Short abstract
The dual sugar absorption test, specifically the lactulose:mannitol test, is used to assess gut health. Lactulose absorption is said to represent gut damage and mannitol absorption is used as a measure of normal small bowel function and serves as normalizing factor for lactulose. A underappreciated limitation of this common understanding of the lactulose:mannitol test is that mannitol is not absorbed to any substantial extent by a transcellular process. Additionally, this interpretation of lactulose:mannitol is not consistent with current understanding of paracellular pathways, where three pathway types exist: pore, leak, and unrestricted. Pore and leak pathways are regulated biological constructions of the small bowel barrier, and unrestricted pathways represent micropathological damage. We analyzed 2334 lactulose:mannitol measurements rigorously collected from 622 young rural Malawian children at high risk for poor gut health in light of the pathway model. An alternative method of normalizing for gut length utilizing autopsy data is described. In our population, absorbed lactulose and mannitol are strongly correlated, r = 0.68 P <0.0001, suggesting lactulose and mannitol are traversing the gut barrier via the same pathways. Considering measurements where pore pathways predominate, mannitol flux is about 14 times that of lactulose. As more leak pathways are present, this differential flux mannitol:lactulose falls to 8:1 and when increased numbers of unrestricted pathways are present, the differential flux of mannitol:lactulose is 6:1. There was no substantial correlation between the lactulose:mannitol and linear growth. Given that mannitol will always pass through a given pathway at a rate at least equal to that of lactulose, and lactulose absorption is a composite measure of flux through both physiologic and pathologic pathways, we question the utility of the lactulose:mannitol test. We suggest using lactulose alone is as informative as lactulose:mannitol in a sugar absorption testing in subclinical gut inflammation.
Impact statement
Our work integrates the standard interpretation of the lactulose:mannitol test (L:M), with mechanistic insight of intestinal permeability. There are three paracellular pathways in the gut epithelium; pore, leak, and unrestricted. Using thousands of L:M measurements from rural Malawian children at risk for increased intestinal permeability, we predict the differential flux of L and M through the pathways. Our findings challenge the traditional notions that little L is absorbed through a normal epithelial barrier and that M is a normalizing factor for L. Our observations are consistent with pore pathways allowing only M to pass. And that substantial amounts of L and M pass through leak pathways which are normal, regulated, cell-junctional adaptations. So M is a composite measure of all pathways, and L is not a measure solely of pathologic gut damage. Using L alone as a probe will yield more information about gut health than L:M.
Keywords: Environmental enteric dysfunction, dual sugar absorption test, lactulose:mannitol test, paracellular permeability, gut health
Introduction
A top priority in global health is to reduce stunting, as it is associated with reduced longevity, economic productivity, and neurocognitive development in a variety of developing world settings.1 In sub-Saharan Africa, 35% of rural children are stunted, in Malawi, 47% of children are stunted.2 Focused, singular interventions to improve dietary intake and reduce enteric infectious exposures in populations vulnerable to stunting have been disappointing, resulting in little or no benefit.3
Good gut health is necessary for optimal growth and development, as it maintains nutrient absorption while protecting the body from excess microbial exposure, infection, and inflammation. Association modeling of characteristics of stunted populations suggests that perturbed gut health plays an important role in the pathogenesis of stunting.4 Perturbed gut health, referred to as environmental enteric dysfunction (EED), alludes to reduced nutrient absorption coupled with a chronic inflammatory state of the small bowel.5 The most reliable methods to assess gut health involve direct (endoscopic) visualization of the gut and biopsy, and the dual sugar absorption test.6 Endoscopy is a resource-intensive procedure that is not suited to mass screening, or to frequent intra-host assessment, especially in developing nations.
The dual sugar absorption test, in its most commonly used form, the lactulose:mannitol (L:M) test, is administered by orally ingesting a solution with an excess of both sugars, collecting all urine over a timed period of several hours and quantifying the excreted sugars. Both sugars are chemically inert and uncharged molecules. Lactulose (L), a 13 Å in diameter disaccharide, is absorbed only through cell junctions, while mannitol (M), an 8 Å monosaccharide, is thought to be absorbed across cell membranes and across normal cell junctions.7 Once absorbed, these sugars are excreted unmetabolized in the urine. The results of the L:M test are expressed as a ratio of absorbed L and M, and it said that M normalizes the L value for intestinal surface area and/or intestinal transit time.8–10 This explanation implies that healthy guts will absorb less L and more M, and that an inverse relationship between L and M exists.
Animal data do not support the notion that M absorption increases with improved gut health.11 M does not bind to monosaccharide transporters in the gut epithelium with appreciable affinity, and there is no evidence for its active transport in large quantities across cell membranes. Endocytosis, another form of transmembrane absorption, is a process that captures very large molecules, often antigens, and occurs at rates orders of magnitude less than active transport, and is unlikely to be a mechanism of substantial M absorption. These observations suggest that differential absorption of L and M in children with EED and good gut health are properties of the permeability of cell junctions. A cell junction that is permeable to a disaccharide, L, on the basis of size and configuration, will also be permeable to a monosaccharide, M.
In animal models, the tight junction between epithelial cell membranes forms a selectively permeable barrier that can assume the configuration of either a pore pathway or a leak pathway.12–15 The pore pathway is a charge- and size- selective channel with a diameter of 5–10 Å. The leak pathway is less selective channel with a diameter of 15–125 Å. Permeability increases in the presence of inflammatory cytokines, namely IL-13 and TNF. IL-13 augments paracellular permeability by increasing claudin-2 expression. TNF enhances transcription of myosin light chain kinase (MLCK), multiple claudins (1, 2, 12, 15 and 16), three tight junction-associated MARVEL proteins (TAMPSs; occludin tricellulin, and marvelD3) and ZO-1.16
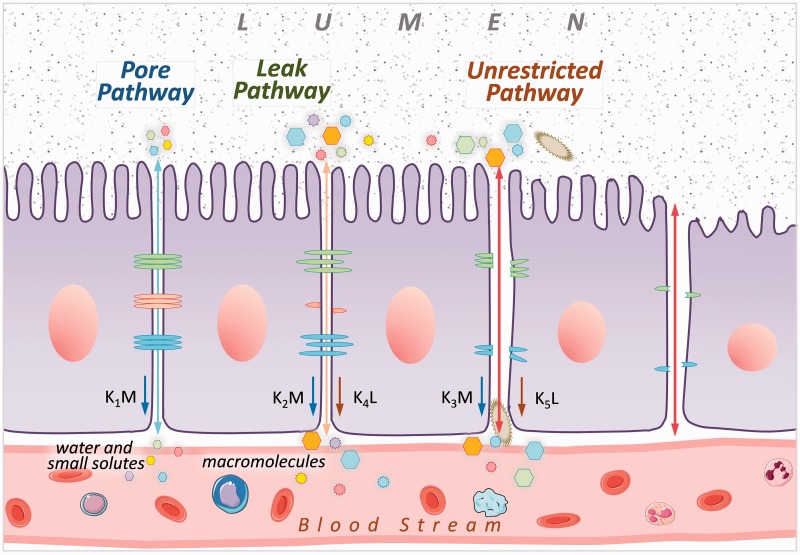
In addition, barrier function may be compromised by epithelial damage, large disruptions which allow for ingress of a wide range of molecules, bacteria, and complex antigens.15 These disruptions are referred to as unrestricted pathways. Unrestricted pathways presumably occur after damage to leak and pore pathways, rather than in isolation from the other pathways. These three cell junction pathways are depicted in a cartoon in Figure 1.
Figure 1.
Model of the three different epithelial permeability pathways. With respect to the dual sugar absorption test, k1, k2 and k3 are the rate constants by which mannitol (M) passes through pore, leak and unrestricted pathways, respectively, and k4 and k5 are the rate constants by which lactulose (L) passes through leak and unrestricted pathways, respectively.
We used data sets collected from rural Malawian children at risk for EED with serial L and M measurements to test the following hypotheses (1) M and L are inversely correlated, which would be consistent with the traditional interpretation of dual sugar permeability tests, (2) that a mathematical modeling based only on the three pathways observed in animal models is consistent with the empiric clinical L and M data, and (3) that the L:M test is a useful indicator of gut health status as a predictor of linear growth.
Materials and methods
Patient population and L:M testing
Data from 622 children from two clinical trials conducted in rural Malawi were used.17–19 This included L:M test measurements from children aged 6–12 months (n = 815) and 12–36 months (n = 1519). These children were apparently healthy, did not have acute malnutrition, a known or obvious chronic disability or disease, nor was diarrhea reported within 48 h of L:M testing. The study was approved by the College of Medicine Research and Ethics Committee, University of Malawi and Washington University Human Studies Committee.
Children were asked to take no food (water and breast milk were permitted) after 10 p.m. on the evening prior to the dual sugar absorption test. The test began when each child drank 20 mL solution containing 1 g M and 5 g L. Children were carefully observed to ensure complete ingestion of this solution; any child who spit, vomited, or refused the solution was brought back for testing on another day. Adhesive urine bags were placed on the child’s perineum and monitored for urine output. As soon as urine was noted in the bag, it was removed and the urine transferred to a clean container containing 10 mg merthiolate to prevent bacterial degradation of the excreted sugars, and a new bag was placed. After 3 h, children were given water to drink to facilitate the collection of urine. After the child voided for the first time after 4 h, the urine collection was complete. The total amount of urine voided during the study was quantified and an aliquot flash frozen for analysis. The concentrations of L and M in urine were determined using HPLC, as described previously.20 Our HPLC method has been previously validated using liquid chromatography-tandem mass spectrometry and the results were found to be highly correlated.21 Previously, this population has had urine tested for M prior to administration of oral M and none was detected by HPLC. Correlation coefficients and best fit regression lines for L and M were determined using SPSS 24.0 (Armonk, NY, 2016).
Model definition and understanding of relative fluxes through paracellular pathways
The model was conceived on the basis of animal observations reported in the literature.11–13 Three distinct pathways of different sizes were assumed; pore (small size, most prevalent), leak (medium sized), and unrestricted (large size, least prevalent) (Figure 1). The pathways were assumed to absorb M and L at different rates, primarily on the basis of the configuration of the pathway. M and L were assumed to be inert and passively traverse the pathway on the basis of a concentration gradient, and thus their rates of absorption through each of the three pathways were fixed. The molar concentration gradient of L was 2.7 times greater than M. Because of disparities in the size of M and L with respect to the pathways, L absorption through the pore pathway was assumed to be negligible. Because the unrestricted pathway is so large compared to L and M, the rate of absorption for each sugar across the unrestricted pathway is likely to be equal. Since M is a smaller molecule than L and the diameter of the leak pathway can approximate the size of L, M is likely to be absorbed at a greater rate across leak pathways than L. The unit of measure of L and M for the purposes of modeling was µmoles. While we have several thousand empiric observations, we cannot uniquely solve for the rate constants because each observation yields two new equations, yet three unknowns. Therefore, we examined selected segments of the data set to offer insight as to what is occurring with L and M in the three pathways.
An important consideration is that the length of the small bowel, and thus the numbers of each pathway type, likely differs with age in pediatric populations. Since our data were drawn from children aged 6–36 months, a factor to normalize sugar absorption to bowel size was needed. Autopsy studies have described that small bowel length, as measured from ligament of Treitz to ileocecal valve, is proportional to stature in a wide range of ages of individuals from the fetus to adult.22 Original measurements of pediatric small bowel length and body height from the most extensive autopsy study were obtained from the principal investigator.22 These data were used to model small bowel length as a function of body length over the limited range of body heights of our study population. Log–log regression was used to model bowel length with respect to height.23 The best fit mathematical model was used to calculate predicted small bowel length for all subjects for the purposes of normalizing %M.
Linear growth in the population studied
For subset of 1669 L:M measurements, linear growth data for the subsequent three-month period were available, and the Spearman’s correlation coefficient between growth and sugar absorption was calculated. In addition, poor linear growth was defined as <−0.3 z scores over the subsequent three months. Random forest modeling is the most appropriate machine-based learning technique suited to predict a dichotomous outcome from a small number of independent variables which are related to each other. Therefore, random forest modeling was used to determine if measurements of L and M could be used to predict adequate or poor linear growth in this population. Linear growth was expressed in change in length-for-age z-score (LAZ) determined from the WHO reference population for children in developing nations.24
Results
Lactulose and mannitol are directly correlated
The study population was rural African preschool aged children, typically living in mud huts, and receiving more than half of their daily dietary energy from corn (Table 1). L and M were directly correlated (Figure 2). Body length was best related to small bowel length in the subjects with a length from 55 to 95 cm by the following equation
Table 1.
Characteristics of Malawian children at risk for environmental enteric enteropathy.
| Characteristic | |
|---|---|
| Household characteristics n = 622 | |
| Sex, male | 332 (53%) |
| Siblings | 2.5 ± 1.9 |
| Mother alive | 618 (99%) |
| Improve roofing material on home (metal sheets) | 139 (22%) |
| Radio in home | 220 (35%) |
| Bicycle in home | 322 (52%) |
| Animals sleep with children in home | 195 (31%) |
| Has a clean source of water | 446 (72%) |
| Home has a pit latrine | 100 (16%) |
| Breastfed at the time of the study | 571 (92%) |
| Demographic and anthropometric characteristics at time of dual sugar absorption testing n =2334 | |
| Age, month | 17.0 ± 8.2 |
| Length-for-age, z score | −1.54 ± 1.08 |
| Weight-for-height, z score | −0.06 ± 0.87 |
| Mid-upper arm circumference, cm | 14.4 ± 1.0 |
Note: Values expressed as mean ± SD or no (%).
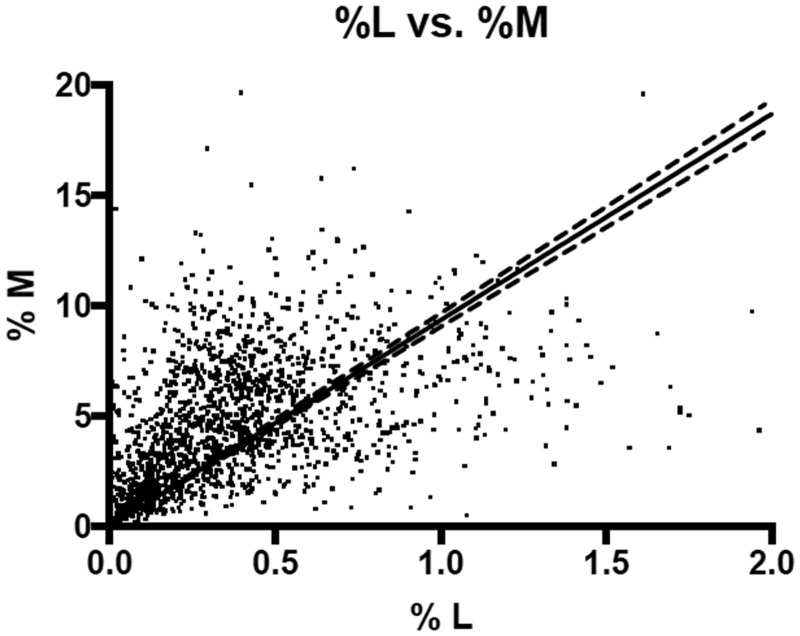
Figure 2.
Lactulose and mannitol excretion in rural Malawian children at risk for environmental enteric dysfunction. The units of lactulose and mannitol are % of oral dose that was excreted by weight. The solid line indicates the best fit line and the dotted lines the 95% CI of the line. A strong positive correlation exists between absorption of the mono- and disaccharide, Pearson’s r = 0.58, P < 0.0001 and Spearman’s r = 0.68 P < 0.0001.
Log (small bowel length) = 0.4538 (log(height)) + 1.731 (r = 0.58, P < 0.0001)
Normalization of M measurements was achieved by calculating small bowel length for each child using the equation above, creating a term ‘relative small bowel length’ and dividing %M by this term. Relative small bowel length = calculated small bowel length/425.3 cm (maximum small bowel length). Normalized %M (%nM) was used for pathway flux and growth comparisons.
Determination of relative flux of lactulose and mannitol across pore, leak, and unrestricted pathways
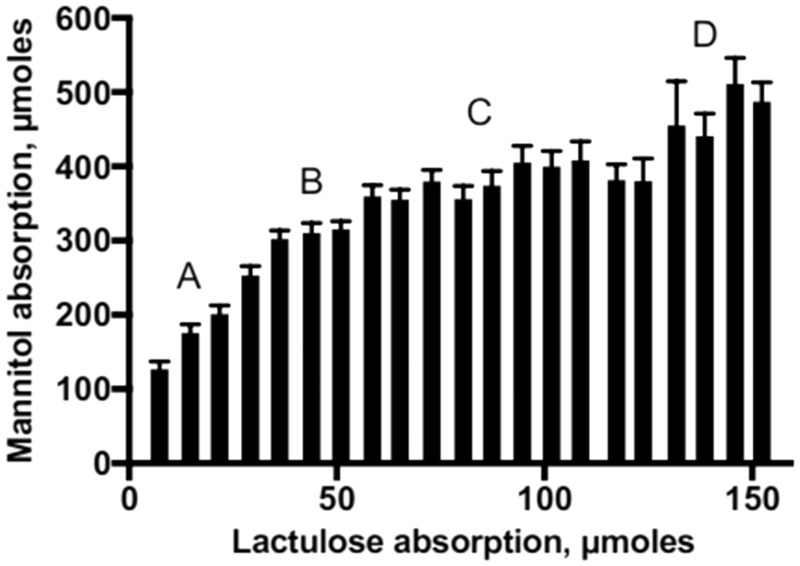
To determine which pathways are most active in different individuals at different time points, we plotted L versus M absorption (Figure 3). All values in which %L < 0.025%, which is extremely low, were not included in this analysis, as they are likely to be associated with a laboratory measurement anomaly. The slopes and plateaus seen on the plot were interpreted in terms abundance of the pore, leak, and unrestricted pathways. In particular, the first plateau was interpreted as a group of measurements in which pore pathways are saturated, and pore pathways account for most of the M absorption. Increases in M relative to L after this first plateau represented increasing numbers of leak pathways and unrestricted pathways. Comparison of L and M absorption provides data that are consistent with the proposed three pathway model of paracellular movement of small sugars (Table 2, Figure 3).
Figure 3.
Lactulose and mannitol excretion in rural Malawian children at risk for environmental enteric dysfunction. The data are expressed as a histogram and the units of lactulose and mannitol are µmoles. The plot was divided visually into four sections: A: where L and M increase at a constant rate and are present in low quantities, B: where L increases by 50% and M is unchanged, C: where L increases by 150% and M changes by 40%, D: where L increases by 15% and M is at least 15% greater than sections A, B or C.
Table 2.
Dual sugar absorption test results segregated by percentage of lactulose (%L).
| % Lactulose absorbed | N | μmole L absorbed (mean) | μmole M absorbed (mean ± SD) | μmole M in excess of first plateau | μmole L in excess of first plateau | L:M, expressed as a ratio of moles absorbed |
|---|---|---|---|---|---|---|
| 0.025–0.075 | 194 | 7.4 | 127 ± 144 | 0 | 0 | 0.11 ± 0.08 |
| 0.075–0.125 | 186 | 14.8 | 176 ± 159 | 0 | 0 | 0.14 ± 0.11 |
| 0.125–0.175 | 189 | 21.8 | 201 ± 157 | 0 | 0 | 0.17 ± 0.13 |
| 0.175–0.225 | 213 | 29.2 | 253 ± 180 | 0 | 0 | 0.17 ± 0.12 |
| 0.225–0.275 | 193 | 36.3 | 302 ± 159 | 0 | 0 | 0.16 ± 0.10 |
| 0.275–0.325 | 183 | 43.9 | 310 ± 187 | 0 | 0 | 0.19 ± 0.12 |
| 0.325–0.375 | 177 | 51.0 | 315 ± 150 | 0 | 0 | 0.21 ± 0.13 |
| 0.375–0.425 | 163 | 58.6 | 360 ± 190 | 45 | 7.6 | 0.20 ± 0.11 |
| 0.425–0.475 | 123 | 65.3 | 355 ± 151 | 40 | 14.3 | 0.22 ± 0.11 |
| 0.475–0.525 | 111 | 72.9 | 380 ± 163 | 65 | 21.9 | 0.24 ± 0.14 |
| 0.525–0.575 | 81 | 80.5 | 356 ± 156 | 41 | 29.5 | 0.28 ± 0.14 |
| 0.575–0.625 | 81 | 87.3 | 374 ± 176 | 59 | 36.3 | 0.29 ± 0.15 |
| 0.625–0.675 | 56 | 94.7 | 405 ± 171 | 90 | 43.7 | 0.28 ± 0.15 |
| 0.675–0.725 | 51 | 101.7 | 400 ± 150 | 85 | 50.7 | 0.29 ± 0.11 |
| 0.725–0.775 | 44 | 108.7 | 408 ± 170 | 93 | 57.7 | 0.32 ± 0.15 |
| 0.775–0.825 | 38 | 117.3 | 382 ± 128 | 67 | 66.3 | 0.35 ± 0.12 |
| 0.825–0.875 | 31 | 123.8 | 380 ± 170 | 65 | 72.8 | 0.40 ± 0.19 |
| 0.875–0.925 | 22 | 131.8 | 455 ± 280 | 140 | 80.8 | 0.38 ± 0.18 |
| 0.925–0.975 | 16 | 138.6 | 441 ± 123 | 126 | 87.6 | 0.33 ± 0.08 |
| 0.975–1.025 | 21 | 146 | 511 ± 160 | 196 | 95.0 | 0.32 ± 0.10 |
| > 1.025 | 91 | 192.3 | 487 ± 249 | 172 | 141.3 | 0.45 ± 0.16 |
The molar ratio of M to L absorption among children with %L < 0.35% is about 5:1. These children are absorbing M primarily through the pore pathway; this corresponds to section A of Figure 3. This suggests the flux of M about 14× times greater than L when pore pathways predominate. For children where both leak and pore pathways are operative, section C of Figure 3, M flux is about 8-fold greater than L. When unrestricted pathways are utilized for L and M absorption as well as pore and leak pathways, section D of Figure 3, M flux is about 6-fold greater than L. The distribution of the 2259% L measurements in the sections of the plot was as follows: A, 780 (35%); B, 552 (24%); C, 777 (34%) and D, 150 (7%).
Sugar correlations with growth
The Spearman correlation coefficients (P value) with change in LAZ and %L, %nM and L:nM were 0.003 (0.90), 0.050 (0.04) and −0.051 (0.03), respectively. The random forest model with the best accuracy using %L, %nM and L:nM as independent variables and change in LAZ as the dependent variable correctly identified 54% of children with poor linear growth correctly and 52% good linear growth. The change in LAZ for the four sections of the histogram in Figure 3(a) to (d) were −0.08 ± 0.42, −0.07 ± 0.41, −0.06 ± 0.37, −0.07 ± 0.37, respectively (mean ± SD).
Discussion
These data challenge the traditional interpretation of the L:M test when used to assess EED among children in rural Africa. The strong direct correlation between L and M suggests that both sugars traverse the gut barrier using the same pathways, and does not support the use of M as factor to normalize L between subjects. More than half of M is likely absorbed through pore pathways in our population, but a substantial amount of M passes through leak and unrestricted pathways in 70% of the children. Thus, total M measurements cannot be used to differentiate the types of paracellular pathways. L traverses the epithelium using the physiologic leak pathway and the pathologic unrestricted pathway, thus L is a combined measure of luminal inflammatory stimuli and small breaches in the epithelial barrier. Both of luminal inflammation and microruptures of the epithelium indicate poorer gut health, they do not inevitably lead to the growth leading characteristic of EED.
A major limitation of our findings is that the model applies only to the population from which the data were derived, rural African children aged 6–36 months at risk for EED, tested under the very same conditions as we rigorously employed. These conditions include the doses of L and M, method of administration of the sugars, and the duration of the urine collection.
The pathway model (Figure 1) is compatible with our empiric data, the relative rate constants are plausible for passive flow based on molecular size and the changes seen in relative flux of M and L are consistent with a model where there are increased numbers of leak and unrestricted pathways in some children. The magnitudes of the M values are not so large to suggest that substantial amounts of M might be absorbed via a transcellular process.
Evidence from inflammatory bowel disease and celiac disease indicates that increased numbers of unrestricted pathways are associated with worse clinical disease, and this is also seen in histologic studies of EED.25 Healthy adults without intestinal disease will absorb less L and more M.26 However, the clinical manifestations of guts with more or less leak pathways are unknown. Leak pathways are a normal component of the gastrointestinal tract tight junctions, as they are intricately regulated and controlled by innate biological processes. An excess of leak pathways might lead to clinical gut inflammation and barrier dysfunction, but it is uncertain at present what might constitute an excess. For EED to be implicated in stunting, it is likely that the presence of barrier disruption and unrestricted pathways is necessary.
A variation of the L:M test was recently described, where rhamnose (R) was substituted for M in African children.27 R was chosen over M as there was less R present in commonly consumed crops, although it was acknowledged that use of R did not address the concern that any monosaccharide that is not absorbed transcellularly is trafficked in a similar manner. The L:R was shown to be much higher in 85 African children when compared to 46 children from South and North America, and multivariate regression modeling demonstrated an association with weight-for-age z score and L:R. The small numbers of children studied from very different demographies indicate that the dual sugar absorption test has utility in discerning substantial differences in gut health. We did not find the dual sugar absorption test as useful when we focused it on a larger group of vulnerable children in whom growth faltering is also important.
Improvements in the dual sugar permeability test might be possible with the use of different sized probes. Currently, the variations of the dual sugar absorption test utilize different mono- and disaccharides, particularly monosaccharides that are or are not actively transported across the epithelium and disaccharides for which human enzymes exist to cleave them, such as sucrose, or no biological enzymes exist to cleave the sugars, such as sucralose. The choice of neutral molecules that are not actively transported allows the dual sugar absorption test to delineate the types and sizes of cell junctions. While radioactive chromium probes have been employed for this purpose and have an excellent safety record, the use of radioactive compounds in normal populations inherently raises concern.28 Larger organic molecules which might serve as small bowel permeability probes might also provoke an antigenic response, so careful testing will be needed before such are accepted. Stable isotope tracers of carefully chosen common molecules that traverse the gut via paracellualr processes might also be useful.29 The development of safe and pathway specific alternative intestinal permeability probes that are particularly chosen to differentiate pore, leak, and unrestricted pathways would allow for better understanding of the clinical significance of each pathway.
In this population, the correlation between linear growth faltering and dual sugar absorption tests was quite weak with r2 < 0.01. Nor could random forest modeling of the dual sugar absorption tests predict growth faltering with sufficient reliability to be useful, even though these data are particularly suited to this machine-based learning method. Yet the population is identified as high risk for EED, and 35% were stunted. This is evidence that the L:M test, as currently implemented, is of limited utility in characterizing the gut health of rural African children.
Examination of Table 2 comparing L measurements to L:M reveals no obvious advantage in including M as a probe as intestinal permeability probe. While L steadily increases with more leak and unrestricted pathways, L:M is less dynamic, and thus it is more difficult to interpret. As is expected from the pathway model, L:M actually decreases with the most severe damage to cell junctions where unrestricted pathways are most numerous. In some populations, M is consumed in the diet and thereby obfuscates interpretation of L:M. Environmental contamination of sample by M in food and skin can confound the analyses as well.27,29 Measurement of two probes rather than one increases cost and complexity. Our previous studies from Malawi have found it is L, not L:M, that is best correlated with linear growth.20 Pretesting of the population for dietary M excretion is also needed to have confidence that the M measured is from the test sugar. Given these limitations, the inclusion of a monosaccharide probe in intestinal permeability testing appears to be of dubious value as a normalizing agent.
If L alone is used in the sugar absorption testing, careful attention to the dosing of L and total time of the urine collection is necessary.
A recent report describes a mouse model where recurrent exposure to a low dose of a pathogenic Salmonella leads to a chronic inflammatory state of the intestinal lining, which persisted long after clearance of the Salmonella.30 This model may yield insight to into the pathogenesis of EED. Such a subclinical state might be best detected by an abundance of leak and unrestricted pathways in the intestine, in the absence of microbial antigens. This model emphasizes the importance of a test that delineates function and structure of the small intestine, such as the dual sugar absorption test, as we strive to understand and ameliorate EED.
In conclusion, improvements in the dual sugar absorption test are needed to allow it to accurately measure the relative numbers of the types of paracellular pathways. These improvements might come from using probes of different sizes or choosing probes that correlate with clinical outcomes. At present the rationale for using a ratio L:M for the detection of EED in a high-risk population is not supported by this empiric data, and the use of a single probe, L, will yield the same information. New methods for efficient and effective evaluation of intestinal health could prove a valuable contribution to the global plight against childhood stunting.
Acknowledgment
The authors thank Prof. Tim J. Cole for providing pediatric small bowel length and body height data from autopsies and his useful suggestions with regard to estimating small bowel length.
Authors’ contributions
MJM, KS and SA designed the study, KS, SA, and OD collected the samples, MIO, CD, NS and MJM analyzed the data, CD, MIO and MJM developed the models, MIO, CD and MJM wrote the first draft of the manuscript. All authors have seen and approve of the publication.
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This work was supported by the United States Agency for International Development (USAID), as part of Feed the Future, the U.S. Government’s global hunger and food security initiative, under the terms of Cooperative Agreement No. EDH-A-00–07-00005–00, and the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital. The opinions expressed herein are those of the authors and do not necessarily reflect the views of USAID or the U.S. Government.
References
- 1.United Nations. Sustainable development goals, www.un.org/sustainabledevelopment/suatinable-development goals/ (accessed 20 March 2018)
- 2.Unicef. Statistics on Malawi, www.unicef.org/infobycountry/malawi_statistics.html (accessed 20 March 2018)
- 3.Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health 2014; 34:250–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trehan I, Kelly P, Shaikh N, Manary MJ. New insights into environmental enteric dysfunction. Arch Dis Child 2016; 101:741–4 [DOI] [PubMed] [Google Scholar]
- 5.Thompson AJ, Hughes M, Anastasova S, Conklin LS, Thomas T, Leggett C, Faubion WA, Miller TJ, Delaney P, Lacombe F, Loiseau S, Meining A, Richards-Kortum R, Tearney GJ, Kelly P, Yang GZ. Position paper: the potential role of optical biopsy in the study and diagnosis of environmental enteric dysfunction. Nat Rev Gastroenterol Hepatol 2017; 14:727–38 [DOI] [PubMed] [Google Scholar]
- 6.Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis 2014; 59:S213–9 [DOI] [PubMed] [Google Scholar]
- 7.Vojdani A. For the assessment of intestinal permeability, size matters. Altern Ther Health Med 2013; 19:12–24 [PubMed] [Google Scholar]
- 8.Nathavitharana KA, Lloyd DR, Raafat F, Brown GA, McNeish AS. Urinary mannitol: lactulose excretion ratios and jejunal mucosal structure. Arch Dis Child 1988; 63:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw D, Gohil K, Basson M. Intestinal mucosal atrophy and adaptation. World J Gastroenterol 2012; 18:6357–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequeira IR, Lentle RG, Kruger MC, Hurst RD. Standaridising the lactulose mannitol test of gut permeability to minimize error and promote comparability. PLoS One 2014; 9:e99256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9:799–809 [DOI] [PubMed] [Google Scholar]
- 12.France MM, Turner JR. The mucosal barrier at a glance. J Cell Sci 2017; 130:307–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 2011; 73:283–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 2014; 36:204–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lingaraju A, Long TM, Wang Y, Austin JR, 2nd, Turner JR. Conceptual barriers to understanding physical barriers. Semin Cell Dev Biol 2015; 42:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 2018; 10:pii:a029314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trehan I, Benzoni NS, Wang AZ, Bollinger LB, Ngoma TN, Chimimba UK, Stephenson KB, Agapova SE, Maleta KM, Manary MJ. Common beans and cowpeas as complementary foods to reduce environmental enteric dysfunction and stunting in Malawian children: study protocol for two randomized controlled trials. Trials 2015; 16:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephenson KB, Agapova SE, Divala O, Kaimila Y, Maleta KM, Thakwalakwa C, Ordiz MI, Trehan I, Manary MJ. Complementary feeding with cowpea reduces growth faltering in rural Malawian infants: a blind, randomized controlled clinical trial. Am J Clin Nutr 2017; 106:1500–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agapova S, Stephenson KB, Divala O, Kaimila Y, Maleta K, Thakwalakwa C, Ordiz MI, Trehan I, Manary M. Additional common bean in the diet of Malawian children does not affect linear growth, but reduces intestinal permeability. J Nutr 2018; 148:267–74 [DOI] [PubMed] [Google Scholar]
- 20.Weisz AJ, Manary MJ, Stephenson K, Agapova S, Manary FG, Thakwalakwa C, Shulman RJ., Manary MJ. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J Pediatr Gastroenterol Nutr 2012; 55:747–50 [DOI] [PubMed] [Google Scholar]
- 21.Ordiz MI, Shaikh N, Trehan I, Maleta K, Stauber J, Shulman R, Devaraj S, Tarr PI, Manary MJ. Environmental enteric dysfunction is associated with poor linear growth and can be identified by host fecal mRNAs. J Pediatr Gastroenterol Nutr 2016; 63:453–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver LT, Austin S, Cole TJ. Small intestinal length: a factor essential for gut adaptation. Gut 1991; 32:1321–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole TJ, Altman DG. Statistics notes: percentage differences, symmetry, and natural logarithms. BMJ 2017; 358:j3683 [DOI] [PubMed] [Google Scholar]
- 24.WHO Multicentre Growth Reference Study Group. Assessment of differences in linear growth among populations in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl 2006; 450:56–65 [DOI] [PubMed] [Google Scholar]
- 25.Bjarnason I, Peters TJ, Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet 1983; 1:323–5 [DOI] [PubMed] [Google Scholar]
- 26.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006; 55:1512–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faubion WA, Camilleri M, Murray JA, Kelly P, Amadi B, Kosek MN, Enders F, Larson J, Boe G, Dyer R, Singh R. Improving the detection of environmental enteric dysfunction: a lactulose, rhamnose assay of intestinal permeability in children aged under 5 years exposed to poor sanitation and hygiene. BMJ Glob Health 2016; 1:e000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elia M, Behrens R, Northrop C, Wraight P, Neale G. Evaluation of mannitol, lactulose and 51Cr-labelled ethylenediaminetetra-acetate as markers of intestinal permeability in man. Clin Sci 1987; 73:197–204 [DOI] [PubMed] [Google Scholar]
- 29.Grover M, Camilleri M, Hines J, Burton D, Ryks M, Wadhwa A, Sundt W, Dyer R, Singh RJ. ( 13) C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 2016; 28:1114–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang WH, Heithoff DM, Aziz PV, Sperandio M, Nizet V, Mahan MJ, Marth JD. Recurrent infection progressively disables host protection against intestinal inflammation. Science 2017; 358:6370. [DOI] [PMC free article] [PubMed] [Google Scholar]