbohydr Polym 2015; 132:311–22 [DOI] [PubMed] [Google Scholar]
- 80.Ao C, Niu Y, Zhang X, He X, Zhang W, Lu C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int J Biol Macromol 2017; 97:568–73 [DOI] [PubMed] [Google Scholar]
- 81.Boyd SA, Su B, Sandy JR, Ireland AJ. Cellulose nanofibre mesh for use in dental materials. Coatings 2012; 2:120–37 [Google Scholar]
- 82.Ramalingam M, Ramakrishna S. Nanofiber composites for biomedical applications. 1st ed Cambridge: Woodhead Publishing, 2017 [Google Scholar]
- 83.Deepak A, Goyal AK, Rath G. Nanofiber in transmucosal drug delivery. J Drug Deliv Sci Technol 2018; 43:379–87 [Google Scholar]
- 84.Tonglairoum P, Ngawhirunpat T, Rojanarata T, Kaomongkolgit R, Opanasopita P. Fabrication of a novel scaffold of clotrimazole-microemulsion-containing nanofibers using an electrospinning process for oral candidiasis applications. Colloids Surf B Biointerfaces 2015; 126:18–25 [DOI] [PubMed] [Google Scholar]
- 85.Thakkar S, Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci 2017; 107:148–67 [DOI] [PubMed] [Google Scholar]
- 86.Veras FF, Roggia I, Pranke P, Pereira CN, Brandelli A. Inhibition of filamentous fungi by ketoconazole-functionalized electrospun nanofibers. Eur J Pharm Sci 2016; 84:70–6 [DOI] [PubMed] [Google Scholar]
- 87.Millas ALG, Mckean R, Stevens B, Yussuf M, Silveira JVW, Puzzi M, Bittencourt E. Fabrication of electrospun scaffolds incorporating an amazonian therapeutic oil from the Copaifera Sp. for wound care applications. J Biomater Tissue Eng 2014; 4:217–20 [Google Scholar]
- 88.Yao D, Liu H, Fan Y. Silk scaffolds for musculoskeletal tissue engineering. Exp Biol Med 2016; 241:238–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao Y, Sagi S, Zhang L, Liao Y, Cowles DM, Sun Y, Fong H. Electrospun nanoscaled glass fiber reinforcement of bisGMA/TEGDMA dental composites. J Appl Polym Sci 2008; 110:2063–70 [Google Scholar]
- 90.Subramani K, Ahmed W. Emerging nanotechnologies in dentistry: processes, materials and applications. 1st ed. Saint Louis: Elsevier, 2012 [Google Scholar]
- 91.De Carvalho LD, Urbanetto Peres B, Maezomo H, Shen Y, Haapasalo M, Manso AP, Ko F, Carvalho RM. Chlorhexidine-containing electrospun nanofibers: effect of production mode on chlorhexidine release. Dent Mater 2017; 33:e17–8 [Google Scholar]
- 92.Anusavice K, Shen C, Rawls HR. Phillips' science of dental materials. 12th ed. St. Louis: Saunders, 2012 [Google Scholar]
- 93.Shahmirzadi NJ, Inanloo SH. Effect of hapnanofibers prepared by electrospinning process on the mechanical properties of dental resins. Biomed Pharmacol J 2015; 8:283–9 [Google Scholar]
- 94.Ratner B, Hoffman A, Schoen F, Lemons J. Biomaterials science: an introduction to materials. 3rd ed Oxford: Academic Press, 2012 [Google Scholar]
- 95.Balusamy B, Senthamizhan S, Uyar T. In vivo safety evaluations of electrospun fibernanofibers for biomedical application In: Uyar T andKny E (eds) Electrospun materials for tissue engineering and biomedical applications: research, design and commercialization. Sawston: Woodhead Publishing, 2017 [Google Scholar]
- 96.Persano L, Camposeo L, Tekmen C, Pisignano D. Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol Mater Eng 2013; 298:504–20 [Google Scholar]