Abstract
Background
Precipitating hydrophobic injectable liquid is a newly introduced liquid embolic agent for endovascular embolization with some technical advantages over other liquid embolic agents. We present our initial experience with precipitating hydrophobic injectable liquid in the endovascular treatment of cerebral arteriovenous malformations.
Methods
From October 2015 to January 2018, 27 patients harboring cerebral arteriovenous malformations underwent endovascular embolization with precipitating hydrophobic injectable liquid 25. Clinical features, angiographic results, procedural details, complications, and follow-up details were retrospectively analyzed.
Results
Twenty-seven patients with cerebral arteriovenous malformations were included. Total obliteration in one endovascular session was confirmed for 14/27 (52%) patients. Partial embolization was attained in 13 patients (48%) in whom staged treatment with following radiosurgery or surgery was planned. No mortality was recorded in this series. Complications during or after the embolization occurred in six of 27 (22.2%) patients.
Conclusion
In our initial experience, precipitating hydrophobic injectable liquid has acceptable clinical outcome comparable to other liquid embolic agents. Although this is the largest reported study in arteriovenous malformation treatment with precipitating hydrophobic injectable liquid, further studies are needed to validate its safety and efficacy.
Keywords: Cerebral arteriovenous malformations (AVMs), embolization, precipitating hydrophobic injectable liquid (PHIL), liquid embolic agents (LEAs), unruptured brain AVMs
Introduction
Embolic agents are the essential tools for the treatment of cerebral arteriovenous malformations (AVMs). Historically, the most commonly used liquid embolic agents (LEAs) in interventional neuroradiology were N-butyl cyanoacrylate (NBCA) and the well known Onyx liquid embolic system (ev3 Neurovascular, Irvine, CA, USA).1
Precipitating hydrophobic injectable liquid (PHIL; Microvention, Inc., CA, USA) is a relatively new LEA. It is non-adhesive and is composed of a biocompatible polymer dissolved in dimethyl sulfoxide (DMSO) solvent and bonded covalently with an iodine component in order to provide radio-opacity for fluoroscopic visualization. It is a ready-to-use sterile system available in three different concentrations and viscosities (PHIL 25, 30 and 35) consisting of two pre-filled 1 mL syringes and one similar DMSO syringe. Given the recent introduction of PHIL, there is very limited published clinical data regarding the potential benefits of this new LEA on embolization efficacy, intraprocedural control, post-procedure complication rate, and post-procedure imaging artifacts.
Here, we report our early experience with PHIL in treating cerebral AVMs. To our knowledge, this is the largest reported study of AVM embolization with PHIL.
Methods
We performed retrospective analysis of prospectively collected data. Between October 2015 and January 2018, 27 patients (16 women and 11 men, mean age 42 years; median 44, range 6–63 years) harboring cerebral AVMs were treated with PHIL 25 in our department.
Clinical presentation and patient characteristics
For all 27 patients, clinical data, AVM angioarchitecture, procedural angiographic details, pre and post-procedure modified Rankin scale scores, and neurological examinations were collected (Table 1). Pre-embolization was aimed as a complement to surgical resection and/or radiosurgery in larger AVMs (Spetzler–Martin grade III or more).2 Endovascular embolization of small AVMs (Spetzler–Martin grade I or II) aimed for radiological cure. Staged treatment was planned for larger AVMs in order to obtain enough size reduction, rendering AVMs suitable for subsequent radiosurgery.
Table 1.
Clinical presentation, angioarchitectural features and patients’ characteristics.
| Case | AVM location and SM scale | Clinical presentation | Amount of PHIL, ml per session | Embolization (%) | Following treatment | mRS prior to discharge | mRS at 3 months |
|---|---|---|---|---|---|---|---|
| Left temporoparietal, grade III | Seizures | >6 | 70 | Following embolization/ radiosurgery | 0 | 0 | |
| Left temporal, grade II | Seizures | <5 | 80 | Radiosurgery | 0 | 0 | |
| Left parietal, grade III | Migraine | >6 | 80 | Following embolization | 0 | 0 | |
| Left frontoparietal, grade III | Headache, seizures | >7 | 90 | Radiosurgery | 0 | 0 | |
| Right temporoparietal, grade III | Seizures | 7 | 60 | Surgery | 0 | 3 | |
| Right occipital, grade II | Asymptomatic MRI findings | 5 | 60 | Following embolization/ radiosurgery | 0 | 0 | |
| Left parietal, grade I | Asymptomatic intranidal aneurysm | 3 | 100 | 0 | 0 | ||
| Pericallosal, grade II | Migraine | 5 | 100 | 0 | 0 | ||
| Right parietal occipital, grade III | Visual disturbance | >7 | 80 | Radiosurgery | 0 | 0 | |
| Left parietal occipital, grade II | Seizures | >3 | 100 | 0 | 0 | ||
| Left frontal parietal, grade I | Asymptomatic MRI – silent rupture data | >2 | 100 | 0 | 0 | ||
| Right temporal, grade II | Headache, seizures | 3 | 100 | 0 | 0 | ||
| Right parietal, grade III | Headache, seizures | >4 | 100 | 0 | N/A | ||
| Left temporo-occipital, grade IV | Seizures | >7 | 40 | Following embolization/ radiosurgery | 0 | N/A | |
| Left parietal, grade I | Migraine | >2 | 100 | 0 | 0 | ||
| Left occipital, grade III | Visual disturbance | >6 | 100 | Following embolization/ radiosurgery | 0 | 0 | |
| Left parieto-occipital, grade III | Migraine with aura | >5 | 70 | Surgery | 0 | 0 | |
| Right frontoparietal, grade III | Seizures | >7 | 80 | Refused any further treatment | 0 | N/A | |
| Left temporal, grade I | Asymptomatic MRI – hemosiderin-stained parenchyma | 2 | 100 | 0 | 0 | ||
| Left frontoinsular, grade I | Asymptomatic hemosiderin-stained parenchyma and gliosis | 3 | 100 | 0 | 0 | ||
| Left temporo-occipital, grade III | Seizures, headache | >6 | 80 | Radiosurgery | 0 | 0 | |
| Right temporal, grade II | Migraine with aura | 4 | 100 | 0 | N/A | ||
| Left parietal, grade I | Asymptomatic aneurysm of feeding artery | 2 | 100 | 0 | 0 | ||
| Left frontal temporal, grade III | Seizures | >5 | 60 | Following embolization/ radiosurgery | 0 | 0 | |
| Left parietal temporal, grade III | Seizures | >5 | 60 | Following embolization/ radiosurgery | 0 | N/A | |
| Left occipital, grade I | Asymptomatic MRI – hemosiderin-stained parenchyma, gliosis | 2 | 100 | 0 | 0 | ||
| Right parietal, grade II | Asymptomatic MRI data for intranidal aneurysm | >3 | 100 | 0 | N/A |
AVM: arteriovenous malformation; SM: Spetzler–Martin; PHIL: precipitating hydrophobic injectable liquid; MRI: magnetic resonance imaging; mRS: modified Rankin scale.
Procedural details
All patients were informed about the procedure according to local institutional policy and the ethics committee, and written informed consent was obtained from every patient. The decision to perform endovascular treatment was based on consensus by a multidisciplinary team of neuroradiologists, neurologists, radiosurgeons and neurosurgeons.
All procedures were performed under general anesthesia on a biplane angiographic unit (Innova GE Healthcare 31 31 IQ bi-plane). During the procedures, systolic blood pressure was controlled between 100 and 110 mmHg to prevent migration of embolic material to the venous side. Catheterization of the right femoral artery was performed in all 27 patients. After diagnostic angiography the diagnostic catheter was exchanged over an exchange-length wire for a Guider Softip XF Guiding catheter (6 French; Boston Scientific). The guiding catheter was positioned in the pre-petrous portion of the internal carotid artery and flushed by a pressure bag with saline containing 2500 U heparin/L.
Roadmap angiography was then performed, and a DMSO-compatible Apollo (Medtronic, USA) microcatheter was navigated to the nidal part of the malformation over a 0.008-inch guidewire. Once microcatheter angiography was performed to confirm optimal and safe positioning, 0.25 mL of DMSO was injected into the microcatheter in order to fill the dead space. A small reflux of PHIL was intentionally allowed to cover a portion of the detachable tip of the microcatheter, creating a PHIL ‘plug’. Slow injections of the liquid agent were then continued under fluoroscopy in the same pattern as the well known ethylene vinyl alcohol copolymer method (Figure 1).3,4 Between injections, control angiograms were obtained to assess nidus occlusion rate and the status of the draining veins. When any unwanted reflux of PHIL into the feeding artery or venous migration was noticed, the injection was stopped for 30–45 seconds to 1 minute to allow solidification of the agent. No pressure-cooker technique or any other anti-reflux modifications were used in these case series.5
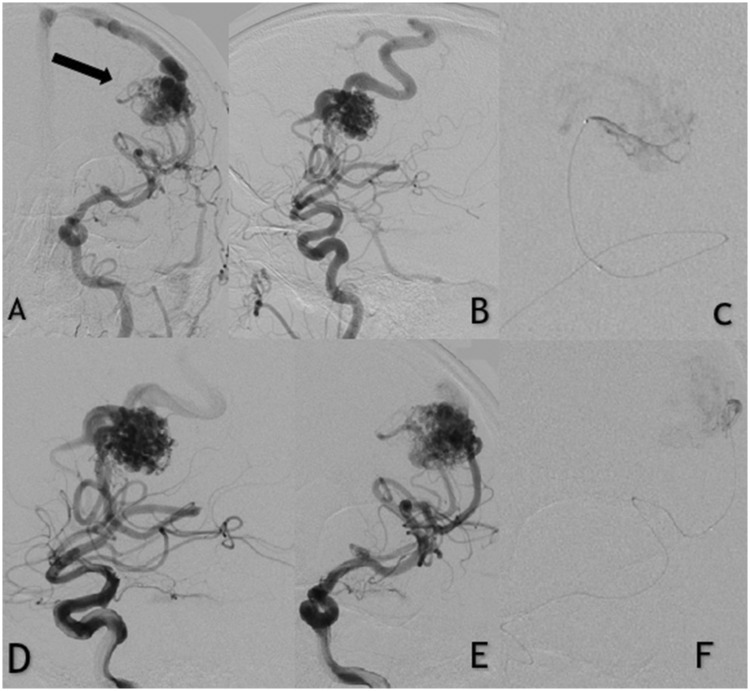
Figure 1.
Patient with unruptured left frontal arteriovenous malformation (AVM). (a and b) Anteroposterior digital subtraction angiography shows the AVM fed by the left middle cerebral artery. (d and e) Selective contrast injections in the left internal carotid artery. (c and f) Apollo microcatheter injection of the middle cerebral artery afferent demonstrating embolization of the nidus with precipitating hydrophobic injectable liquid. Note that the tip of the microcatheter is clearly visible in the cast.
All patients were monitored in the intensive care unit for at least 2 days for following clinical observation. A small dose of corticosteroids for a short period of time was given to all of the patients in order to reduce or prevent edema. Patients with complete nidal embolization had an increased risk of retrograde thrombosis of the feeding artery. As preventive medication low molecular heparin was administered for 2 consecutive days in these patients.6
Results
Twenty-seven patients with cerebral AVMs (19 symptomatic and eight asymptomatic) underwent endovascular embolization using only PHIL – 25% in a total of 43 procedures (mean 1.6 procedures per patient). Classification according to the Spetzler–Martin scale was grade I in seven of 27 (26%) patients, grade II in seven of 27 (26%), grade III in 12/27 (44%) and grade V in one of 27 (4%).2 None had a previous endovascular treatment. Clinical presentation of the cerebral AVMs included seizures in 13 patients (48.1%), migraine in four (14.8%), visual disturbance in two (7.4%), and eight patients (29.6%) were asymptomatic. Five of those eight asymptomatic patients had pre-embolization magnetic resonance imaging and angiography with evidence of previous silent AVM hemorrhages consisting of hemosiderin-stained parenchyma, gliosis and encelephalomalacia adjacent to the nidus. Intranidal or associated aneurysms of the feeding arteries were observed in three of those eight patients.
A total of 67 pedicles were injected, ranging from one to five per case. The mean procedure time was 95 ± 45 minutes, with an average fluoroscopy time of 51 ± 23 minutes. The mean amount of PHIL 25 used was 5.07 ± 6.9 ml. The average estimated size reduction of the AVMs was 85%, ranging from 40% to 100%. The evaluation of the AVM volume was calculated using the known method of Pasqualin et al.7 Adequate nidal penetration was achieved in all cases. Follow-up angiograms (mean 4 months; range 3–6 months) were obtained after the last endovascular treatment session in 21/27 (77.7%) cases.
Total obliteration in one endovascular session was confirmed for 14/27 (52%) patients, all of whom had small AVMs (Spetzler–Martin grades I and II). The clinical success and complete occlusion were confirmed with at least one follow-up angiography in all of the 14 patients (Figure 2).
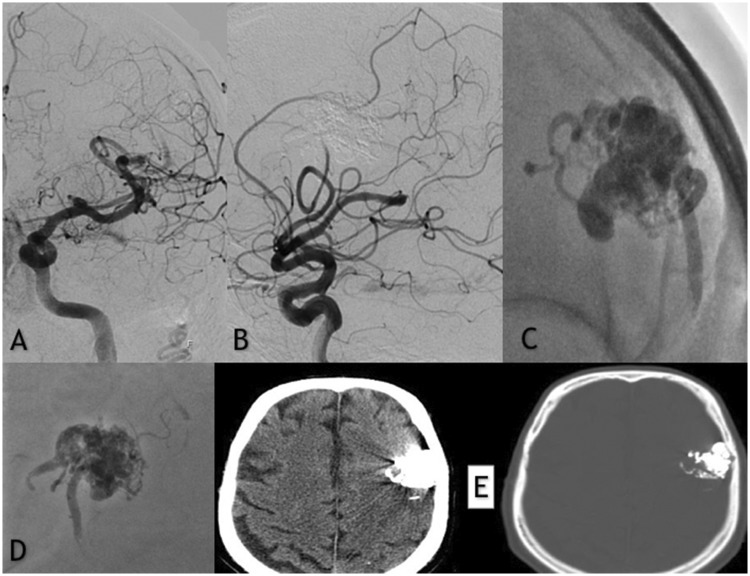
Figure 2.
(a and b) Frontal and lateral subtracted digital subtraction angiography injections showing the complete embolization of the left frontal unruptured arteriovenous malformation. (c and d) Native image demonstrating the opacities of the precipitating hydrophobic injectable liquid cast used for the embolization. (e) Axial non-contrast enhanced post-embolization computed tomography (CT) scan with minimal artifacts noted.
In the remaining 13 patients (48%) a total number of 29 procedures (including the initial embolization) were performed in order to achieve sufficient AVM nidal occlusion suitable for following treatment (Figure 3).
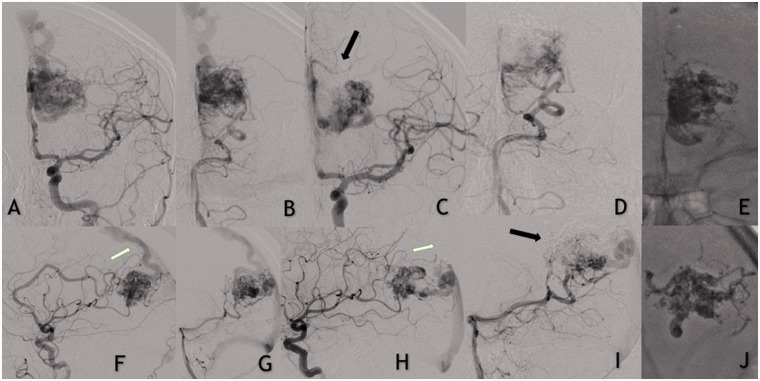
Figure 3.
(a and f) Left internal carotid and left vertebral artery (b and g) contrast injections demonstrating a left-sided unruptured occipito-parietal arteriovenous vascular malformation (AVM). Frontal (c and d) and lateral (h and i) views showing partial embolization of the AVM. Note the AVM remnant with reduced number of arterial feeders and changes in the venous drainage. (e and j) Precipitating hydrophobic injectable liquid.
Ten of these patients later had radiosurgery and two underwent microvascular surgical resection. However, one patient refused any further treatment.
Complications
Complications during or after the embolization occurred in six of 27 (22.2%) patients as summarized in Table 2. In our series there was no mortality. However, complications led to permanent neurological deficit in one particular patient (Glasgow outcome scale 3, permanent morbidity 3.7%). This patient presented with an AVM grade III and had a post-procedure intraparenchymal hemorrhage requiring surgical intervention and evacuation of the hematoma.
Table 2.
Complications.
| AVM location and SM scale | Volume of PHIL used (mL) | Complication | Additional therapy | Outcome, GOS |
|---|---|---|---|---|
| Left temporoparietal, grade III | >6 | Transient sensomotor aphasia | Dexamethasone/mannitol | No deficit at 3 months, GOS 5 |
| Left temporal, grade II | <5 | Transient sensomotor aphasia | Radiosurgery | No deficit at 3 months, GOS 5 |
| Left parietal, grade III | >6 | Severe headache | Dexamethasone/mannitol | No deficit at 3 months, GOS 5 |
| Left frontoparietal, grade III | >7 | Headache, mood changes | Dexamethasone/mannitol radiosurgery | No deficit at 3 months, GOS 5 |
| Right temporoparietal, grade III | 7 | Hemorrhage 1 day after embolization | Acute surgical removal of hematoma and AVM | Left hemiparesis GOS 3 |
| Right occipital, grade II | 5 | Hemianopia | Dexamethasone/mannitol, following embolization | GOS 5 |
AVM: arteriovenous malformation; SM: Spetzler–Martin; PHIL: precipitating hydrophobic injectable liquid; MRI: magnetic resonance imaging; GOS: Glasgow outcome scale.
Apart from the intended detachment of the microcatheter tip, no catheter breakage or retention occurred in our cases. No adverse physiological changes, or technical complications such as vessel injury, embolic agent extravasation, or microcatheter rupture were observed.
Discussion
Continued experience with endovascular techniques and technologies with cyanoacrylate derivatives such as NBCA have led to a greater pursuit of stand-alone curative endovascular embolization of AVMs.8,9 NBCA is a highly adhesive agent and while this allows reliable vessel occlusion, it also leads to a high risk of intravascular catheter adhesion. Therefore, the significantly high learning curve and ill-tolerated reflux led to the development of newer cohesive agents that offer several advantages over cyanoacrylates.10
Although first described in the 1990s, Onyx (Medtronic, Dublin, Ireland) still remains one of the most frequently used materials for cerebral AVMs.11,12 This LEA consists of ethylene vinyl alcohol copolymer, DMSO and tantalum powder, the latter causing radiopacity.13 Contrary to previous/ancestral LEAs Onyx is cohesive but not adhesive. This results in less adherence to microcatheters and vessel walls, and therefore it was intended to reduce the risk of complications.14 The specific possibility of multiple, repeated and longer injections through the same catheter have led to higher endovascular cure rates for brain AVMs.15 However, the most commonly reported disadvantage of Onyx is the production of diagnostic imaging artifacts in terms of streak artifacts in conventional and in cone-beam CT.16,17 Reported complications with the use of tantalum-based agents may be due to rapid sedimentation of the powder, which could cause blocking and sealing the microcatheter, specifically during long injections.18
Introduced in 2015, PHIL is a non-adhesive, inherently radiopaque, copolymer-based LEA that is intended for use in the embolization of lesions in the peripheral and microvasculature including AVMs, arteriovenous fistulas and hypervascular tumors. Composed of a non-adhesive copolymer that uses DMSO as a solvent PHIL offers stable radiopacity throughout prolonged injections due to covalently bonded iodine, which requires no preparation prior to use.19 Several small case series have demonstrated the effectiveness and safety of this novel LEA for embolization of cerebral AVMs and dural arteriovenous fistulas. Despite the promising initial experience, the results of these small case series require validation in larger cohorts.20–22 These preliminary studies demonstrated potential advantages of PHIL over well-established LEAs including faster and safer plug formation, a constant degree of embolization effect, and low intraoperative hazards. Other reported potential benefits are its consistent visibility, and fewer artifacts on post-interventional imaging.
In our experience, the embolized lesions were better visualized in comparison to Onyx. PHIL is slightly less radiopaque when compared to Onyx, thus allowing better visualization through the copolymer cast. We had no difficulties in determining the intravascular penetration of the agent or the tip of the microcatheter during fluoroscopy or even magnified subtracted images. As expected, PHIL 25 demonstrated no changes in radiopacity during all of the procedures. In our case series, we observed that PHIL has a lower visibility than Onyx when passing through the lumen of the microcatheter. Although this feature of lower radiopacity may theoretically be disadvantageous when used in high-flow shunts and arteriovenous fistulas, we did not have issues with visualization during AVM embolization.
Another minor technical advantage that we have encountered during our case series is that PHIL comes in sterile, prepared glass syringes and does not require shaking or additional preparation prior to injection because its visibility is not based on tantalum powder. This unique feature eliminates the risk of tantalum precipitation and potential occlusion of the microcatheter that can occur during the usage of Onyx.
In addition, given its iodine-based nature, we observed less prominent beam-hardening artifacts on post-procedure follow-up CT and susceptibility artifacts in MRI scans as compared to other tantalum-based embolic agents. The low degree of artifacts was mentioned in all recent reported clinical and preclinical studies. A recently published article assesses qualitatively and quantitatively the artifacts produced by Onyx and PHIL on conventional CT, cone-beam CT and MRI in an animal model.23 Technically speaking, the underlying reason behind the lesser artifact production is the lower atomic number of iodine. For example, the relatively high degree of artifacts produced by Onyx can/could be explained by the higher atomic number of its radiopaque composer – tantalum.24,25
Our initial experience with the use of PHIL for embolization of cerebral AVMs yielded promising results. In our study of 27 consecutive patients, the estimated post-embolization average volume reduction of the nidus using the ABC/2 formula was 85%. Total occlusion in one endovascular session was achieved in half of all treated patients (52%, 14). Similar results in terms of complete occlusion and the rate of adverse events were observed in studies conducted in patients treated with Onyx.26–28
In our experience, PHIL behaves in a similar fashion to Onyx in terms of delivery during embolization and clinical results. We find the required proximal plug formation and flow penetration comparable to the well known Onyx technique, even though it advances into the vasculature as a ‘block’ instead of forming layers.29,30 In comparison to Onyx, PHIL appears to fill different nidal compartments on different injections. It also maintains a constant forward flow when injected at a steady rate, ultimately leading to successful nidal occlusion. We did not observe an increased risk of inadvertent embolization of potentially dangerous anastomoses, extra-nidal vasculature, or ivenous reflux rates in comparison with other LEAs. In our series, there were no major complications related to PHIL injections or microcatheter occlusions and retraction failures as the above-mentioned studies have documented. We did not observe any occasions in which PHIL has precipitated/any instances of PHIL precipitating within the syringes over time during embolization.
Limitations
Despite being the largest clinical study of treatment of cerebral AVMs with PHIL reported to date, our data have several important limitations. First, this is a single-center experience and the technical results are limited by authors’ individual technique and experience. Second, the sample is relatively small. Third, our cohort involves only unruptured AVMs. Thus the results if our study should be interpreted with caution as they may not be widely applicable to general practice.
Conclusion
In our initial experience with endovascular embolization of cerebral AVMs, PHIL has a similar technical profile and comparable clinical outcome with other LEAs. Moreover, PHIL appears to be a safe and promising option for endovascular treatment for cerebral AVMs. The noticeably less post-embolization imaging artifact seems to be a potential advantage of this embolic agent. Larger multicenter series are needed to confirm the clinical benefits of this new embolic system.
Authorship
SSS, AS and AG: conception and design of the work, data acquisition, writing of the manuscript. KM, KN, MP, DK and HH: analysis and interpretation of the data for the work. RR, KO and VK: critical review of the work. SSS and RR: final approval of the version to be published.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Brassel F, Meila D. Evolution of embolic agents in interventional neuroradiology. Clin Neuroradiol 2015; 25(Suppl 2): 333–339. [DOI] [PubMed] [Google Scholar]
- 2.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986; 65: 476–483. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Dabus G, Rabinov JD, et al. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. Am J Neuroradiol 2008; 29: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saatci I, Geyik S, Yavuz K, et al. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: Long-term results in 350 consecutive patients with completed endo-vascular treatment course. J Neurosurg 2011; 115: 78–88. [DOI] [PubMed] [Google Scholar]
- 5.Chapot R, Stracke P, Velasco A, et al. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol 2014; 41: 87–91. [DOI] [PubMed] [Google Scholar]
- 6.Sobh K, Hegazy A. Feasibility and outcomes of endovascular embolization of cerebral arteriovenous malformations at a low-volume centre. J Vasc Interv Neurol 2013; 5: 4–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Pasqualin A, Barone G, Cioffi F, et al. The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 1991; 28: 370–379. [DOI] [PubMed] [Google Scholar]
- 8.Gross BA, Du R. Diagnosis and treatment of vascular malformations of the brain. Curr Treat Options Neurol 2014; 16: 279. [DOI] [PubMed] [Google Scholar]
- 9.Rosen RJ, Contractor S. The use of cyanoacrylate adhesives in the management of congenital vascular malformations. Semin Interv Radiol 2004; 21: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol 1990; 11: 163–168. [PMC free article] [PubMed] [Google Scholar]
- 11.Terada T, Nakamura Y, Nakai K, et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene vinyl alcohol copolymer. Report of three cases. J Neurosurg 1991; 75: 655–660. [DOI] [PubMed] [Google Scholar]
- 12.Saatci I, Geyik S, Yavuz K, et al. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: Long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 2011; 115: 78–88. [DOI] [PubMed] [Google Scholar]
- 13.Pierot L, Cognard C, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: Results of a prospective, multicentre study (BRAVO). Eur Radiol 2013; 23: 2838–2845. [DOI] [PubMed] [Google Scholar]
- 14.Lv X, Wu Z, Jiang C, et al. Complication risk of endovascular embolization for cerebral arteriovenous malformation. Eur J Radiol 2011; 80: 776–779. [DOI] [PubMed] [Google Scholar]
- 15.Bruno CA, Meyers PM. Endovascular management of arteriovenous malformations of the brain. Interv Neurol 2013; 1: 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saatci I, Cekirge HS, Ciceri EF, et al. CT and MR imaging findings and their implications in the follow-up of patients with intracranial aneurysms treated with endosaccular occlusion with onyx. AJNR Am J Neuroradiol 2003; 24: 567–578. [PMC free article] [PubMed] [Google Scholar]
- 17.Loy DN, Rich KM, Simpson J, et al. Time-of-flight magnetic resonance angiography imaging of a residual arteriovenous malformation nidus after Onyx embolization for stereotactic radiosurgery planning. Technical note. Neurosurg Focus 2009; 26: E13. [DOI] [PubMed] [Google Scholar]
- 18.Kulcsár Z, Karol A, Kronen PW, et al. A novel, non-adhesive, precipitating liquid embolic implant with intrinsic radiopacity: Feasibility and safety animal study. Eur Radiol 2016; 27: 1248–1256. [DOI] [PubMed] [Google Scholar]
- 19.Leyon JJ, Chavda S, Thomas A, et al. Preliminary experience with the liquid embolic material agent PHIL (precipitating hydrophobic injectable liquid) in treating cranial and spinal dural arteriovenous fistulas: Technical note. J Neurointerv Surg 2016; 8: 596–602. [DOI] [PubMed] [Google Scholar]
- 20.Koçer N, Hanımoğlu H, Batur Ş, et al. Preliminary experience with precipitating hydrophobic injectable liquid in brain arteriovenous malformations. Diagn Interv Radiol 2016; 22: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samaniego EA, Kalousek V, Abdo G, et al. Preliminary experience with precipitating hydrophobic injectable liquid (PHIL) in treating cerebral aVMs. J Neurointerv Surg 2016; 8: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 22.Lamin S, Chew HS, Chavda S, et al. Embolization of intracranial dural arteriovenous fistulas using PHIL liquid embolic agent in 26 patients: A multicenter study. AJNR Am J Neuroradiol 2017; 38: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollherbst DF, Otto R, Do T, et al. Imaging artifacts of Onyx and PHIL on conventional CT, cone-beam CT and MRI in an animal model. Interv Neuroradiol. Epub ahead of print 4 July 2018. DOI: 1591019918782692. [DOI] [PMC free article] [PubMed]
- 24.Giantsoudi D, De Man B, Verburg J, et al. Metal artifacts in computed tomography for radiation therapy planning: Dosimetric effects and impact of metal artifact reduction. Phys Med Biol 2017; 62: R49–R80. [DOI] [PubMed] [Google Scholar]
- 25.Barrett JF, Keat N. Artifacts in CT: Recognition and avoidance. Radiographics 2004; 24: 1679–1691. [DOI] [PubMed] [Google Scholar]
- 26.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM Embolization with Onyx. AJNR Am J Neuroradiol 2007; 28: 172–177. [PMC free article] [PubMed] [Google Scholar]
- 27.Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001; 48: 984–995. [DOI] [PubMed] [Google Scholar]
- 28.Pierot L, Januel C, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformation using Onyx: Preliminary results of a prospective multicenter study. Interv Neuroradiol 2005; 11: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollherbst DF, Otto R, von Deimling A, et al. Evaluation of a novel liquid embolic agent (precipitating hydrophobic injectable liquid (PHIL)) in an animal endovascular embolization model. J Neurointerv Surg 2018; 10: 268–274. [DOI] [PubMed] [Google Scholar]
- 30.Samaniego EA, Derdeyn CP, Hayakawa M, et al. In vivo evaluation of the new PHIL low viscosity in a swine rete mirabile model. Interv Neuroradiol. Epub ahead of print 4 July 2018. DOI: 1591019918784915. [DOI] [PMC free article] [PubMed]