Abstract
Objective
To estimate the cost‐effectiveness of surveillance schedules for non‐muscle‐invasive bladder cancer (NMIBC) amongst older adults.
Patients and Methods
We developed a MIcrosimulation SCreening ANalysis (MISCAN) microsimulation model to compare the cost‐effectiveness of various surveillance schedules (every 3 months to every 24 months, for 2, 5 or 10 years or lifetime) for older adults (aged 65–85 years) with NMIBC. For each surveillance schedule we calculated total costs per patient and the number of quality adjusted life‐years (QALYs) gained. Incremental cost‐effectiveness ratios (ICERs), as incremental costs per QALY gained, were calculated using a 3% discount.
Results
As age increased, the number of QALYs gained per patient decreased substantially. Surveillance of patients aged 65 years resulted in 2–7 QALYs gained, whereas surveillance at age 85 years led to <1 QALY gained. The total costs of the surveillance schedules also decreased as age increased. The ICER of 6‐monthly surveillance at age 65 years for lifetime was $4999 (American dollars)/QALY gained. Amongst patients aged >75 years, the incremental yield of QALY gains for any increase in surveillance frequency and/or duration was quite modest (<2 QALYs gained).
Conclusion
With increasing age, surveillance for recurrences leads to substantially fewer QALYs gained. These data support age‐specific surveillance recommendations for patients treated for NMIBC.
Keywords: bladder cancer, surveillance, cost‐effectiveness
Introduction
About 75% of all bladder cancer diagnoses are non‐muscle‐invasive bladder cancer (NMIBC) 1. NMIBC is generally treated surgically by transurethral resection (TUR). The 10‐year cancer‐specific survival is relatively high, ranging from 70% to 85% for high‐grade to 88–98% for low‐grade cancers 2, 3. However, the probability of recurrence is high (up to 75%) and 10–20% can progress to muscle‐invasive bladder cancer (MIBC), which is potentially lethal 2, 4, 5, 6. Therefore, surveillance by cystoscopy is offered to patients with a history of NMIBC to facilitate early diagnosis and treatment before progression.
All international guidelines on the management of NMIBC recommend cystoscopy at regular intervals, generally based on the patient's risk, but the recommended intervals differ 7. The AUA and the European Association of Urology (EAU) recommend low‐risk patients to have two cystoscopies in the first year, followed by annual cystoscopy for 5 years. The EAU recommends discontinuing surveillance after 5 years, whereas the AUA recommends shared decision‐making to stop or continue annual surveillance 8, 9. The National Institute for Health and Care Excellence (NICE) guideline recommends surveillance only at 3 and 12 months for low‐risk patients, discharging low‐risk patients to primary care if recurrence‐free at 12 months 10. For high‐grade patients, various guidelines generally recommend surveillance every 3 months for the first 2 years, followed by every 6 months for 2 or 3 years, followed by annual and even lifelong surveillance.
Surveillance cystoscopy is an invasive procedure. Patients may experience anxiety and pain, or complications such as painful urination and UTI, which influence quality of life (QoL) 11, 12. Furthermore, bladder cancer is largely a disease of older adults, and has the highest median patient age at diagnosis (73 years) of all cancer sites 13, 14. Despite relatively stable age‐adjusted incidence rates, the overall burden of bladder cancer has increased dramatically with the ageing of the population, increasing from stable figures of ~50 000 cases/year in the USA in the 1990s to >80 000 cases/year projected for 2018 15, 16. Over this same period, the median age at diagnosis of bladder cancer has increased from 67 to 73 years.
Given substantial competing causes of death in this predominantly elderly population, the intensity and duration of the surveillance programme should be evaluated for different age groups. Surveillance programmes should be optimised in terms of frequency of cystoscopy and duration of the surveillance, to simultaneously maximise the life‐years gained and minimise the harms and the costs.
The present study objective was to determine the most cost‐effective surveillance protocol for older adults with NMIBC. We evaluated surveillance strategies of varying frequency and duration.
Patients and Methods
MIcrosimulation SCreening ANalysis (MISCAN) model
We developed a microsimulation model using the MISCAN framework to simulate the impact of surveillance on survival in a patient with NMIBC. MISCAN is a microsimulation model developed for the evaluation of screening and has been used previously to estimate the harms, benefits and cost‐effectiveness of breast, colorectal, cervical and prostate cancer screening 17, 18, 19, 20. In this model, individual life histories of patients are simulated by modelling the transitions between possible health states (Fig. 1). All patients start in the no bladder cancer state after having been diagnosed and treated for bladder cancer. Some patients will have a NMIBC recurrence, which will be treated by TUR, or will progress to the MIBC state. An assumption in the model is that progression can only take place after recurrence. MIBC is assumed to be treated with cystectomy, which ends surveillance. In each subsequent state, there is an increasing risk of death from bladder cancer, therefore preventing progression to the MIBC state by surveillance using cystoscopy will decrease the risk of death and will increase the number of quality adjusted life‐years (QALYs).
Figure 1.
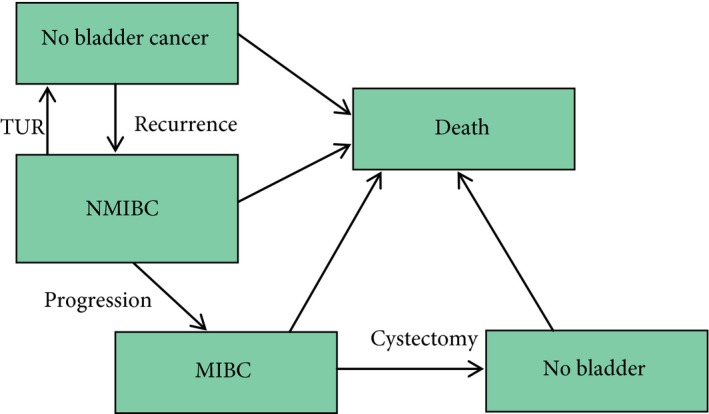
Possible transitions in the MISCAN model.
Model inputs were drawn from a previously developed Markov model with time steps of 3 months 21. This semi‐Markov model was based on the Dutch Cost‐Effectiveness of Follow‐up of Urinary Bladder Cancer (CEFUB) trial, which included 448 patients with non‐muscle‐invasive urothelial cancer (NMI‐UC) 22. In that trial, the efficacy of microsatellite analysis on voided urine for detecting tumour recurrences in the follow‐up of patients was evaluated. Patients included had a primary or recurrent NMI‐UC [pTa (85%), pT1 (15%), Grade 1 (43%) or Grade 2 (57%)] of the urinary bladder, based on histopathological examination of the surgically removed tumour. About half of the cohort (118/228) was newly diagnosed, whereas the remainder was enrolled at the time of recurrence (45 at first recurrence, 30 at second recurrence, 16 at third recurrence, 19 at the fourth or more recurrence). The probability of bladder cancer death by time after progression is calibrated to data from the Dutch Cancer Registry 21. The probability of other causes of death is based on the USA life table for 2010 for the general population. The parameters used in the MISCAN model are described in Table 1 21, 23.
Table 1.
| Parameter | Value |
|---|---|
| Recurrence time, years, mean | 4.18 (exponential distribution) |
| Time until progression, years, mean | 1.46 (exponential distribution) |
| Time until death after progression, years, mean | 2.57 |
| Sensitivity cystoscopy, % | 98 |
| Specificity cystoscopy, % | 88 |
| Costs of cystoscopy, $ | 168 |
| Costs of TUR (after recurrence), $ | 1409 |
| Costs of cystectomy (after progression), $ | 7997 |
| Disutility of cystoscopy | 0.025 for 1 month |
| Disutility of TUR | 0.03 for 1 month |
| Utility in NMIBC | 0.94 |
| Utility in MIBC | 0.80 for lifetime |
$, American dollars.
Screening strategies
We used MISCAN to simulate five cohorts of 1 million patients aged 65, 70, 75, 80 and 85 years at diagnosis and start of surveillance. To each cohort we applied 16 surveillance protocols, which comprised all combinations of frequencies of 3 months, 6 months, 12 months or 24 months and surveillance durations of 2, 5 and 10 years, or lifetime: 80 surveillance protocols with variable age of diagnosis, intensity and duration were investigated, assuming 100% attendance to surveillance.
Cost‐effectiveness
For each surveillance protocol, we calculated the number of cystoscopies, recurrences, progressions, and QALYs gained for a lifetime horizon. The utility estimates to calculate QALYs were based on Zhang et al. 23. The total costs of each surveillance protocol were calculated using cystoscopy and intervention costs (TUR and cystectomy) from the literature 21. We used incremental cost‐effectiveness ratios (ICERs) as the ratio of incremental costs to incremental life‐years gained. An annual discount of 3% was used, and the threshold for the ICER was set on $100 000 (American dollars).
Sensitivity analysis
We performed one‐way sensitivity analyses, in which we varied the comorbidity status, disutilities, and costs. Comorbidity status was based on the life tables for the USA population having no comorbidity and severe comorbidity 24. The disutility of cystoscopy and TUR were increased to 0.1 for 1 month and the costs for cystoscopy, TUR and cystectomy were all lowered by 10%.
Results
The discounted QALYs gained per patient vs costs per patient are presented for all simulated surveillance scenarios in Fig. 2. The younger the patient was when entering a surveillance programme, the more QALYs gained. The duration of surveillance increased both QALYs gained and the associated costs. However, the difference in QALYs gained between a surveillance protocol running for 10 years and one running for a lifetime decreased as patients aged and was negligible for patients aged >80 years: e.g., screening every 3 months for 10 years or lifetime both resulted in 0.92 QALY gained amongst 85‐year olds and ~1.7 QALYs amongst individuals aged 80 years. The frequency of a surveillance protocol had a similar effect across starting ages.
Figure 2.
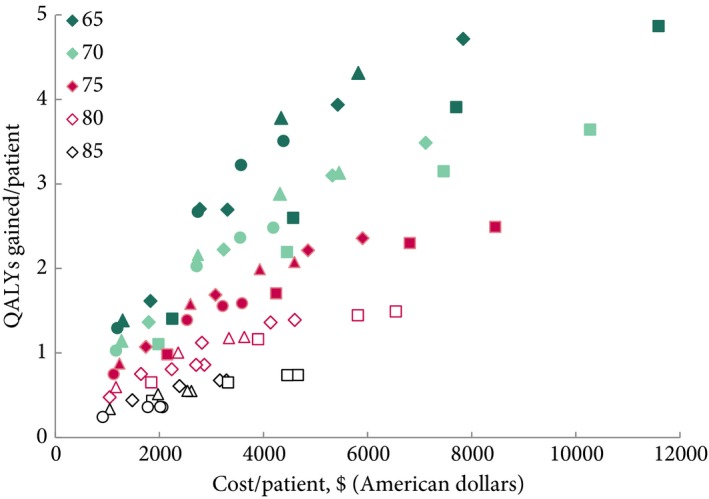
The costs and QALYs gained per patient of all evaluated surveillance strategies. Both costs and QALYs are discounted. The colours of the symbols represent the ages at the start of the surveillance strategy and the symbols the different frequencies of surveillance (square every 3 months, diamond every 6 months, triangle every 12 months, and circle every 24 months).
In Table 2 only the cost‐effective strategies within each cohort are presented. Surveillance strategies that allow for more surveillance (in both ways – by more frequent cystoscopy or by longer monitoring) resulted in larger cost increases. The number of diagnosed recurrences increased with the number of cystoscopies. In contrast, the overall number of diagnosed cancer progressions followed a parabolic trend, where it increased with the duration of the surveillance protocol but started to decrease when more than one test per year was performed. This suggests that frequent surveillance can detect recurrences before they progress to muscle invasion. Detecting recurrences has a major effect on the costs of a protocol, as the treatment of progression is over five‐times as expensive as treatment for NMIBC.
Table 2.
Effects and costs (in American dollars) of the cost‐effective strategies per age cohort. The number of screens, recurrences and progression, costs and QALYs gained are given per patient over a lifetime, without discount. In the calculation of the ICER a 3% discount rate was used
| Start age, years | Frequency, months | Duration, years | Screens, n | Recurrence, n | Progression, n | Costs, $ | QALYs | ICER, $ |
|---|---|---|---|---|---|---|---|---|
| 65 | 24 | 2 | 0.91 | 0.17 | 0.11 | 1 264 | 1.87 | 920 |
| 65 | 24 | 5 | 2.46 | 0.39 | 0.26 | 3 050 | 3.99 | 1 123 |
| 65 | 12 | 5 | 4.51 | 0.55 | 0.19 | 3 017 | 3.86 | 1 145 |
| 65 | 12 | 10 | 8.14 | 0.87 | 0.30 | 4 984 | 5.60 | 1 450 |
| 65 | 12 | Lifetime | 14.34 | 1.19 | 0.41 | 7 374 | 6.60 | 2 786 |
| 65 | 6 | Lifetime | 30.01 | 1.78 | 0.31 | 10 050 | 7.12 | 4 999 |
| 65 | 3 | Lifetime | 60.59 | 2.27 | 0.20 | 14 973 | 7.29 | 24 758 |
| 70 | 12 | 2 | 1.86 | 0.25 | 0.08 | 1 329 | 1.53 | 1 112 |
| 70 | 12 | 5 | 4.36 | 0.53 | 0.18 | 2 924 | 2.98 | 1 445 |
| 70 | 12 | 10 | 7.66 | 0.82 | 0.28 | 4 709 | 4.15 | 2 177 |
| 70 | 12 | Lifetime | 11.66 | 1.05 | 0.36 | 6 327 | 4.62 | 4 579 |
| 70 | 6 | Lifetime | 24.49 | 1.54 | 0.27 | 8 429 | 5.06 | 4 675 |
| 70 | 3 | Lifetime | 49.65 | 1.92 | 0.17 | 12 405 | 5.22 | 20 195 |
| 75 | 12 | 2 | 1.81 | 0.24 | 0.08 | 1 288 | 1.13 | 1 410 |
| 75 | 12 | 5 | 4.13 | 0.50 | 0.17 | 2 770 | 2.11 | 1 932 |
| 75 | 12 | 10 | 6.90 | 0.75 | 0.26 | 4 276 | 2.76 | 3 237 |
| 75 | 6 | 10 | 14.38 | 1.03 | 0.18 | 5 306 | 3.00 | 4 147 |
| 75 | 6 | Lifetime | 19.13 | 1.27 | 0.22 | 6 770 | 3.26 | 7 340 |
| 75 | 3 | Lifetime | 38.94 | 1.56 | 0.14 | 9 841 | 3.40 | 19 065 |
| 80 | 12 | 2 | 1.71 | 0.23 | 0.08 | 1 217 | 0.75 | 1 958 |
| 80 | 12 | 5 | 3.74 | 0.46 | 0.16 | 2 517 | 1.30 | 2 918 |
| 80 | 6 | 5 | 7.76 | 0.60 | 0.10 | 2 990 | 1.42 | 3 895 |
| 80 | 6 | 10 | 12.10 | 0.88 | 0.15 | 4 494 | 1.78 | 5 502 |
| 80 | 6 | Lifetime | 14.09 | 0.98 | 0.17 | 5 122 | 1.83 | 15 572 |
| 80 | 3 | Lifetime | 28.83 | 1.19 | 0.11 | 7 364 | 1.94 | 19 402 |
| 85 | 12 | 2 | 1.53 | 0.20 | 0.07 | 1 092 | 0.41 | 3 112 |
| 85 | 6 | 2 | 3.86 | 0.31 | 0.05 | 1 528 | 0.53 | 4 118 |
| 85 | 6 | 5 | 6.54 | 0.51 | 0.09 | 2 526 | 0.75 | 5 404 |
| 85 | 6 | 10 | 9.07 | 0.67 | 0.12 | 3 406 | 0.85 | 11 558 |
| 85 | 3 | 10 | 18.63 | 0.80 | 0.07 | 4 824 | 0.92 | 20 914 |
For example, 12‐monthly surveillance from the age of 65 years is expected to result in 6.60 QALYs gained per patient (without discount), whilst 6‐monthly surveillance from the same age gained an extra 0.52 QALYs at an increased cost of $10 050/patient. The corresponding ICER was $4999 (using a 3% discount). This small effect is achieved by more than doubling the number of surveillance cystoscopies.
The sensitivity analyses showed that the results were robust to the parameters varied (Appendix S1). Only in a few cases, a different strategy was amongst the cost‐effective strategies. Comorbidity status had the largest, although still limited, impact on the results.
Discussion
Our present findings suggest that cystoscopy‐based surveillance for NMIBC is cost‐effective for younger patients. However, we found that, even in the youngest cohort (65 years), lifelong quarterly cystoscopy was associated with high‐costs for modest potential QALY gains. Perhaps more importantly, within the context of the high, and increasing, median age at bladder cancer diagnosis, we found that the incremental yield of QALY gains for any increase in surveillance frequency and/or duration amongst patients aged ≥75 years was modest. Given the changing age structure of the population and the disproportionately high incidence rates in the oldest ages 25, the burden of bladder cancer in the elderly is projected to increase by 54% in the next 15 years 26, underscoring the public health impact of these findings.
In contrast to other urological cancers, in particular prostate cancer 27, age and competing risks have not, to date, been explicitly considered in bladder cancer guidelines. A multidisciplinary stakeholder group of clinicians, patients, payers and patient advocates identified cancer care for the elderly and post‐treatment surveillance as two of the top three cancers comparative effectiveness research priorities 28. Post‐treatment surveillance for NMIBC is uniquely invasive, intensive, and under current recommendations often lifelong. Given the rapidly growing and ageing population of patients with bladder cancer, it is critically important to define age‐based surveillance schedules for this group.
There are two other studies that evaluated different surveillance protocols. Overall, the results were comparable: for younger patients an intensive surveillance strategy was more cost‐effective than for older patients. The analysis of Zhang et al. 23 focused on low‐grade NMIBC, which accounts for about two‐thirds of all NMIBCs. International guidelines for surveillance were simulated and the number of cystoscopies and the QALYs were calculated for men and women separately. The authors concluded that patient‐specific factors such as the presence of comorbidity, or perception of utility loss from cystoscopy, should play an important role in determining the best surveillance protocol. Kent et al. 29 calculated the expected delay in detection of the next tumour based on several surveillance scenarios and concluded that the optimal surveillance schedule is less frequent for low‐risk patients than for high‐risk patients. In our present study, we considered the full spectrum of NMIBC, including the relatively higher risk subset with multiple recurrences, in contrast to Zhang et al. 23, and found relatively small incremental benefits of QALYs gained for more intensive or lengthy surveillance programmes amongst patients aged ≥75 years. As a result of advanced chronological age, patients with bladder cancer have a high burden of multiple chronic conditions 30 and the American Society for Clinical Oncology has called for more explicit consideration of these factors in the guideline development process 31. A previous analysis found that in patients with the highest rates of comorbidity, the optimal surveillance protocol was no surveillance 23. In our present analysis, even for patients with severe comorbidity surveillance could still be cost‐effective.
Strong points of the present study are that we included lifetime surveillance and evaluated the results over a lifetime horizon. Furthermore, we applied cost‐effectiveness analysis to determine the optimal surveillance protocol. This study also has several limitations.
We did not explicitly model heterogeneity in NMIBC. Surveillance can be stratified by pathology findings, treatment and recurrence history into low‐, intermediate‐ and high‐risk disease 9. Individuals with high‐grade tumours have a higher risk of recurrence and progression to MIBC and may require a more intensive surveillance protocol 5, 6. However, to reduce the burdens and costs of surveillance, patients at low‐risk of recurrence could follow a less intensive surveillance strategy 32.
Risk‐stratified surveillance has had slow uptake in the USA, with evidence of a mismatch between patient‐level risk and intensity of surveillance 33. The CEFUB cohort, which the model is based on, did not include the highest‐risk minority of cases with Grade 3 tumours. However, it reflects the majority of incident and prevalent cases, including both incident and recurrent cases, and is relevant for the subset where there is lesser consensus in surveillance guidelines. As such, our present analysis is one of the first to apply modelling to examine these areas of uncertainty, especially the questions of age, intensity and duration of surveillance. Whilst the inclusion criteria of the CEFUB trial reflected relatively lower grade, the fact that half of the cohort included recurrent tumours (with about half of those being multiply recurrent cases), the risks of progression in that cohort was nontrivial, higher than might be inferred from inclusion criteria of Grades 1/2 only. The inclusion in the present study population of newly diagnosed and recurrent cases reflects a broader spectrum of risk.
Another limitation is that we assumed that all patients with progression will undergo cystectomy; however, in practice, a proportion of patients will not undergo cystectomy. This will impact survival, QoL and costs, but the magnitude is unclear. Future work will apply the MISCAN model to these questions. Also, we did not consider adjuvant intravesical therapies and whilst these adjuncts may reduce risks of recurrence, implementation in real‐world practice is infrequent 34.
Finally, we did not stratify by gender. Bladder cancer is three‐times more common in men 1, and there is some uncertainty about the potential for varying recurrence and progression patterns by sex 35. Zhang et al. 23 found that women should be screened more intensively. However, currently the guidelines do not stratify recommended surveillance schedules by sex.
Another cost‐effective possibility to reduce the number of cystoscopies might be the utilisation of biomarker tests [e.g. fibroblast growth factor receptor 3 (FGFR3) mutation analysis] in voided urine samples 21, 36. A previous analysis concluded that the effectiveness of surveillance with the FGFR3 urine test was similar to that of surveillance entirely by cystoscopy, and the costs were substantially lower. The utility of such strategies, specifically amongst older patients where we found more modest benefits of regular cystoscopy, warrants further study.
In conclusion, various schedules for surveillance cystoscopy amongst patients with NMIBC are cost‐effective. However, recognising that bladder cancer has the highest median age at diagnosis amongst all cancer sites, our present finding of modest QALY gains amongst patients aged ≥75 years calls into question the utility of intensive or lengthy surveillance schedules amongst this large and growing subset of patients. Future studies should focus on surveillance stratified by age and competing risks, and evaluate the cost and QoL tradeoffs of innovative urine‐based biomarker tests.
Conflicts of Interest
Dr Nielsen has research funding from the National Institutes of Health, not directly related to the submitted work. Dr Nielsen is on the Medical Advisory Board for Grand Rounds and has research funding from the Patient‐Centered Outcomes Research Institute (PCORI). All other authors do not have conflicts of interest.
Abbreviations
- (N)MIBC
(non‐)muscle‐invasive bladder cancer
- CEFUB
Cost‐Effectiveness of Follow‐up of Urinary Bladder Cancer
- FGFR3
fibroblast growth factor receptor 3
- ICER
incremental cost‐effectiveness ratio
- MISCAN
MIcrosimulation SCreening Analysis
- NMI‐UC
non‐muscle‐invasive urothelial cancer
- QALY
quality adjusted life‐year
- QoL
quality of life
- TUR
transurethral resection
Supporting information
Appendix S1. Sensitivity analyses.
Acknowledgements
This work was funded by a grant from the Lineberger Comprehensive Cancer Center at the University of North Carolina at Chapel Hill. In addition, we would like to thank the Cancer Intervention and Surveillance Modeling Network (CISNET consortium; http://cisnet.cancer.gov) for important background discussions relevant to the model used in this paper.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30 [DOI] [PubMed] [Google Scholar]
- 2. Palou J, Sylvester RJ, Faba OR et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease‐specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette‐Guerin. Eur Urol 2012; 62: 118–25 [DOI] [PubMed] [Google Scholar]
- 3. American Cancer Society . Survival Rates for Bladder Cancer 2016. Available at: https://www.cancer.org/cancer/bladder-cancer/detection-diagnosis-staging/survival-rates.html . Accessed February 2018
- 4. Leblanc B, Duclos AJ, Benard F et al. Long‐term followup of initial Ta grade 1 transitional cell carcinoma of the bladder. J Urol 1999; 162: 1946–50 [DOI] [PubMed] [Google Scholar]
- 5. Donat SM. Evaluation and follow‐up strategies for superficial bladder cancer. Urol Clin North Am 2003; 30: 765–76 [DOI] [PubMed] [Google Scholar]
- 6. Sylvester RJ, van der Meijden AP, Oosterlinck W et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49: 466–77 [DOI] [PubMed] [Google Scholar]
- 7. Power NE, Izawa J. Comparison of guidelines on non‐muscle invasive bladder cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer 2016; 2: 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Babjuk M, Böhle A, Burger M et al. Guidelines on non‐muscle‐invasive bladder cancer (Ta, T1 and CIS) European Association of Urology, 2015. Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-muscle-invasive-Bladder-Cancer-2015-v1.pdf. Accessed August 2018 [Google Scholar]
- 9. Chang SS, Boorjian SA, Chou R et al. Diagnosis and treatment of non‐muscle invasive bladder cancer: AUA/SUO guideline. J Urol 2016; 196: 1021–9 [DOI] [PubMed] [Google Scholar]
- 10. National Collaborating Centre for Cancer (UK) . Bladder cancer: diagnosis and management . NICE guideline [NG2], 2015. Available at: https://www.nice.org.uk/guidance/ng2. Accessed August 2018 [Google Scholar]
- 11. Soomro KQ, Nasir AR, Ather MH. Impact of patient's self‐viewing of flexible cystoscopy on pain using a visual analog scale in a randomized controlled trial. Urology 2011; 77: 21–3 [DOI] [PubMed] [Google Scholar]
- 12. Wilson L, Ryan J, Thelning C, Masters J, Tuckey J. Is antibiotic prophylaxis required for flexible cystoscopy? A truncated randomized double‐blind controlled trial. J Endourol 2005; 19: 1006–8 [DOI] [PubMed] [Google Scholar]
- 13. Abdollah F, Gandaglia G, Thuret R et al. Incidence, survival and mortality rates of stage‐specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 2013; 37: 219–25 [DOI] [PubMed] [Google Scholar]
- 14. American Cancer Society . Cancer Facts & Figures 2017. 2017. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Accessed February 2018
- 15. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30 [DOI] [PubMed] [Google Scholar]
- 16. Silverberg E, Boring CC, Squires TS. Cancer statistics, 1990. CA Cancer J Clin 1990; 40: 9–26 [PubMed] [Google Scholar]
- 17. de Kok IM, van Rosmalen J, Dillner J et al. Primary screening for human papillomavirus compared with cytology screening for cervical cancer in European settings: cost effectiveness analysis based on a Dutch microsimulation model. BMJ 2012; 344: e670 10.1136/bmj.e670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heijnsdijk EA, de Carvalho TM, Auvinen A et al. Cost‐effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst 2015; 107: 366 10.1093/jnci/dju366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sankatsing VD, Heijnsdijk EA, van Luijt PA, van Ravesteyn NT, Fracheboud J, de Koning HJ. Cost‐effectiveness of digital mammography screening before the age of 50 in The Netherlands. Int J Cancer 2015; 137: 1990–9 [DOI] [PubMed] [Google Scholar]
- 20. van Hees F, Habbema JD, Meester RG, Lansdorp‐Vogelaar I, van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost‐effectiveness analysis. Ann Intern Med 2014; 160: 750–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Bekker‐Grob EW, van der Aa MN, Zwarthoff EC et al. Non‐muscle‐invasive bladder cancer surveillance for which cystoscopy is partly replaced by microsatellite analysis of urine: a cost‐effective alternative? BJU Int 2009; 104: 41–7 [DOI] [PubMed] [Google Scholar]
- 22. van der Aa MN, Zwarthoff EC, Steyerberg EW et al. Microsatellite analysis of voided‐urine samples for surveillance of low‐grade non‐muscle‐invasive urothelial carcinoma: feasibility and clinical utility in a prospective multicenter study (Cost‐Effectiveness of Follow‐Up of Urinary Bladder Cancer trial [CEFUB]). Eur Urol 2009; 55: 659–67 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Denton BT, Nielsen ME. Comparison of surveillance strategies for low‐risk bladder cancer patients. Med Decis Making 2013; 33: 198–214 [DOI] [PubMed] [Google Scholar]
- 24. Lansdorp‐Vogelaar I, Gulati R, Mariotto AB et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med 2014; 161: 104–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nielsen ME, Smith AB, Meyer AM et al. Trends in stage‐specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer 2014; 120: 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009; 27: 2758–65 [DOI] [PubMed] [Google Scholar]
- 27. National Comprehensive Cancer Network. NCCN Guidelines Prostate Cancer Early Detection Version 2.2016: NCCN, 2016. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed February 2018
- 28. Greenberg CC, Wind JK, Chang GJ, Chen RC, Schrag D. Stakeholder engagement for comparative effectiveness research in cancer care: experience of the DEcIDE Cancer Consortium. J Comp Eff Res 2013; 2: 117–25 [DOI] [PubMed] [Google Scholar]
- 29. Kent DL, Nease RA, Sox HC Jr, Shortliffe LD, Shachter R. Evaluation of nonlinear optimization for scheduling of follow‐up cystoscopies to detect recurrent bladder cancer. The Bladder Cancer follow‐up Group. Med Decis Making 1991; 11: 240–8 [DOI] [PubMed] [Google Scholar]
- 30. Garg T, Young AJ, Kost KA et al. Burden of multiple chronic conditions among patients with urological cancer. J Urol 2017; 199: 543–50 [DOI] [PubMed] [Google Scholar]
- 31. Somerfield MR, Bohlke K, Browman GP et al. Innovations in American society of clinical oncology practice guideline development. J Clin Oncol 2016; 34: 3213–20 [DOI] [PubMed] [Google Scholar]
- 32. Svatek RS, Hollenbeck BK, Holmäng S et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014; 66: 253–62 [DOI] [PubMed] [Google Scholar]
- 33. Schroeck FR, Smith N, Shelton JB. Implementing risk‐aligned bladder cancer surveillance care. Urol Oncol 2018; 36: 257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamie K, Saigal CS, Lai J et al. Quality of care in patients with bladder cancer: a case report? Cancer 2012; 118: 1412–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boorjian SA, Zhu F, Herr HW. The effect of gender on response to bacillus Calmette‐Guerin therapy for patients with non‐muscle‐invasive urothelial carcinoma of the bladder. BJU Int 2010; 106: 357–61 [DOI] [PubMed] [Google Scholar]
- 36. van Kessel KE, Kompier LC, de Bekker‐Grob EW et al. FGFR3 mutation analysis in voided urine samples to decrease cystoscopies and cost in nonmuscle invasive bladder cancer surveillance: a comparison of 3 strategies. J Urol 2013; 189: 1676–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Sensitivity analyses.


