Short abstract
Bone loss is one of the important extra-intestinal manifestations in patients with inflammatory bowel diseases (IBDs). Compounds derived from natural products have been used to treat IBDs. However, the role of natural products on IBD-induced bone loss is not completely clarified. In the present study, we observed the effects of dihydroartemisinin (DHA), an antimalaria drug, on IBD and IBD-induced bone loss in a rat model. Chronic IBD model was established in Sprague-Dawley rats by giving them 2.5% dextran sodium sulfate in drinking water. DHA was given by intraperitoneal injection. Blood, colon, and bone samples were collected for biomarker assay and histological analysis. There was an obvious increase in tumor necrotic factor (TNF) α and receptor activator of nuclear factor (NF)-kB ligand (RANKL), and decrease in procollagen type 1 N-terminal propeptide (P1NP) level in IBD groups compared with the normal control (p < 0.05). The disease activity score of IBD rats was significantly higher than the control (p < 0.01). Obvious decrease in disease activity score, TNFα, and RANKL level and increase in P1NP were observed in DHA-treated IBD rats. Bone loss, shown as the decrease in bone mineral density, bone volume fraction, and trabecular number and increase in trabecular separation were observed in IBD rats compared with control (p < 0.01). DHA treatment obviously abolished the bone loss, in particular in the high-dose group (p < 0.05). DHA treatment also inhibited the excessive osteoclast formation; RANKL protein expression; and RANK, TRAF6, Fra-1, NFATc1 mRNA expression induced by IBD. Our data indicated that DHA may be a potential therapeutic agent for IBD and IBD-induced bone loss.
Impact statement
Bone loss is one of the important extra-intestinal manifestations in patients with inflammatory bowel diseases (IBDs). Studies have shown that compounds derived from natural products are useful in the treatment of IBDs. However, few studies have investigated the role of compounds derived from natural products in treatment of osteoporosis in IBDs. The current study aimed to show the effects of dihydroartemisinin (DHA), antimalaria drug, on bone loss in a rat model of IBD. The findings showed that DHA intervention dose dependently protected against bone loss in IBD rats by inhibiting tumor necrotic factor α production and osteoclast formation. These findings highlights that DHA may be beneficial for bone health in those patients with IBD.
Keywords: Inflammatory bowel diseases, bone loss, tumor necrotic factor α, osteoclast, dihydroartemisinin, receptor activator of nuclear factor-kB ligand
Introduction
Inflammatory bowel diseases (IBDs) are common chronic gastrointestinal disorders characterized by inflammation and tissue degeneration in both children and adults.1 The pathogenic mechanism of IBD is still unknown. Genetic, immune, and environmental factors may both play critical roles in IBD.2 IBD patients suffer from chronic intestinal inflammation characterized by diarrhea, abdominal pain, and weight loss.3 During the inflammatory process, the stimulated inflammatory cells, such as macrophages, dendritic cells, and antigen-presenting cells, secrete pro-inflammatory cytokines and chemokines, such as interleukin (IL)-6, IL-12, and tumor necrosis factor (TNF),4 which can cause systemic inflammation and extra-intestinal manifestations.5–7
Bone loss is one of the important extra-intestinal manifestations in IBD patients. Low bone mass was observed in 40–50% of IBD patients.8 Many studies have shown that IBD patients have a high risk of osteoporosis and bone fractures.7–9 Bone loss is related to a variety of factors, including weight loss,7,10,11 systemic inflammation,6,9,12 and treatment with corticosteroids.9 Recent studies showed that IBD-induced bone loss was independent upon weight loss.13 In addition, Card et al.14 reported that the more than half of hip fracture in IBD patients was not due to the steroid use. Inflammation may be the main determinant of bone loss in IBD patients.6,13 The cytokines released by the inflammatory cells, especially for TNFα can stimulate osteoclast formation and activity, which cause excessive bone resorption and bone loss,15 and anti-TNFα therapy can decrease the bone loss.16,17 In addition, bone formation is also inhibited in IBD patients,18 particularly in children.13
Compounds derived from natural products, such as Asian herbs, can protect against bone loss by inhibiting osteoclast formations.19–22 Recent studies also indicated that DHA21 and artemisinin,23–25 antimalaria drug, can inhibit estrogen deficiency- or lipopolysaccharide-induced bone loss by suppressing osteoclast formation or inducing osteoclast apoptosis. Compounds derived from natural products are also useful for the treatment of IBD.26 Previous studies indicated that Artesunate treatment decreased the high TNFα level and the colon damage induced by dextran sodium sulfate (DSS).27 However, the effect of DHA on IBD and IBD-induced bone loss is not known. In addition, the overactivation of osteoclasts is a critical way in IBD-induced bone destruction.28,29 In the present study, we aimed to investigate the role of DHA in IBD-induced bone loss in an adult rat model and explored the potential mechanisms from the aspect of bone formation and bone-resorbing osteoclasts.
Materials and methods
Experimental design
Thirty-two 12-week-old male Sprague-Dawley rats weighing 360–372 g were fed in the specific pathogen-free animal facilities and maintained under conventional conditions (21 ± 1°C, 50–80% relative humidity) in a 12 h light–12 h dark cycle. All rats were allowed free access to standard laboratory food and water. After one-week acclimatization to laboratory conditions, the animals were randomly divided into four groups of eight mice: a non-IBD control group, IBD group, and two DHA treatment groups. The dose of DHA for antimalarial treatment was 60 mg/d. The equivalent dose ratio of human to rat is 6.3. Therefore, the calculated dose for rat is 60 mg/60 kg × 6.3 = 6.3 mg/kg. Therefore, the two doses, 10 and 20 mg/kg which were close to the estimated dose were adopted. The protocol employed here was approved by the Animal Care Committee of Affiliated Hospital of Guangdong Medical University.
Preparation of IBD models and DHA treatment
The chronic IBD model was established as described in previous studies by using DSS (molecular weight 36,000–50,000 MW).30,31 Briefly, the rats were exposed to 2.5% DSS for five days and then had free access to normal water for one week. Next, the same procedure was performed as above once again. Then, the rats had free access to normal water for four weeks. Control rats were given normal water. For the two treatment groups, the DHA (10 and 20 mg/kg) was given at the second week for a total of five weeks (five times per week). The rats in IBD and control group were also intraperitoneally injected with 3% DMSO five times per week. The data of body weight were obtained every week.
Sample collection
At the sixth week, all rats were sacrificed by anesthesia (7% chloral hydrate, 0.5 mL/100 g body weight). We collected the blood from carotid artery without anticoagulant and it was centrifuged for serum isolation. The serum was divided into aliquots and stored at −80°C until analysis. The colon was collected for hematoxylin and eosin (HE) staining. Lumbar spines were collected for bone mineral density (BMD) determination after removing excess soft tissues. Left tibia was harvested for histological examination after decalcified by 10% EDTA. The right tibia was obtained for microCT analysis.
Colitis evaluation
Colon was fixed with 10% formalin solution for 24 h and embedded in paraffin. Five micrometer sections were obtained for HE staining. The severity of colitis was evaluated by using disease activity index (DAI) as previous studies described.31,32 The DAI includes degree of inflammation (0: none; 1: mild; 2: moderate; 3: severe) and mucosal damage (0: none, 1: mucous layer, 2: submucosa, 3: muscularis and serosa), crypt damage (0: none, 1: basal, 1/3: damaged, 2: basal, 2/3: damaged, 3: entire crypt damaged, 4: epithelium lost), and range of lesions (0: none, 1: 0–25%, 2: 26–50%, 3: 51–75%, 4: 76–100%). All the parameters were summed.
Bone densitometry and microCT analysis
Lumbar spines were wrapped in tissue soaked in normal saline chloride. The BMD was determined by using dual energy X-ray absorptiometry (Hologic Discovery Wi, USA) with small animal software.
Excess soft tissue was removed from tibias and wrapped in tissue soaked in normal saline chloride. The samples were placed in Eppendorf tubes to maintain position. The tibia was imaged with a microCT system (GE Healthcare, eXplore Locus, USA). The imaging parameters were as following: X-ray tube potential of 80 kV, X-ray intensity of 350 µA, field of view 3.0 cm, and 45 µm isotropic resolutions. Tibia images were then reconstructed using software with a constant threshold value. The 3D images were loaded into the analysis program for further analysis. The following parameters were analyzed: bone volume fractions (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp). The region of interest was set at 1 mm below the growth plate and extending a further longitudinal distance of 2 mm in the distal direction.
Bone histologic assay
Left tibia decalcified by 10% EDTA, dehydrated through a series of ascending ethanol solution (40–100%) and embedded in paraffin. Five micrometer sections were obtained. The sections were dewaxed by using dimethylbenzene and then hydrating by passage through 100–40% ethanol solution. The osteoclasts in bone tissues were identified by tartrate-resistant acid phosphate (TRAP) staining which was performed by using a commercial kit (Sigma 387-A, St Louis, USA). Then, the sections were viewed with a microscope. TRAP-positive length/bone surface were analyzed by using imaging software.
In addition, receptor activator of nuclear factor (NF)-kB ligand (RANKL) expression in bone was determined by immunohistochemical methods. After dewaxing in dimethylbenzene and rehydrating in PBS, the sections were heated in retrieval solution. Then, the sections were incubated in 1% H2O2. Subsequently, they were incubated with primary antibodies (Mouse monoclonal anti-RANKL, 1:100 dilutions, Catalog no. sc-59982, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) overnight at 4°C, following incubation with secondary antibodies (1:1000, Catalog no. ab193651, Abcam, MA, USA) at room temperature. Finally, the antigen–antibody complex was visualized by 3,30-diaminobenzidine kit. The RANKL expression was evaluated by the positive area (%) by using the following equation= the area of RANKL-positive growth plate divided by the area of growth plate.
Biochemical marker assay
Serum tumor necrosis factor α (TNFα, Catalog no. RTA00, R&D Systems), RANKL (Quantikine colorimetric sandwich ELISA; Catalog no. MTR00, R&D Systems), tartrate-resistant acid phosphatase 5b (Tracp5b) (Rat TRAP Assay, Catalog no. SB-TR201A, IDS, UK), and procollagen type 1 N-terminal propeptide (P1NP) (Catalog no. AC-33F1, IDS, UK) were determined by using enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions. The detection limits were both 5.0 pg/mL for TNFα and RANKL. The intraassay and interassay were both lower than 6%. For Tracp5b, the manufacturer supplied a standard sample (1.9 U/L) and the obtained result in our laboratory was 1.7–1.9 U/L.
In addition, the alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine were determined by using commercial kit (Jiancheng Bio Inc., Nanjing, China; Catalog no. c010-3, c009-3, c013-2, c011-2).
Reverse transcription polymerase chain reaction in vivo
Tibias were crushed under liquid nitrogen and total RNA was extracted by using Trizol reagent. Furthermore, cDNA was synthesized by reverse transcription using a using a Quantscript RT kit (Catalog no. KR103, Tiangen Biotech Co., China). The individual cDNA species were amplified in a reaction mixture containing cDNA aliquot, the relevant sense and antisense primers (RANK: forward 5′ccaggacagggctgatgagaa′3, reverse 5′tggctgacatacaccacgatga′3, TRAF6: 5′agcccacgaaagccagaagaa′3, reverse 5′cccttatggatttgatgatga′3, Fra-1: 5′agagctgcagaagcagaagg′3, reverse 5′caagtacgggtcctggagaa′3; NFATc1: forward 5′-ctcaccacagggctcactatg-3′, reverse 5′-ttcttcctcccgatgtccgt-3′), and SYBR Premix Ex Taq Mix(Takara Bio Inc., Otsu, Japan). Reactions were initiated by incubation at 94°C for 5 min, and real-time polymerase chain reaction was performed for 40 cycles. Each cycle consisted of 94°C for 15 s, 60°C for 30 s, and 72°C for 20 s.
Statistical analysis
The data were managed and analyzed by suing SPSS16.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± SD. One-way analysis of variance followed by Student–Newman–Keuls post hoc was used to compare the mean values of quantitative variables among groups. The score of histopathological lesions was analyzed by Mann–Whitney U test. Spearman correlation analysis was used to show the association between disease score and bone microstructural parameters. P value below 0.05 was regarded as significance of differences.
Results
Body weight
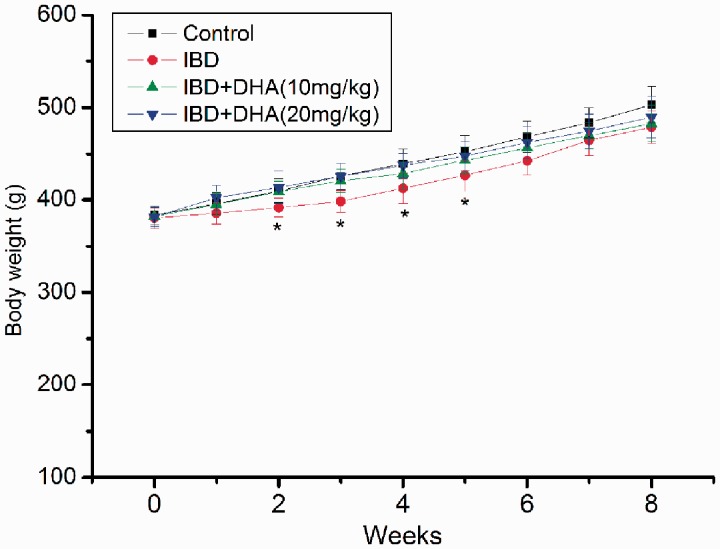
The body weight during the whole experiment is listed in Figure 1. Overall, the body weight of rats in every group increased during the whole experiment. The body weight of 2.5% DSS-treated rat did significantly differ with control rats from the second week (all p < 0.05). However, the weight loss is less than 10%. The body weights of rats in DHA groups were also lower than control rats, but no significant differences were observed.
Figure 1.
Body weight of rats in control, IBDs, and DHA-treated group. The body weight of 2.5% DSS-treated rat did significantly differ with control rats from the second week (all p < 0.05). * control versus IBD, p < 0.05. DHA: dihydroartemisinin; IBD: inflammatory bowel disease. (A color version of this figure is available in the online journal.)
Disease activity score
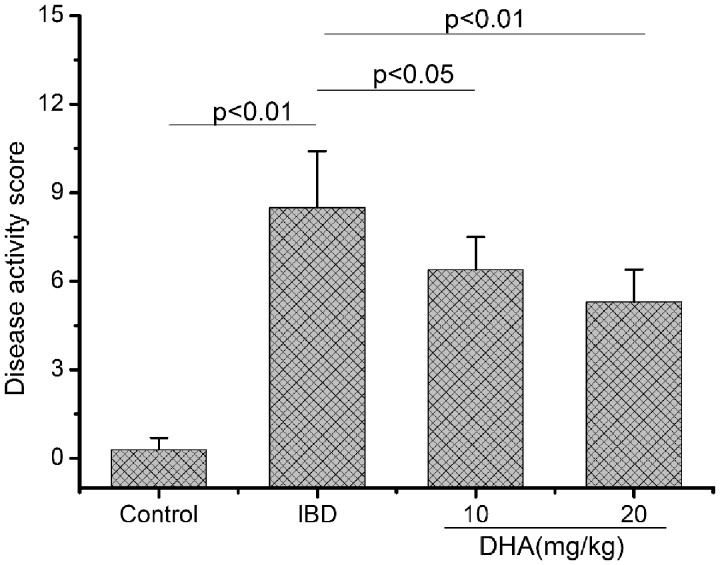
DSS induced IBD in our study as shown in histologic examination and disease activity score (Figure 2). The score of DSS-treated rats was significantly higher than that of control rats (p < 0.01). Intervention with DHA obviously reduced the damage as shown by the decrease in disease activity score, in particular at 20 mg/kg (p < 0.05).
Figure 2.
Disease activity score in control, IBDs, and DHA-treated rats. The score of IBD rats was higher than control rats. DHA reduced the damage as shown by the decrease in disease activity score. DHA: dihydroartemisinin; IBD: inflammatory bowel disease.
Serum biomarker
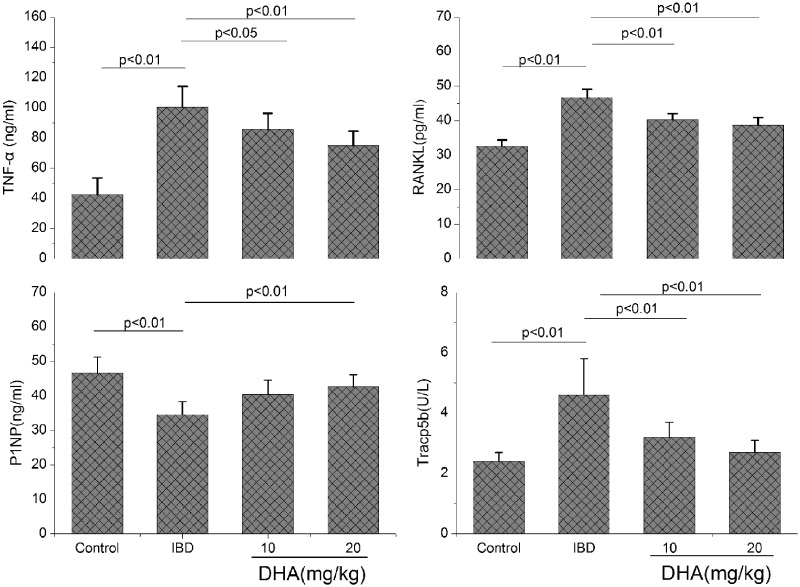
Four biomarkers related with IBD or bone loss were determined in this study (Figure 3). The serum TNFα, Tracp5b, and RANKL were all significantly increased in DSS-treated rats compared with the control (all p < 0.01). The treatments with DHA obviously abolished the increase of TNFα, Tracp5b, and RANKL compared with the rats treated with DSS alone. The P1NP level was decreased in IBD rats. The treatments with DHA diminished the decrease of P1NP level caused by IBD, in particular at 20 mg/kg (p < 0.01). In addition, AST and ALT were increased in IBD rats compared to the control, but no significant differences in AST, ALT, BUN, and creatinine were observed between IBD rats and those treated with DHA (Table 1).
Figure 3.
Serum TNFα, RANKL, tartrate-resistant acid phosphatase 5b (Tracp5b), and P1NP levels in control, IBDs, and DHA-treated rats. The serum TNFα, Tracp5b, and RANKL were increased and P1NP was decreased in IBD rats compared with the control. The treatments with DHA obviously abolished the changes of TNFα, Tracp5b, RANKL, and P1NP compared with the rats treated with DSS alone. DHA: dihydroartemisinin; P1NP: procollagen type 1 N-terminal propeptide; RANKL: receptor activator of nuclear factor (NF)-kB ligand; TNFα: tumor necrotic factor α.
Table 1.
Liver and renal dysfunction.
| Control | IBD | IBD + DHA (10 mg/kg) | IBD + DHA (20 mg/kg) | |
|---|---|---|---|---|
| ALT (U/L) | 42.3 ± 10.6 | 58.7±11.3* | 64.5 ± 12.5 | 65.7 ± 11.8 |
| AST (U/L) | 76.4 ± 11.4 | 87.9 ± 12.3* | 92.3 ± 13.7 | 94.6 ± 12.4 |
| BUN (mmol/L) | 5.7 ± 1.5 | 5.9 ± 1.3 | 5.9 ± 1.4 | 6.2 ± 1.7 |
| Creatinine (umol/L) | 24.6 ± 4.3 | 25.3 ± 3.8 | 26.4 ± 2.4 | 26.8 ± 3.7 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; DHA: dihydroartemisinin; IBD: inflammatory bowel disease.
*p < 0.05 versus control.
BMD and bone microstructure
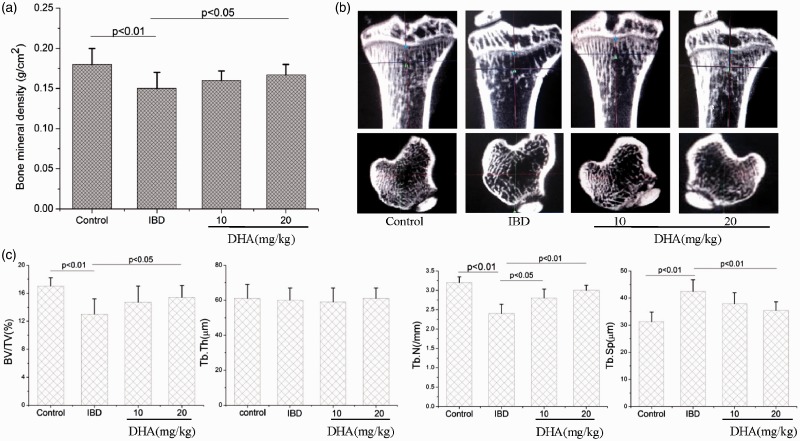
The BMD of DSS-treated rats was obviously decreased compared with that of control rats (p < 0.05). The BMD increased in the presence of DHA (Figure 4(a)), in particular at a dose of 20 mg/kg (p < 0.05).
Figure 4.
BMD (a) and bone microstructure (b, c) in control, IBDs, and DHA-treated rats. (b) MicroCT image of tibia; (c) quantitative analysis of BV/TV, Tb.N, Tb.Th, and Tb.Sp. The BMD, BV/TV, and Tb.N of IBD rats were decreased and Tb.Sp was increased compared to the control. The treatments with DHA obviously abolished these changes. BV/TV: bone volume fractions; DHA: dihydroartemisinin; IBD: inflammatory bowel disease; Tb.N: trabecular number; Tb.Sp: trabecular separation; Tb.Th: trabecular thickness.
Subsequently, we evaluated the bone microstructure by using microCT (Figure 4(b)). Two-dimensional and 3D images both showed the bone loss in DSS-treated rats. The quantitative data further indicated the BV/TV and Tb.N reduce and Tb.Sp increase in DSS rats compared with control (Figure 4(c)). The treatment with DHA protected the rats against bone loss associated with IBD, as shown by increased BV/TV, Tb.N, and decreased Tb.Sp, in particular at a dose of 20 mg/kg (Figure 4(b) and (c)).
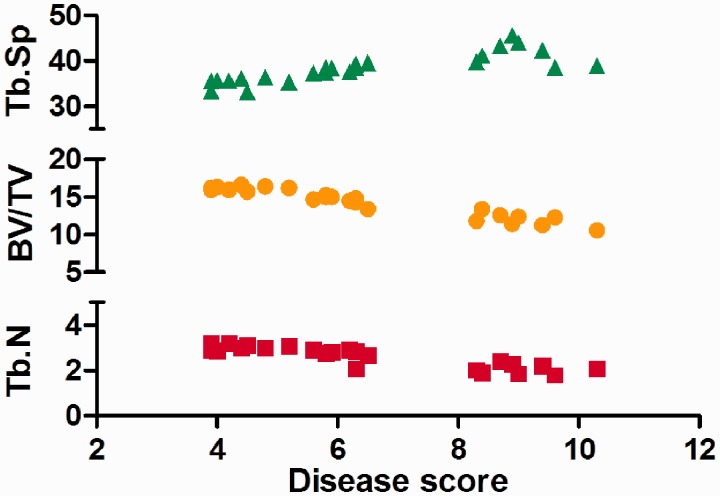
We also showed the association between disease score with bone microstructural parameters (Figure 5). The disease score was positively correlated with Tb.Sp (r = 0.76) and negatively correlated with BV/TV (r = 0.92) and Tb.N (r = 0.86).
Figure 5.
The correlations between disease score and bone microstructural parameters. The disease score was positively correlated with Tb.Sp (r = 0.76) and negatively correlated with BV/TV (r = 0.92) and Tb.N (r = 0.86). BV/TV: bone volume fractions; Tb.N: trabecular number; Tb.Sp: trabecular separation. (A color version of this figure is available in the online journal.)
Bone histological examinations
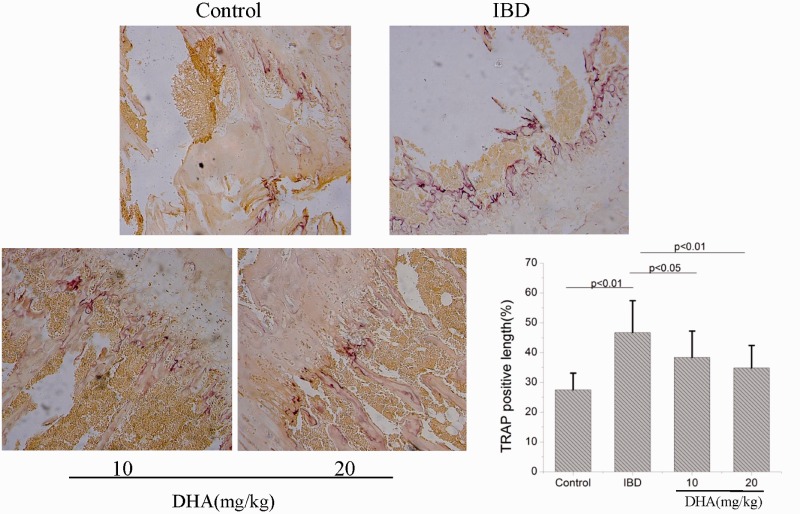
Histochemical stains showed that TRAP-positive cells were located at bone surface (Figure 6). The TRAP-positive length/bone surface in DSS-treated rats was significantly longer than in control rats (p < 0.05). However, the TRAP-positive length/bone surface was decreased in DHA-treated IBD rats compared with IBD group (p < 0.05). This result indicated that DHA may protect IBD-induced bone loss by inhibiting osteoclast formation.
Figure 6.
Histochemical stains show TRAP-positive cells in control, IBDs, and DHA-treated rats. The TRAP-positive length/bone surface in IBD rats was significantly longer than control rats. DHA treatment inhibited the osteoclast formation induced by IBD. DHA: dihydroartemisinin; IBD: inflammatory bowel disease; TRAP: tartrate-resistant acid phosphate. (A color version of this figure is available in the online journal.)
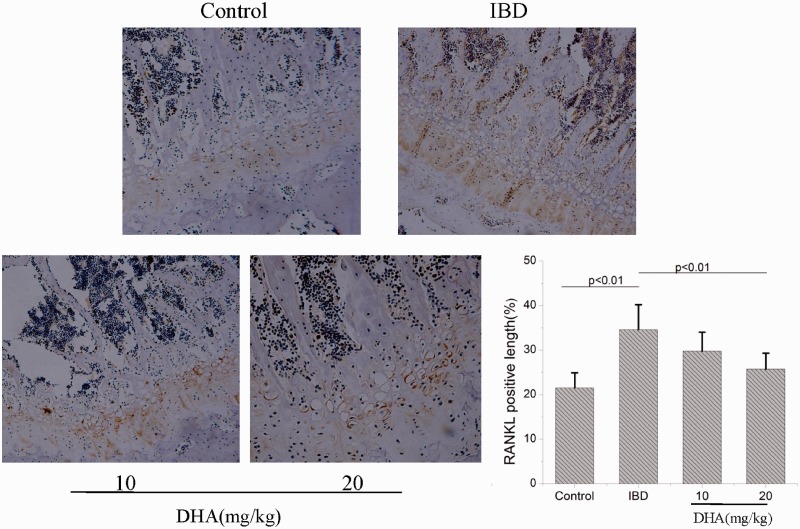
Subsequently, we observed the RANKL expression in bone tissues (Figure 7). Control rats showed weak level of RANKL expression. The RANKL expression in DSS-treated rats was markedly increased compared with the control. The RANKL expression was decreased in DHA-treated IBD rats compared with IBD group, in particular at a dose of 20 mg/kg (p < 0.01).
Figure 7.
Immunochemical stains show RANKL expression in control, IBDs, and DHA-treated rats. The RANKL-positive length in IBD rats was significantly longer than control rats. DHA treatment inhibited the RANKL expression induced by IBD. DHA: dihydroartemisinin; IBD: inflammatory bowel disease; RANKL: receptor activator of nuclear factor (NF)-kB ligand. (A color version of this figure is available in the online journal.)
mRNA expression
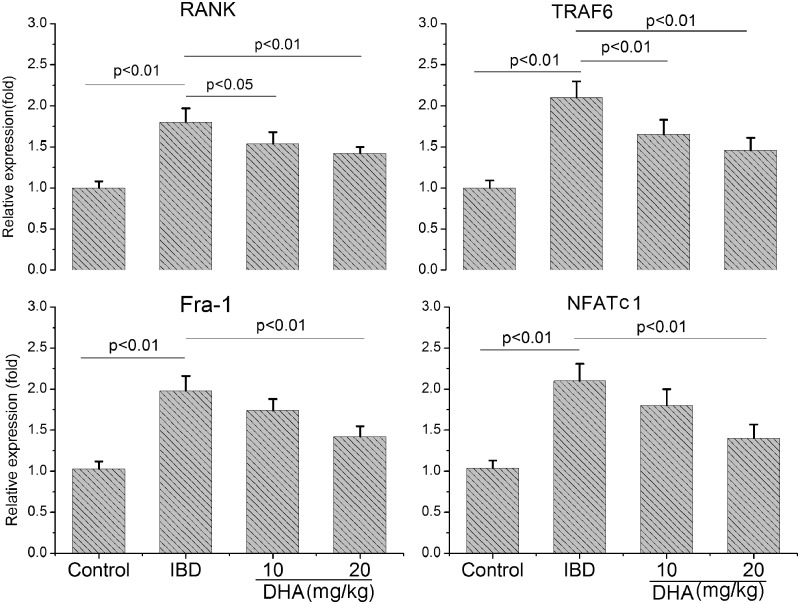
Subsequently, we examined the influence of DHA on the RANK, TRAF6, Fra-1, and NFATc1 mRNA (Figure 8) expression. The RANK, TRAF6, Fra-1, and NFATc1 expression in IBD rats were significantly higher than the control (p < 0.01). The DHA treatment dose dependently abolished the up-regulation of these mRNA expressions in IBD rats (p < 0.05).
Figure 8.
The mRNA expression of RANK, TRAF6, Fra-1, and NFATc1 in control, IBD and DHA-treated rats. The RANK, TRAF6, Fra-1, and NFATc1 expression in IBD rats were higher than the control. The DHA treatment abolished the up-regulation of these mRNA expressions in IBD rats. DHA: dihydroartemisinin; IBD: inflammatory bowel disease; RANK: receptor activator of nuclear factor (NF)-kB.
Discussion
Bone loss induced by IBD is the major extra-intestinal cause of morbidity.33 Low bone mass and osteoporosis can occur in about 50% of IBD patients.8,33 Compounds derived from natural products have great potential for the treatment of IBD.26 However, its role in IBD-induced bone loss is not completely clarified. In the present study, we showed that DHA reduced the colon damage induced by DSS. Moreover, we found that DHA also protected against the bone loss in IBD rats via promoting bone formation and inhibiting osteoclastic bone resorption.
Many agents or drugs have been adopted to treat IBD, including steroid and anti-TNFα monoclonal antibody. However, they also have significant adverse effects.34 More and more studies indicated that natural products and herbal medicines have exhibited efficacy for IBD in animal model or clinical trials.26 DHA and artemisinin, antimalaria drug, have been found to have potential to inhibit cancer cells and inflammation via modulating the NF-κB pathway.35–37 NF-κB is also a critical transcription factor in inflammatory process.38 In addition, a previous study indicated that artesunate was available as a potential therapy for IBD.27 Therefore, we hypothesize that DHA may have the same role. In this study, we demonstrated that DHA administration significantly reduced the colon damage induced by DSS. Further studies showed that DHA inhibited the serum TNFα which was a critical cytokine in IBD. Previous several studies have shown that TNFα inhibition is effective in reducing the severity of IBD.9,34 Our data indicated that DHA may abolish the colon damage induced by DSS through suppression of TNFα production.
Previous studies have shown that DHA inhibited RANKL-induced NF-κB activity21,25 and osteoclast formation21,25 or promoted osteoclast apoptosis.39 Therefore, we speculate that DHA may also protect against bone loss induced by IBD. Interestingly, bone loss was reduced in IBD rats treated with DHA, as shown by improvement in BMD and bone microstructure parameters. To the best of our knowledge, few studies have observed the effects of natural products on IBD and IBD-induced bone loss together. Our data indicate that DHA not only have potential to treat IBD, but also inhibit IBD-induced bone loss.
TNFα plays one of key roles in bone loss in IBD patients.13,16,17 TNFα can inhibit bone formation via suppression of osteoblast.40,41 TNFα suppresses serum insulin-like growth factor-1 which is a stimulator of bone formation.41 A recent study showed that osteoblast activity was suppressed in DSS-treated mice.13 In our study, inhibition in bone formation was also observed in IBD rats as shown by the decrease of P1NP which is a biomarker of bone formation. DHA treatment can attenuate the inhibition in bone formation. Zhou et al.21 showed that DHA did not affect formation, differentiation, and mineralization of osteoblast. Therefore, the improvement in bone formation in IBD rats may be due to the decrease of TNFα. In addition, TNFα may stimulate RANKL expression or cooperate with RANKL to stimulate osteoclast formation.42,43 RANKL is the critical cytokine associated with osteoclasts formation and differentiation.44,45 In the present study, excessive osteoclast formation was observed in IBD rats. In addition, our data showed that DHA intervention inhibited IBD-induced osteoclast formation and high RANKL expression in bone tissues. Our data also showed that osteoclast formation-related gene expressions, including RANK, TRAF6, Fra-1, and NFATc1, were increased in bone tissues of IBD rats. DHA treatment significantly inhibited those gene expressions. Hotokezaka et al.46 showed that TNFα induced TRAP-positive mononuclear cells fusion in the absence of RANKL. We speculated that the decrease in osteoclast formation may be due to the inhibition of RANKL/RNAK signal pathway by DHA or by the decrease of TNFα. In addition, the decrease of TNFα will reduce intestinal inflammation. Consequently, bone loss will be reduced. However, Irwin et al.13 showed percentage of osteoclast surface or markers of osteoclast maturation were not increased in DSS-treated mice which indicated that inflammation predominantly affected osteoblast activity. A young mice IBD model in which bone formation was predominant was used in their study. However, we established an adult rat model in which bone formation and resorption were both active in the present study.
There are several limitations in our study. First, several chemical compounds have been used to induce colitis, such as 2,4-dinitrobenzene sulfonic acid and acetic acid. Only DSS-induced IBD was used in our study. Second, the focus of our study is IBD-induced bone loss. The therapeutic effect of DHA on IBD is just primarily observed. Third, whether the roles of DHA on IBD-induced bone loss are mediated by TNFα or by its direct effects on osteoclast need further study. In addition, other cytokine related to bone loss in IBD, such as IL-17 and interferon γ, was not evaluated in this study. Finally, for a therapeutic study, the sample sizes were relatively small and the dose–response relationships were not observed.
In conclusion, our data indicated that DHA may protect against DSS-induced IBD and IBD-induced bone loss. DHA suppresses the osteoclast formation in bone of IBD rats. The protective role may be due to its inhibitory effects on TNFα or RANKL. DHA may be a potential therapeutic agent for IBD and IBD-induced bone loss.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; XG, ZC, ZX performed experiments; XG, ZC, and FL analyzed data; XG and ZC prepared figures; XG and ZC drafted manuscript; KZ and YY edited and revised manuscript. XG and ZC contributed equally to this paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Benchimol EI, Guttmann A, Griffiths AM, Rabeneck L, Mack DR, Brill H, Howard J, Guan J, To T. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut 209;58:1490–7 [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006; 3:390–407 [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, LeMair AW, Malfertheiner Ouyang Q, Rey JF, Sood A, Steinwurz F, Thomsen OO, Thomson A, Watermeyer G. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010; 16:112–24 [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011; 140:1756–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med 2010; 42:97–114 [DOI] [PubMed] [Google Scholar]
- 6.Paganelli M, Albanese C, Borrelli O, Civitelli F, Canitano N, Viola F, Passariello R, Cucchiara S. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2007; 13:416–23 [DOI] [PubMed] [Google Scholar]
- 7.Semeao EJ, Jawad AF, Zemel BS, Neiswender KM, Piccoli DA, Stallings VA. Bone mineral density in children and young adults with Crohn’s disease. Inflamm Bowel Dis 1999; 5:161–6 [DOI] [PubMed] [Google Scholar]
- 8.Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, Hawker G, Griffiths AM. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis 2007; 13:42–50 [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Sands BE, Pazianas M. Prevention and treatment of osteoporosis in inflammatory bowel disease. Inflamm Bowel Dis 2006; 12:797–813 [DOI] [PubMed] [Google Scholar]
- 10.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 2006; 118:1950–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siffledeen JS, Fedorak RN, Siminoski K, Jen H, Vaudan E, Abraham N, Steinhart H, Greenberg G. Randomized trial of etidronate plus calcium and vitamin D for treatment of low bone mineral density in Crohn’s disease. Clin Gastroenterol Hepatol 2005; 3:122–32 [DOI] [PubMed] [Google Scholar]
- 12.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998; 115:182–205 [DOI] [PubMed] [Google Scholar]
- 13.Irwin R, Raehtz S, Parameswaran N, McCabe LR. Intestinal inflammation without weight loss decreases bone density and growth. Am J Physiol Regul Integr Comp Physiol 2016; 311:R1149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Card T, West J, Hubbard R, Logan RF. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut 2004; 53:251–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol 2014; 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan BM, Russel MG, Schurgers L, Wichers M, Sijbrandij J, Stockbrugger RW, Schoon E. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther 2004; 20:851–7 [DOI] [PubMed] [Google Scholar]
- 17.Pazianas M, Rhim AD, Weinberg AM, Su C, Lichtenstein GR. The effect of anti-TNFa therapy on spinal bone mineral density in patients with Crohn’s disease. Ann NY Acad Sci 2006; 1068:543–6 [DOI] [PubMed] [Google Scholar]
- 18.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 2012; 11:234–50 [DOI] [PubMed] [Google Scholar]
- 19.Hwang JK, Noh EM, Moon SJ, Kim JM, Kwon KB, Park BH, You YO, Hwang BM, Kim HJ, Kim BS, Lee SJ, Kim JS, Lee YR. Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology 2013; 52:1583–91 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Yan M, Yu QF, Yang PF, Zhang HD, Sun YH, Zhang ZF, Gao YF. Puerarin prevents LPS-induced osteoclast formation and bone loss via inhibition of Akt activation. Biol Pharm Bull 2016; 39:2028–35 [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Liu Q, Yang M, Wang T, Yao J, Cheng J, Yuan J, Lin X, Zhao J, Tickner J, Xu J. Dihydroartemisinin, an anti-malaria drug, suppresses estrogen deficiency-induced osteoporosis, osteoclast formation, and RANKL-induced signaling pathways. J Bone Miner Res 2016; 31:964–74 [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Ren S, Zhu G, Wang Z, Wen X. Emodin suppresses cadmium-induced osteoporosis by inhibiting osteoclast formation. Environ Toxicol Pharmacol 2017; 54:162–8 [DOI] [PubMed] [Google Scholar]
- 23.Lee SK, Kim H, Park J, Kim HJ, Kim KR, Son SH, Park KK, Chung WY. Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts. Sci Rep 2017; 7:17332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Zhang Y, Wang S, Wang K, Tao L, Zou M, Chen N, Xu J, Liu S, Li X. Artesunate suppresses RANKL-induced osteoclastogenesis through inhibition of PLCγ1-Ca2+-NFATc1signaling pathway and prevents ovariectomy-induced bone loss. Biochem Pharmacol 2017; 124:57–68 [DOI] [PubMed] [Google Scholar]
- 25.Wei CM, Liu Q, Song FM, Zholudev A, Horr B, Shih MC, Grand RJ. Artesunate inhibits RANKL-induced osteoclastogenesis and bone resorption in vitro and prevents LPS-induced bone loss in vivo. J Cell Physiol 2018; 233:476–85 [DOI] [PubMed] [Google Scholar]
- 26.Guo BJ, Bian ZX, Qiu HC, Wang YT, Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann NY Acad Sci 2017; 1401:37–48 [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Ding J, Yang C, Gao Y, Li X, Chen X, Peng Y, Fang J, Xiao S. Immunomodulatory and anti-inflammatory properties of artesunate in experimental colitis. Curr Med Chem 2012; 19:4541–51 [DOI] [PubMed] [Google Scholar]
- 28.Ciucci T, Ibanez L, Boucoiran A, Birgy-Barelli E, Pène J, Abou-Ezzi G, Arab N, Rouleau M, Hébuterne X, Yssel H, Blin-Wakkach C, Wakkach A. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 2015; 64:1072–81 [DOI] [PubMed] [Google Scholar]
- 29.Ibáñez L, Abou-Ezzi G, Ciucci T, Amiot V, Belaïd N, Obino D, Mansour A, Rouleau M, Wakkach A, Blin-Wakkach I. Osteoclasts prime TNFα-producing CD4+ T cells and express CX3 CR1. J Bone Miner Res 2016; 31:1899–908 [DOI] [PubMed] [Google Scholar]
- 30.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor-B (p65) antisense oligonucleotide on mouse dextransulphate sodium (DSS)-induced colitis. Clin Exp Immunol 2010; 120:51–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Zhong L, Sun D, Rong L. Magnesium lithospermate B acts against dextran sodiumsulfate-induced ulcerative colitis by inhibiting activation of the NRLP3/ASC/Caspase-1 pathway. Environ Toxicol Pharmacol 2016; 41:72–7 [DOI] [PubMed] [Google Scholar]
- 32.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993; 69:238–49 [PubMed] [Google Scholar]
- 33.Wakkach A, Rouleau M, Blin-Wakkach C. Osteoimmune interactions in inflammatory bowel disease: central role of bone marrow Th17 TNFα cells in osteoclastogenesis. Front Immunol 2015; 6:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009; 136:1182–97 [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Zhang G, Wu X, Xu F, Zhou J, Zhang X. Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-kappaB modulation. J Cancer Res Clin Oncol 2012; 138:2095–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X, Xue D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J Cancer Res Clin Oncol 2010; 136:897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, Yang X, Lian F, Sun L. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology 2007; 46:920–6 [DOI] [PubMed] [Google Scholar]
- 38.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007; 12:1641–57 [DOI] [PubMed] [Google Scholar]
- 39.Dou C, Ding N, Xing J, Zhao C, Kang F, Hou T, Quan H, Chen Y, Dai Q, Luo F, Xu J, Dong S. Dihydroartemisinin attenuates lipopolysaccharide-induced osteoclastogenesis and bone loss via the mitochondria-dependent apoptosis pathway. Cell Death Dis 2016; 7:e2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sylvester FA, Wyzga N, Hyams JS, Gronowicz GA. Effect of Crohn’s disease on bone metabolism in vitro: a role for interleukin-6. J Bone Miner Res 2002; 17:695–702 [DOI] [PubMed] [Google Scholar]
- 41.Nanes MS. Tumor necrosis factor-α: molecular and cellular mechanisms in skeletal pathology. Gene 2003; 321:1–15 [DOI] [PubMed] [Google Scholar]
- 42.Komine M, Kukita A, Kukita T, Ogata Y, Hotokebuchi T, Kohashi O. Tumor necrosis factor-a cooperates with receptor activator of nuclear factor kB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone 2001; 28:474–83 [DOI] [PubMed] [Google Scholar]
- 43.Zou W, Hakim I, Tschoep K, Endres S, Bar-Shavit Z. Tumor necrosis factor-a mediates RANK ligand stimulation of osteoclast differentiation by an autocrine mechanism. J Cell Biochem 2001; 83:70–83 [DOI] [PubMed] [Google Scholar]
- 44.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423:337–42 [DOI] [PubMed] [Google Scholar]
- 45.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93:165–76 [DOI] [PubMed] [Google Scholar]
- 46.Hotokezaka H, Sakai E, Ohara N, Hotokezaka Y, Gonzales C, Matsuo K, Fujimura Y, Yoshida N, Nakayama K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-alpha, lipopolysaccharide, or peptidoglycan. J Cell Biochem 2007; 101:122–34 [DOI] [PubMed] [Google Scholar]