Abstract
Background
Previous studies have demonstrated that short interpregnancy interval (the interval between delivery and estimated last menstrual period of a subsequent pregnancy) is associated with small for gestational age birth. It is controversial if this association is causal, as few studies have accounted for likely confounding factors such as unintended pregnancy. We examined the association between interpregnancy interval and infant birthweight, adjusting for pregnancy intention and other socio‐economic and obstetrical risk factors.
Methods
We used data from the Scandinavian Successive Small‐for‐Gestational‐Age births study (1986‐1988). Birthweight was expressed as a gestational age‐standardised z‐score.
Results
Among 1406 women, a trend towards lower birthweight z‐score with short interpregnancy interval was not statistically significant (unadjusted difference in birthweight z‐score of −0.25, 95% confidence interval (CI) −0.55, 0.05). After adjusting for pregnancy intention, detailed measures of socio‐economic status, and other covariates, the estimated magnitude of effect between interpregnancy interval and birthweight z‐score was further attenuated (adjusted difference in birthweight z‐score of −0.13, 95% CI −0.46, 0.20).
Conclusions
In this cohort study with detailed information on pregnancy intention and socio‐economic status, short interpregnancy interval was not associated with lower birthweight. These findings suggest that previously observed associations between short interpregnancy interval and lower birthweight may reflect confounding by socio‐economic and/or other unmeasured confounders.
Keywords: birth spacing, fetal growth, fetal growth restriction, interpregnancy interval, pregnancy intention
1. INTRODUCTION
Meta‐analyses of observational studies have demonstrated that short interpregnancy interval (the time interval between delivery and estimated last menstrual period of a subsequent pregnancy) increases the risk of adverse obstetrical outcomes, including small for gestational age (SGA) birth.1, 2 This has led national and international organisations such as the World Health Organization to recommend interpregnancy intervals of at least 18‐24 months following a livebirth3, 4 and at least 6 months following a miscarriage or induced abortion.3
Despite these recommendations, it is controversial if the link between interpregnancy interval and adverse outcomes is actually causal.5 Previous studies adjusted their analyses for some demographic and socio‐economic risk factors such as age, parity, race/ethnicity, and smoking status,6 but it is unlikely that these variables fully captured the circumstances or conditions that influence both interpregnancy interval and adverse obstetrical outcomes. In particular, a large fraction of pregnancies conceived after a short interpregnancy interval are unintended,7 and unintended pregnancy is also a risk factor for preterm birth, low birthweight, SGA birth, and other adverse obstetrical outcomes.8, 9 Pregnancy intention, socio‐economic and obstetrical risk factors, and interpregnancy interval may affect separate, partially overlapping, or completely overlapping pathways towards increased risk for SGA birth. However, few previous studies have examined the association between short interpregnancy interval and SGA birth, accounting for potential confounding by pregnancy intention. Disentangling the consequences of short interpregnancy interval on adverse obstetrical outcomes from the contributions of pregnancy intention and other socio‐economic influences is critical for informing evidence‐based public health policies and clinical recommendations for family planning.
The aim of this study was to examine the association between short interpregnancy interval and infant birthweight, adjusted for pregnancy intention and other socio‐economic and obstetrical risk factors.
2. METHODS
2.1. Study population
We conducted a cohort study using data from the U.S. National Institute of Child Health and Human Development Scandinavian Successive Small‐for‐Gestational‐Age births study.10 This multicentre prospective cohort recruited mothers and children from three counties in Norway and Sweden from 1986 to 1988. Women were eligible for enrolment if they had a singleton pregnancy and had 1 or 2 previous births greater than 20 weeks’ gestation (live or stillborn), were of Caucasian origin, spoke one of the Scandinavian languages, and were registered by the study centre before 20 weeks’ gestation. Of the women who were eligible and attended the first study visit (N = 5722), a 10% random sample (n = 561) was selected to represent the general population of multiparae women. Of the remaining participants, those with any of the following risk factors for SGA birth were selected to be in the study group: a prior low birthweight infant, maternal cigarette smoking around the time of conception, a low prepregnancy weight, a previous perinatal death, or the presence of a chronic maternal disease (renal disease, essential hypertension, or heart disease). Among women who reported cigarette smoking around the time of conception, 50% were randomly selected to the study group. A total of 1945 women were invited for detailed visits at 17, 25, 33, and 37 weeks’ gestation, as well as for collection of birth and neonatal outcomes.
Gestational age was calculated based on either the reported last menstrual period (LMP) or the obstetric gestational age determined by ultrasound at approximately 17 weeks’ gestation. The reported LMP was used if it could be recalled within 3 days. Biparietal diameter (BPD) at the first study visit (approximately 17 weeks’ gestation) was used to date the pregnancy if the discrepancy between the estimated dates of delivery based on LMP and BPD was more than 14 days, or if the LMP could not be recalled. Prior low birthweight infant was defined as a prior first birth of a female<2700 g, or male less than 2800 g; or prior second birth of a female less than 2800 g or male less than 2900 g (accounting for increased expected birthweight of a second child).10
The analyses conducted in the current study were restricted to women who did not have an intervening abortion (ie induced or spontaneous delivery prior to 20 weeks’ gestation) between births defining the interpregnancy interval examined.
2.2. Exposures and outcomes
The primary exposure was interpregnancy interval, which was defined as the time in completed months from the date of birth of the previous child (live or stillborn) to the beginning of the current pregnancy. The beginning of the current pregnancy was calculated as either the reported last menstrual period or the beginning of the gestation as determined by the first ultrasound (at approximately 17 weeks’ gestation). Interpregnancy interval was categorically defined as less than 12 months, 12‐17.9 months, 18‐23.9 months, and greater than or equal to 24 months, based on previous reports of a reverse J‐shaped association with SGA birth.1
Details of the previous pregnancy and current socio‐economic characteristics were self‐reported at the first study visit. Pregnancy intention and details describing maternal social support were self‐reported at the third study visit (approximately 33 weeks’ gestation). Regarding pregnancy intention, participants were asked: “Was this pregnancy planned?” and those who answered “yes” were classified as having a planned pregnancy whereas those who answered “no” were classified as having an unplanned pregnancy.
The primary outcome was birthweight‐for‐gestational age z‐score based on an internal standard created from the random sample (n = 561). The z‐scores were created by expressing fetal weight as a function of gestational age using a multilevel model,11 which estimated the average population growth pattern throughout gestation and variability in growth between fetuses.12 SGA birth is commonly used as a proxy for fetal growth restriction and is typically defined as an estimated fetal weight or birthweight less than the tenth percentile.13 In the randomly selected group, this corresponded to a birthweight z‐score of <−1.28.12 However, we used birthweight z‐score as continuous outcome since we aimed to examine the biological continuum of fetal growth, and clinical relevance of SGA is limited by the fact that it does not differentiate between fetuses which are constitutionally small vs those which are pathologically small.13, 14 To address this limitation, we conducted a sensitivity analysis using the outcome of conditional birthweight z‐scores. Conditional birthweight z‐scores identified newborns who deviated from their anticipated individual growth trajectory, by comparing their weight at birth to that expected based on ultrasound estimated fetal weights at 25 and 33 weeks’ gestation.12 Thus, they better describe fetal growth instead of fetal size. Conditional z‐scores were calculated using the same internal standard used to calculate birthweight‐for‐gestational age z‐scores using previously described methods.11
2.3. Statistical analyses
A directed acyclic graph (DAG) based on existing literature was created to represent the putative causal pathway between short interpregnancy interval and poor fetal growth.15 Based on the DAG, covariates fulfilling the minimally sufficient adjustment set were selected. The DAG was created using DAGitty version 2.3.16 Multivariable regression was used to calculate the crude and adjusted difference in birthweight z‐scores with 95% confidence intervals. Individuals who were missing data for demographic or socio‐economic variables were included under a missing category. The reference interpregnancy interval category of 18‐23 months was used since previous literature suggests this interval has the lowest risk for SGA birth.1 Estimated birthweight z‐scores by interpregnancy interval were calculated with 95% confidence intervals. Sensitivity analyses were performed using conditional birthweight z‐scores as stated above. Since pregnancy intention was collected at approximately 33 weeks’ gestation, we also conducted a sensitivity analysis excluding those delivering prior to 33 weeks’ gestation. All analyses were completed using Stata version 15.1.17
To reflect the general population of women who were eligible for the study, we accounted for the oversampling of high‐risk women in the study's enrolment by utilising frequency weights. These weights were based on the original Scandinavian Successive Small‐for‐Gestational‐Age study inclusion criteria (as noted above). Therefore, each participant who was selected through the random sample of the population counted for 10 women, each participant who smoked around the time of conception counted for two women, and each participant who had a specific risk factor for SGA counted for one woman in the descriptive analyses and our regression models.
3. RESULTS
3.1. Directed acyclic graph
Based on previous literature, the directed acyclic graph contained the following maternal factors: parity, income, education, occupation, maternal age, smoking status, previous stillbirth or neonatal death, maternal morbidity, unintended pregnancy, maternal stress, and maternal BMI. Factors were classified as those influencing the exposure (short interpregnancy interval), those influencing the outcome (poor fetal growth), and those influencing both the exposure and the outcome (see Figure 1, or Appendix A for classification assumptions). The minimally sufficient adjustment set included maternal age, parity, income, education, occupation, smoking status, previous stillbirth or neonatal death, and unintended pregnancy. These factors were therefore used as covariates in our multivariable regression models.
Figure 1.
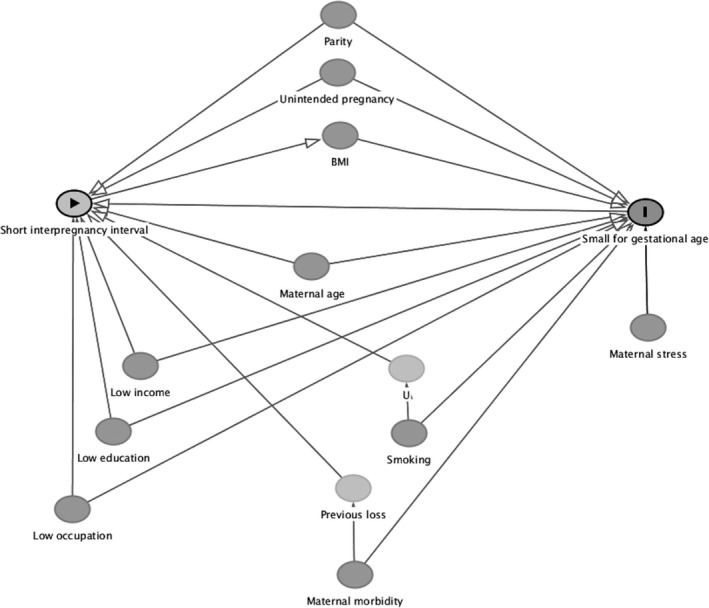
Directed acyclic graph representing the putative relationship between interpregnancy interval and SGA birth. Appendix A provides a rationale for the inclusion and inter‐relationships between variables
3.2. Description of the cohort
A total of 1945 women were followed in the original Scandinavian Successive Small‐for‐Gestational‐Age births study.10 Among these, 300 participants were excluded since their most recent pregnancy was an abortion less than 20 weeks’ gestation. A further 239 women were missing data required to calculate interpregnancy interval (n = 238) or birthweight z‐score (n = 1). This left a total of 1406 participants available for the current analyses.
Baseline characteristics are summarised in Tables 1 and 2. The mean age of participants was 25.7 years, and the majority of participants were para 1. The average gestational age at delivery was 280 days (40 weeks) and the mean birthweight was 3578 g. Forty‐three per cent of women reported smoking around the time of conception of the current pregnancy, and the proportion of those who smoked was consistent across interpregnancy interval categories. Approximately 3% of women reported a stillbirth or neonatal death in their previous pregnancy. Stillbirth or neonatal death was more common among those with short interpregnancy intervals (<12 months and 12‐17 months) compared to those with longer interpregnancy intervals.
Table 1.
Descriptive characteristics of the Scandinavian Successive Small‐For‐Gestational‐Age Birth cohort, 1986‐88: Demographics, birth outcomes, obstetrical risk factorsa
| Total N = 1406 | Interpregnancy interval, months | ||||
|---|---|---|---|---|---|
| <12 n = 197 | 12–17 n = 175 | 18–23 n = 195 | ≥24 n = 839 | ||
| Demographics | |||||
| Maternal age, mean years (SE)b | 25.7 (0.1) | 26.5 (0.4) | 25.8 (0.3) | 26.3 (0.3) | 25.4 (0.2) |
| Parity, % | |||||
| 1 | 71.8 | 71.5 | 83.3 | 86.5 | 66.0 |
| 2 | 28.1 | 28.5 | 16.7 | 13.5 | 34.0 |
| Birth Outcomes | |||||
| GA at delivery, mean days (SE) | 279.8 (0.4) | 279.6 (1.1) | 280.6 (1.1) | 280.1 (0.7) | 279.6 (0.6) |
| Birth weight, mean grams (SE) | 3577.7 (21.7) | 3587.0 (51.8) | 3552.3 (53.8) | 3673.3 (56.7) | 3559.4 (29.3) |
| Stillbirth or neonatal death, % | 2.1 | 1.0 | 0.1 | 3.1 | 2.5 |
| Obstetrical Risk factors | |||||
| Maternal smoking at the time of conception, % | |||||
| Yes | 43.3 | 42.4 | 43.3 | 38.1 | 44.7 |
| No | 56.2 | 57.4 | 56.5 | 60.6 | 55.0 |
| Missing | 0.4 | 0.3 | 0.1 | 1.4 | 0.3 |
| Stillbirth or neonatal death in the previous pregnancy, % | 3.4 | 12.8 | 4.0 | 2.3 | 1.5 |
| Was this pregnancy planned, % | |||||
| Yes | 70.7 | 43.1 | 63.5 | 74.5 | 77.4 |
| No | 18.2 | 40.3 | 17.8 | 15.3 | 14.3 |
| Missing | 11.0 | 16.6 | 18.7 | 10.2 | 8.4 |
Values are weighted to account for oversampling of higher‐risk women.
Standard error (SE) values are presented to correspond with weighted analysis.
Table 2.
Descriptive characteristics of the Scandinavian Successive Small‐For‐Gestational‐Age Birth cohort, 1986‐88: Socioeconomic factorsa
| Total N = 1406 | Interpregnancy interval, months | ||||
|---|---|---|---|---|---|
| <12 n = 197 | 12–17 n = 175 | 18–23 n = 195 | ≥24 n = 839 | ||
| Wealth | |||||
| Able to raise NOK 5000 in 1 week, % | |||||
| Yes | 8.2 | 5.8 | 9.1 | 9.8 | 8.2 |
| No | 84.3 | 82.2 | 75.5 | 85.0 | 86.5 |
| Missing | 7.5 | 11.9 | 15.4 | 5.2 | 5.3 |
| Own description of family's economic situation, % | |||||
| Very good | 7.2 | 5.8 | 6.8 | 7.5 | 7.6 |
| Good | 38.1 | 34.5 | 35.1 | 31.0 | 41.1 |
| Medium | 43.3 | 40.3 | 40.5 | 51.8 | 42.6 |
| Bad | 3.9 | 7.6 | 3.8 | 2.9 | 3.4 |
| Missing | 7.5 | 11.8 | 13.7 | 6.8 | 5.4 |
| Education | |||||
| Highest maternal education level, % | |||||
| <9 years | 0.9 | 1.9 | 0.4 | 0.1 | 1.1 |
| 9 + 1‐2 years (9‐11 years) | 42.0 | 43.2 | 36.7 | 36.3 | 44.3 |
| 9+3 years | 18.6 | 11.4 | 25.4 | 16.4 | 19.2 |
| Higher education, non‐university | 23.9 | 19.7 | 18.8 | 37.0 | 22.9 |
| Higher education, university level | 8.6 | 13.4 | 6.9 | 5.6 | 8.6 |
| Missing | 6.0 | 11.4 | 11.8 | 4.6 | 3.8 |
| Occupation | |||||
| Occupation, % | |||||
| Full time work | 26.6 | 21.7 | 21.5 | 27.6 | 28.4 |
| Part time work | 43.0 | 30.9 | 41.6 | 47.3 | 44.9 |
| Not working/ other | 23.7 | 34.5 | 24.4 | 20.3 | 22.0 |
| Missing | 6.7 | 13.0 | 12.5 | 4.8 | 4.6 |
Values are weighted to account for oversampling of higher‐risk women.
3.3. Interpregnancy interval, pregnancy intention, and socio‐economic status
Among the total cohort, 71% per cent of women reported their current pregnancy as planned. Those with shorter interpregnancy intervals had lower rates of planned pregnancy compared to those with longer intervals: 43% of pregnancies were planned among those with an interval of less than 12 months, 64% of pregnancies with an interval of 12‐17 months, 75% of pregnancies with an interval of 18‐23 months, and 77% of pregnancies with an interval of greater than or equal to 24 months (Table 1). The majority of participants reported that they would not be able to raise 5000 Norwegian kroner (NOK; approximately equivalent to 800 Canadian dollars) in 1 week, but still described their family's economic situation as “good” or “medium” (Table 2). However, among those with an interpregnancy interval of <12 months, 8% reported having a “bad” economic situation, compared to 3% among those with an interval of 18‐23 months, and 4% in the entire cohort. Approximately two‐thirds of participants reported nine to 13 years of formal education, one‐third reported more than 13 years, and less than one per cent had fewer than 9 years of formal education. This was relatively consistent across interpregnancy interval categories, although those with an interpregnancy interval of 18‐23 months had the greatest proportion of participants with more than 13 years of formal education (43%). Across interpregnancy interval categories, the majority of participants reported working outside the home either full time or part time. However, 35% of participants with an interpregnancy interval of <12 months reported not working, compared with 20% of participants with an interval of 18‐23 months, and 24% of participants in the entire cohort. In addition, bivariate analysis of demographic and socio‐economic variables by pregnancy intention showed that those who were para 2, smoking around the time of conception, and reported less advantageous socio‐economic positions were more likely to report their current pregnancy as unplanned (Table S1).
Factors which described maternal social support are summarised in Table 3. In the total cohort and across interpregnancy intervals, most participants reported being married and living with the father of the expected child. Among those who were married or living with the father of the expected child, 85% stated their partner approved of the current pregnancy, with small variations (76%‐88%) across interpregnancy intervals. In the total cohort and across interpregnancy intervals, the majority of women (>70%) reported having a person she regarded as a main support in the pregnancy, having a person that had her confidence during the pregnancy, and having a person who could give her a helping hand if needed. However, women with longer interpregnancy intervals (≥18 months) generally reported higher rates of these support variables compared to women with shorter intervals (<18 months). Across interpregnancy intervals, 55‐61% of participants reported their partner was planning to take a care leave. Although two‐thirds of women in the total cohort planned on taking a care leave themselves, the proportion of women planning to take a care leave increased with increasing interpregnancy interval.
Table 3.
Descriptive characteristics of the Scandinavian Successive Small‐For‐Gestational‐Age Birth cohort, 1986‐88: Factors influencing maternal supporta
| Total N = 1406 | Interpregnancy interval, months | ||||
|---|---|---|---|---|---|
| <12 n = 197 | 12–17 n = 175 | 18–23 n = 195 | ≥24 n = 839 | ||
| Civil Status, % | |||||
| Married | 70.7 | 65.6 | 61.3 | 76.1 | 72.7 |
| Cohabitating | 22.1 | 21.3 | 25.5 | 18.8 | 22.3 |
| Single | 1.4 | 1.7 | 1.7 | 0.5 | 1.4 |
| Missing | 5.8 | 11.4 | 11.5 | 4.5 | 3.7 |
| Person(s) the pregnant woman is living with, % | |||||
| Father of the child expected | 92.2 | 86.9 | 84.0 | 95.0 | 94.5 |
| Another man | 0.03 | 0 | 0 | 0 | 0.06 |
| Family (parents, sibling, other relatives) | 0.2 | 1.5 | 0.3 | 0 | 0.03 |
| Friends or in a collective, other | 0.2 | 0.3 | 0 | 0 | 0.3 |
| Missing | 7.3 | 11.4 | 15.7 | 5.0 | 5.1 |
| If she is married/ lives with a partner, does he approve of this pregnancy?, % | |||||
| No | 0.8 | 0.3 | 0.3 | 0.5 | 1.0 |
| Yes | 84.8 | 76.4 | 76.5 | 87.2 | 87.9 |
| Uncertain | 3.3 | 6.0 | 4.1 | 1.9 | 2.9 |
| Missing | 11.1 | 17.3 | 19.1 | 10.4 | 8.2 |
| Does she have a person she regards as a support for this pregnancy?, % | |||||
| No | 5.0 | 3.8 | 4.5 | 10.9 | 3.9 |
| Yes | 81.7 | 74.2 | 72.9 | 78.0 | 86.1 |
| Uncertain | 2.3 | 5.2 | 3.5 | 0.8 | 1.7 |
| Missing | 11.0 | 16.7 | 19.0 | 10.2 | 8.3 |
| Does any one person have her confidence?, % | |||||
| No | 0.7 | 1.6 | 0 | 1.5 | 0.5 |
| Yes | 87.2 | 78.3 | 80.9 | 86.4 | 90.6 |
| Uncertain | 1.6 | 3.2 | 0.1 | 3.5 | 1.1 |
| Missing | 10.6 | 16.9 | 19.0 | 8.6 | 7.8 |
| Does she have any one person who can give her a helping hand if needed?, % | |||||
| No | 0.9 | 1.7 | 1.4 | 1.5 | 0.4 |
| Yes | 88.0 | 83.0 | 78.0 | 89.2 | 91.0 |
| Uncertain | 0.8 | 0.3 | 1.6 | 0.4 | 0.8 |
| Missing | 10.3 | 15.0 | 19.0 | 8.9 | 7.8 |
| Does her partner plan to take a care leave?, % | |||||
| No | 20.3 | 11.8 | 20.0 | 23.2 | 21.6 |
| Yes | 58.3 | 55.3 | 50.4 | 56.8 | 61.1 |
| Don't know | 10.2 | 17.0 | 9.2 | 10.5 | 8.9 |
| Missing | 11.1 | 15.9 | 20.4 | 9.5 | 8.4 |
| Does the pregnant woman plan to take a care leave?, % | |||||
| No | 0.6 | 0.6 | 1.4 | 0 | 0.5 |
| Yes | 62.1 | 47.7 | 54.0 | 62.8 | 66.9 |
| Plan to quit working | 3.8 | 6.3 | 5.1 | 6.1 | 2.4 |
| Don't know | 2.0 | 0.4 | 0.6 | 4.0 | 2.1 |
| Missing | 31.6 | 45.0 | 39.0 | 27.1 | 28.1 |
Values are weighted to account for oversampling of higher‐risk women.
3.4. Primary outcomes
Compared to those with an interpregnancy interval of 18‐23 months, crude birthweight z‐score was −0.13 lower (95% CI −0.47 to 0.21) for those with an interpregnancy interval of <12 months; −0.25 lower (95% CI −0.55 to 0.05) with an interpregnancy interval of 12‐17 months and −0.25 lower (95% CI −0.52 to 0.02) with an interpregnancy interval of 24 months or more. When adjusted for maternal age, parity, maternal smoking, last pregnancy ending in stillbirth or neonatal death, and measures of financial means, education, and occupation, the associations between interpregnancy interval and birthweight z‐score were attenuated (adjusted birthweight z‐score differences of −0.07, −0.15, and −0.23 for <12, 12‐17, and ≥24 months, respectively). For an infant born at 40 weeks, these z‐score differences would correspond to weight differences −31.9, −68.0, and −103.8 g, respectively. Additionally adjusting for pregnancy intention did not further attenuate the z‐scores (Table 4). The crude and adjusted estimated mean birthweight z‐scores are plotted in Figure 2 and displayed in Appendix B. Sensitivity analysis using conditional birthweight z‐scores rather than birthweight z‐scores produced similar conclusions (Appendices C and D). In addition, a sensitivity analysis excluding the 19 participants who delivered prior to 33 weeks’ gestation (and therefore prior to having information on pregnancy intention collected) did not meaningfully change our conclusions.
Table 4.
Regression coefficients for birthweight z‐score by interpregnancy interval category in the Scandinavian Successive Small‐for‐Gestational‐Age Birth cohort, 1986‐88 (N = 1406)
| Interpregnancy interval, in months | Model 1: Unadjusted | Model 2a | Model 3b |
|---|---|---|---|
| Birthweight z‐score regression coefficient (95% CI) | |||
| <12 | −0.13 (−0.47, 0.21) | −0.07 (−0.46, 0.31) | −0.05 (−0.47, 0.36) |
| 12‐17 | −0.25 (−0.55, 0.05) | −0.15 (−0.46, 0.17) | −0.13 (−0.46, 0.20) |
| 18‐23 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 24 or more | −0.25 (−0.52, 0.02) | −0.23 (−0.50, 0.05) | −0.23 (−0.52, 0.05) |
Adjusted for maternal age at last delivery, parity, smoking at time of conception, last pregnancy stillbirth or neonatal death, occupation, ability to raise 5000 NOK in 1 wk, rating of own/family wealth, education level.
Adjusted for all Model 2 covariates plus pregnancy intention.
Figure 2.
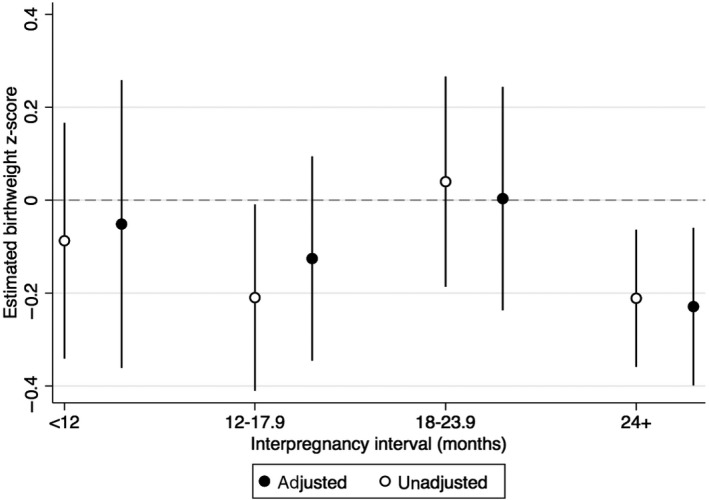
Estimated mean birthweight z‐score by interpregnancy interval category, before and after adjusting for maternal age at last delivery, parity, smoking at time of conception, last pregnancy stillbirth or neonatal death, occupation, can raise 5000 NOK in 1 wk, rating of own/family wealth, pregnancy intention
4. COMMENT
4.1. Principal findings
In this population‐based cohort of women from countries with high social support and relatively low inequality,18 we found that short interpregnancy interval was not associated with a decrease in birthweight z‐score. Adjusting for pregnancy intention, detailed socio‐economic factors and obstetrical risk factors further attenuated the risk estimates.
4.2. Strengths of the study
Our study has several strengths. First, the study was conducted in a setting where women have reasonably equitable access to health care and contraception.19 In addition, although maternal support variables were not adjusted for in our comparative analysis, our descriptive analysis indicated that levels of social support were similar across interpregnancy interval categories. Finally, we were able to account for a large number of socio‐economic and pregnancy risk factors, including pregnancy intention. Unintended pregnancy is an important potential confounder as it has been associated with both interpregnancy interval20 and small for gestational age birth.9 In addition, pregnancy intention may not be a time‐invariant confounder which can be accounted for using within‐woman analyses.21 In our study, controlling for unintended pregnancy in addition to other socio‐economic and obstetrical risk factors did not alter the association between interpregnancy interval and infant birthweight. This may be because unintended pregnancy does not significantly confound the relationship between interpregnancy interval and birthweight‐for‐gestational age in our cohort, or because the confounding effect of unintended pregnancy was already accounted for by our relatively uniform study population and ability to adjust for the other socio‐economic factors. However, unintended pregnancy is a complex construct, including both unwanted and mistimed pregnancies.22 Our measure, which asked women if their current pregnancy was planned, may not have fully captured this construct, thus potentially limiting the interpretation of our results with regard to the effect of pregnancy intention. Finally, our primary results are corroborated by the analyses using conditional birthweight z‐scores as an outcome, which may be a more accurate marker of poor fetal growth compared to measurement of birthweight alone.12
4.3. Limitations of the data
Our study also has several limitations. The study size of 1406 women may have limited our ability to detect a significant association between interpregnancy interval and poor fetal growth. However, using birthweight z‐score as a continuous variable makes type II error less likely. We excluded approximately 12% of the initial Scandinavian Successive Small‐for‐Gestational‐Age births study population due to missing interpregnancy interval. The birthweight of the current pregnancy was similar among women for whom the interpregnancy interval was available, compared to those for whom it was missing. In general, those with a missing interpregnancy interval were more likely to be missing information on other study variables such as pregnancy intention and socio‐economic characteristics. This may limit the generalisability of our results, if there were systematic differences in the association between interpregnancy interval and fetal size among those excluded due to missing data. In addition, within our included cohort we were missing data on 11% of participants regarding pregnancy intention (Table 1) and on 6%‐7.5% of participants regarding socio‐economic factors (Table 2). As a result, we cannot rule out residual confounding due to individuals with missing values for these variables. Although it is possible that the effect of a short interpregnancy interval on fetal growth may differ according to different maternal factors identified in our DAG (such as maternal age or socio‐economic status), we did not test for possible effect measure modification in our analysis as we were likely underpowered to explore this given our sample size. In addition, the extent to which the prevalence of intended pregnancies in a population is linked with differences in the association between interpregnancy interval and fetal growth is unclear and warrants further investigation. Our analysis is based on data collected between 1986 and 1988, which may limit the generalisability of our results to more contemporary populations. For example, the proportion of IPIs greater than or equal to 24 months is higher in our study compared to a recent population‐based Norwegian study using data from 2006 to 2014 (60% vs 37%, respectively),23 and we found a slightly longer mean IPI compared to a population‐based study from the United States using data from 2006 to 2010 (34 months vs 37 months, respectively).7 In addition, in our cohort, 70% of pregnancies were intended, compared to 49% of pregnancies in the United States in 2008,24 and 80% of pregnancies in a cross‐sectional European study of women attending routine prenatal care between 2008 and 2010.25 These variations may due to the inherent complexity of pregnancy intention which make it difficult to measure.22, 25 However, our results are consistent with the recent study by Class et al.,26 which performed a sensitivity analysis that did not find significant cohort effects in a Scandinavian study population ranging from 1973 to 2009. In addition, previous studies have proposed possible causal mechanisms between short interpregnancy interval and adverse pregnancy outcomes, for example that maternal nutrition remains depleted from a prior pregnancy after a short interval,27 which would not be expected to exert differential effects at the time of the original study period compared to the time of our analyses. These mechanisms, however, may suggest that our results would not be generalisable to settings in which access to adequate nutrition is limited.
4.4. Interpretation
Previous research has demonstrated conflicting results with respect to associations between interpregnancy interval and SGA birth. A systematic review and meta‐analysis by Conde‐Agudelo et al.1 found interpregnancy intervals of less than 18 months and greater than 60 months to be significantly associated with small for gestational age birth, despite adjustment for at least maternal age and one marker of socio‐economic status. However, a recent expert working group on birth spacing convened by the US office of population affairs concluded that many of the studies included in this review had serious methodological concerns.28 Several studies have since attempted to address possible confounding using within‐woman and within‐family analyses, which compare multiple interpregnancy intervals in the same woman (ie between siblings), or in the same family (ie between first cousins). This approach aims to reduce confounding by factors that tend to remain consistent within one woman or family (eg socio‐economic status). These studies found significantly attenuated or no associations between short interpregnancy interval and small for gestational age birth, suggesting that unmeasured confounding may account for the associations seen in previous studies.21, 26, 29 Comparing a traditional between‐woman analysis with a within‐woman analysis, Ball et al.21 found that long interpregnancy intervals remained associated with small for gestational age birth while short interpregnancy intervals did not, suggesting that the impact of long interpregnancy intervals may not be fully explained by confounders that remain constant within the same woman across pregnancies. These findings were supported by Class et al,26 who found that despite within‐woman analysis, within‐family analysis, and controlling for post‐birth intervals, the associations between long interpregnancy intervals and small for gestational age birth remained significant while those between short interpregnancy intervals and small for gestational age birth were fully attenuated or showed reversed associations. Similarly, Hanley et al found that although short interpregnancy intervals were associated with small for gestational age birth in traditional between‐woman analyses, this association reversed direction without statistical significance in the within‐woman analyses. In addition, they found short interpregnancy intervals to remain significantly associated with gestational diabetes and obesity at the beginning of a subsequent pregnancy, in both between‐woman and within‐woman analyses.29 This may be relevant since both gestational diabetes and obesity have been linked to large for gestational age birth.30
Within‐woman or within‐family analyses may have limited generalisability as they are restricted to women who have had discrepant birth outcomes among their second and third livebirth.21, 26 Our findings, which are estimated from a cohort derived through population‐based recruitment in three Scandinavian counties, lend support to these previous studies which challenge the causal link between short interpregnancy interval and adverse perinatal outcomes.21, 26, 29
5. CONCLUSION
Our study findings are compatible with the hypothesis that the associations between interpregnancy interval and poor fetal growth or small‐for‐gestational birth observed in previous studies may be due to uncontrolled confounding by socio‐economic and obstetrical risk factors. Our results support those found by previously published within‐woman and within‐family analyses, and may overcome some limitations regarding generalisability inherent to these methodologies. Our findings further support policy emphasis on reducing socio‐economic and obstetrical risk factors instead of solely aiming to modify short interpregnancy intervals if subsequent pregnancies are desired.
CONFLICT OF INTEREST
All authors report no conflict of interests.
DETAILS OF ETHICS APPROVAL
This study was approved by the University of British Columbia/BC Children's and Women's Hospital Research Ethics Board as a minimal risk study (REB# H17‐02410).
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Rønnaug Ødegård for her contribution to the data analysis. Collection of study data was financed by the US Eunice Kennedy Shriver National Institute of Child Health and Human Development, Contract No 1‐HD‐4‐2803. JL is supported by the University of British Columbia Clinician Investigator Program. JAH is the recipient of New Investigator Awards from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research.
Appendix A. Assumptions about direct causes for formation of the directed acyclic graph
A.1 Assumptions about direct causes of short interpregnancy interval (exposure)
| Cause | Reported association/mechanism | Mediator | Direct cause label | Mediator label | Effect |
|---|---|---|---|---|---|
| Unintended pregnancy | Unintended pregnancy has been associated with short IPI, independent of age, educational attainment and income7, 31 | NA | Unintend_preg | NA | Short_IPI |
| Low income | Low income has been associated with short IPI independent of educational attainment or age31 | NA | Low_income | NA | Short_IPI |
| Low educational attainment | Low educational attainment has been associated with short IPI31 | NA | Low_education | NA | Short_IPI |
| Low occupation class/unemployment | Low occupation class/unemployment has been associated with short IPI32 | NA | Low_occupation | NA | Short_IPI |
| Extreme maternal age | Younger15, 16, 17, 18, 19 or older (>30) maternal age has been associated with short IPI7, 32 | NA | Mat_age | NA | Short_IPI |
| Maternal morbidity | Underlying maternal factors may contribute to previous loss, which may result in short IPI33 | Previous fetal/neonatal loss | Mat_morbid | Prev_loss | Short_IPI |
| Parity | Short IPI has been associated with high parity7, 32 | NA | High_Parity | NA | Short_IPI |
| Smoking | Maternal smoking has been associated with short IPI32 | NA | Smoking | NA | Short_IPI |
A.2 Assumptions about direct causes poor fetal growth/SGA birth (outcome)
| Cause | Reported association/mechanism | Mediator | Direct cause label | Mediator label | Effect |
|---|---|---|---|---|---|
| Unintended pregnancy | Unintended pregnancy has been associated with SGA8, 9 | NA | Unintend_preg | NA | SGA |
| Smoking | Maternal smoking has been associated with SGA13 | NA | Smoking | NA | SGA |
| Low BMI | Low prepregnancy BMI has been associated with SGA13 | NA | Low_BMI | NA | SGA |
| Low parity | Low parity has been associated with SGA birth34 | NA | Low_parity | NA | SGA |
| Maternal stress | Maternal distress is associated with SGA birth35 | NA | Mat_stress | NA | SGA |
| Low educational attainment | Low educational attainment has been associated with SGA birth36 | NA | Low_education | NA | SGA |
| Low occupation class | Low occupation class has been associated with SGA birth36 | NA | Low_occupation | NA | SGA |
| Low income | Low income has been associated with SGA birth36 | NA | Low_income | NA | SGA |
| Extreme maternal age | Young (<16) and old (>35) maternal age has been associated with SGA birth13 | NA | Mat_age | NA | SGA |
| Maternal morbidity | Women with vascular morbidity (renal disease, heart disease, diabetes, hypertension) are more likely to have an SGA birth13 | NA | Mat_morbid | NA | SGA |
IPI, interpregnancy interval; SGA, SGA birth
Appendix B. Estimated birthweight z‐scores by interpregnancy interval category in the Scandinavian Successive Small‐for‐Gestational‐Age Birth cohort, 1986‐88, (N = 1406)
B.1.
| Interpregnancy interval, in months | Model 1: Unadjusted | Model 2a | Model 3b |
|---|---|---|---|
| Estimated birthweight z‐score (95% CI) | |||
| <12 | −0.09 (−0.34, 0.17) | −0.07 (−0.35, 0.21) | −0.05 (−0.36, 0.26) |
| 12‐17 | −0.21 (−0.41, −0.01) | −0.14 (−0.35, 0.07) | −0.13 (−0.35, 0.09) |
| 18‐23 | 0.04 (−0.19, 0.27) | −0.00 (−0.23, 0.24) | −0.00 (−0.24, 0.24) |
| 24 or more | −0.21 (−0.36, −0.06) | −0.22 (−0.39, −0.06) | −0.23 (−0.40, −0.06) |
Adjusted for maternal age at last delivery, parity, smoking at time of conception, last pregnancy stillbirth or neonatal death, occupation, ability to raise 5000 NOK in 1 wk, rating of own/family wealth, education level.
Adjusted for all Model 2 covariates plus pregnancy intention.
Appendix C. Regression coefficients for birthweight z‐score (conditional on 25‐wk ultrasound estimated fetal weight) by interpregnancy interval category in the Scandinavian Successive Small‐for‐Gestational‐Age Birth cohort, 1986‐88 (N = 1200), reference category 18‐23.9 mo
C.1.
| Interpregnancy interval, in months | Model 1: Unadjusted | Model 2a | Model 3b |
|---|---|---|---|
| Conditional birthweight z‐score regression coefficient (95% CI) | |||
| <12 | −0.19 (−0.54, 0.17) | −0.24 (−0.59, 0.12) | −0.20 (−0.59, 0.18) |
| 12‐17 | −0.25 (−0.60, 0.11) | −0.23 (−0.58, 0.11) | −0.23 (−0.57, 0.12) |
| 24 or more | −0.24 (−0.51, 0.04) | −0.19 (−0.47, 0.08) | −0.19 (−0.49, 0.07) |
Adjusted for maternal age at last delivery, parity, smoking at time of conception, last pregnancy stillbirth or neonatal death, occupation, ability to raise 5000 NOK in 1 wk, rating of own/family wealth, education level.
Adjusted for all Model 2 covariates plus pregnancy intention.
Appendix D. Regression coefficients for birthweight z‐score by interpregnancy interval category (conditional on 33‐wk ultrasound estimated fetal weight) in the Scandinavian Successive Small‐for‐Gestational‐Age Birth cohort, 1986‐88 (N = 1222), reference category 18‐23 mo
D.1.
| Interpregnancy interval, months | Model 1: Unadjusted | Model 2a | Model 3b |
|---|---|---|---|
| Conditional birthweight z‐score regression coefficient (95% CI) | |||
| <12 | −0.17 (−0.49, 0.15) | −0.21 (−0.54, 0.12) | −0.21 (−0.55, 0.14) |
| 12‐17 | −0.28 (−0.60, 0.03) | −0.29 (−0.61, 0.02) | −0.29 (−0.60, 0.02) |
| 24 or more | −0.27 (−0.52, −0.02) | −0.28 (−0.54, 0.01) | −0.28 (−0.54, 0.02) |
Adjusted for maternal age at last delivery, parity, smoking at time of conception, last pregnancy stillbirth or neonatal death, occupation, ability to raise 5000 NOK in 1 wk, rating of own/family wealth, education level.
Adjusted for all Model 2 covariates plus pregnancy intention.
Liauw J, Jacobsen GW, Larose TL, Hutcheon JA. Short interpregnancy interval and poor fetal growth: Evaluating the role of pregnancy intention. Paediatr Perinat Epidemiol. 2019;33:O73–O85. 10.1111/ppe.12506
References
- 1. Conde‐Agudelo A, Rosas‐Bermudez A, Kafury‐Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta‐analysis. JAMA. 2006;295:1809‐1823. [DOI] [PubMed] [Google Scholar]
- 2. Wendt A, Gibbs CM, Peters S, Hogue CJ. Impact of increasing inter‐pregnancy interval on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:239‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Report of a WHO Technical Consultation on Birth Spacing [Internet]. Geneva: World Health Organization; 2007. [updated 15/06/2007; cited 01/09/2017]. Available from: http://apps.who.int/iris/bitstream/10665/69855/1/WHO_RHR_07.1_eng.pdf.
- 4. Healthy People 2020: Family Planning [Internet]. Washington: US Department of Health and Human Services; 2017 [updated Sept 1, 2017; cited September 1, 2017]. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/family-planning/objectives.
- 5. Klebanoff MA. Interpregnancy interval and pregnancy outcomes: causal or not? Obstet Gynecol. 2017;129:405‐407. [DOI] [PubMed] [Google Scholar]
- 6. Ahrens KA, Nelson HD, Stidd R, Moskosky S, Hutcheon JA. Short interpregnancy intervals and adverse perinatal outcomes in high‐resource settings: an updated systematic review. Paediatr Perinat Epidemiol. 2018, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122:64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah PS, Balkhair T, Ohlsson A, Beyene J, Scott F, Frick C. Intention to become pregnant and low birth weight and preterm birth: a systematic review. Matern Child Health J. 2011;15:205‐216. [DOI] [PubMed] [Google Scholar]
- 9. Mohllajee AP, Curtis KM, Morrow B, Marchbanks PA. Pregnancy intention and its relationship to birth and maternal outcomes. Obstet Gynecol. 2007;109:678‐686. [DOI] [PubMed] [Google Scholar]
- 10. Bakketeig LS, Jacobsen G, Hoffman HJ, et al. Pre‐pregnancy risk factors of small‐for‐gestational age births among parous women in Scandinavia. Acta Obstet Gynecol Scand. 1993;72:273‐279. [DOI] [PubMed] [Google Scholar]
- 11. Patrick R. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;14:1417‐1436. [DOI] [PubMed] [Google Scholar]
- 12. Hutcheon JA, Jacobsen GW, Kramer MS, Martinussen M, Platt RW. Small size at birth or abnormal intrauterine growth trajectory: which matters more for child growth? Am J Epidemiol. 2016;183:1107‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lausman A, Kingdom J, Maternal Fetal Medicine Committee , et al. Intrauterine growth restriction: screening, diagnosis, and management. J Obstet Gynaecol Can. 2013;35:741‐748. [DOI] [PubMed] [Google Scholar]
- 14. Gordijn SJ, Beune IM, Ganzevoort W. Building consensus and standards in fetal growth restriction studies. Best Pract Res Clin Obstet Gynaecol. 2018;49:117‐126. [DOI] [PubMed] [Google Scholar]
- 15. Morgan SL, ed. Handbook of Causal Analysis for Social Research, 1st edn Dordrecht: Springer; 2013. [Google Scholar]
- 16. Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. [DOI] [PubMed] [Google Scholar]
- 17. StataCorp . Stata Statistical Software: Release 15. College Station, TX: StatCorp LLC; 2017. [Google Scholar]
- 18. GINI index (World Bank estimate) [Internet].: The World Bank Group; 2018 [cited April 10, 2018]. Available from: https://data.worldbank.org/indicator/SI.POV.GINI?locations.
- 19. Hognert H, Skjeldestad FE, Gemzell‐Danielsson K, et al. High birth rates despite easy access to contraception and abortion: a cross‐sectional study. Acta Obstet Gynecol Scand. 2017;96:1414‐1422. [DOI] [PubMed] [Google Scholar]
- 20. Ahrens KA, Thoma M, Copen C, Frederiksen B, Decker E, Moskosky S. Unintended pregnancy and interpregnancy interval by maternal age, national survey of family growth. Contraception. 2018;98:52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ball SJ, Pereira G, Jacoby P, de Klerk N, Stanley FJ. Re‐evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ. 2014;349:g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santelli J, Rochat R, Hatfield‐Timajchy K, et al. The measurement and meaning of unintended pregnancy. Perspect Sex Reprod Health. 2003;35:94‐101. [DOI] [PubMed] [Google Scholar]
- 23. Sorbye LM, Skjaerven R, Klungsoyr K, Morken NH. Gestational diabetes mellitus and interpregnancy weight change: a population‐based cohort study. PLoS Med. 2017;14:e1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001‐2008. Am J Public Health. 2014;104:S43‐S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lukasse M, Laanpere M, Karro H, et al. Pregnancy intendedness and the association with physical, sexual and emotional abuse ‐ a European multi‐country cross‐sectional study. BMC Pregnancy Childbirth. 2015;15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Class QA, Rickert ME, Oberg AS, et al. Within‐family analysis of interpregnancy interval and adverse birth outcomes. Obstet Gynecol. 2017;130:1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conde‐Agudelo A, Rosas‐Bermudez A, Castano F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43:93‐114. [DOI] [PubMed] [Google Scholar]
- 28. Ahrens KA, Hutcheon JA, Ananth CV, et al. Report of the office of population affairs’ expert work group meeting on short birth spacing and adverse pregnancy outcomes: methodological quality of existing studies and future directions for research. Paediatr Perinat Epidemiol. 2018, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;129:408‐415. [DOI] [PubMed] [Google Scholar]
- 30. Davies GAL, Maxwell C, McLeod L. Maternal fetal medicine committee, clinical practice obstetrics. Obesity in pregnancy. J Obstet Gynaecol Can. 2010;32:165‐173. [DOI] [PubMed] [Google Scholar]
- 31. Masinter LM, Dina B, Kjerulff K, Feinglass J. Short interpregnancy intervals: results from the first baby study. Womens Health Issues. 2017;27:426‐433. [DOI] [PubMed] [Google Scholar]
- 32. Kaharuza FM, Sabroe S, Basso O. Choice and chance: determinants of short interpregnancy intervals in Denmark. Acta Obstet Gynecol Scand. 2001;80:532‐538. [PubMed] [Google Scholar]
- 33. Erickson JD, Bjerkedal T. Interpregnancy interval. Association with birth weight, stillbirth, and neonatal death. J Epidemiol Community Health. 1978;32:124‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah PS, Knowledge synthesis group on determinants of LBW/PT births . Parity and low birth weight and preterm birth: a systematic review and meta‐analyses. Acta Obstet Gynecol Scand. 2010;89:862‐875. [DOI] [PubMed] [Google Scholar]
- 35. Khashan AS, Everard C, McCowan LM, et al. Second‐trimester maternal distress increases the risk of small for gestational age. Psychol Med. 2014;44:2799‐2810. [DOI] [PubMed] [Google Scholar]
- 36. Kramer MS, Seguin L, Lydon J, Goulet L. Socio‐economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14:194‐210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


