Summary
Objective
Epilepsy is a progressive neurological disease characterized by recurrent seizures and behavioral comorbidities. We investigated the antiseizure effect of cannabidiol (CBD) in a battery of acute seizure models. Additionally, we defined the disease‐modifying potential of chronic oral administration of CBD on associated comorbidities in the reduced intensity status epilepticus–spontaneous recurrent seizures (RISE‐SRS) model of temporal lobe epilepsy (TLE).
Methods
We evaluated the acute antiseizure effect of CBD in the maximal electroshock seizure, 6‐Hz psychomotor seizure, and pentylenetetrazol acute seizure tests, as well as the corneal kindling model of chronic seizures in mice following intraperitoneal administration. Median effective or behavioral toxic dose was determined in both mice and rats. Next, we tested an intravenous preparation of CBD (10 mg/kg single dose) in a rat model of pilocarpine‐induced status epilepticus. We defined the effect of chronic CBD administration (200 mg/kg orally) on spontaneous seizures, motor control, gait, and memory function in the rat RISE‐SRS model of TLE.
Results
CBD was effective in a battery of acute seizure models in both mice and rats following intraperitoneal administration. In the pilocarpine‐induced status epilepticus rat model, CBD attenuated maximum seizure severity following intravenous administration, further demonstrating CBD's acute antiseizure efficacy in this rat model. We established that oral CBD attenuated the time‐dependent increase in seizure burden and improved TLE‐associated motor comorbidities of epileptic rats in the RISE‐SRS model without affecting gait. Chronic administration of CBD after the onset of SRS ameliorated reference memory and working memory errors of epileptic animals in a spatial learning and memory task.
Significance
The present study illustrates that CBD is a well‐tolerated and effective antiseizure agent and illustrates a potential disease‐modifying effect of CBD on reducing both seizure burden and associated comorbidities well after the onset of symptomatic seizures in a model of TLE.
Keywords: cannabinoids, gait, maximal electroshock, memory, motor function, pilocarpine
1.
Key Points.
CBD is effective in a battery of acute seizure models following intraperitoneal administration
CBD is effective in attenuating maximum seizure severity following intravenous administration in rats
Oral CBD can attenuate time‐dependent increase in seizure burden and motor comorbidities in a rat model of TLE
Oral CBD can reverse epilepsy‐induced cognitive deficits in a rat model of TLE
This is the first study to demonstrate the disease‐modifying effect of CBD on spontaneous recurrent seizure and associated comorbidities
2. INTRODUCTION
Epilepsy is a progressive, chronic neurological disorder characterized by recurrent seizures.1 Approximately 65 million people worldwide live with epilepsy, of whom ~30% are considered pharmacoresistant to the currently available antiseizure drugs (ASDs).2 Seizures, although the primary symptom, are not the only aspect of epilepsy that affects a patient's quality of life. Several comorbidities (eg, depression, anxiety, motor disorder, cognitive deficits, social dysfunction) also contribute to a reduced quality of life in addition to the poor prognosis associated with the disease.3 Furthermore, currently available ASDs are also known to produce a variety of cognitive, psychiatric, and motor adverse effects4, 5; thus, therapies that do not carry the potential to increase the adverse effects liability are in significant clinical demand.
Cannabis has been used since prehistoric times to treat several diseases, including epilepsy.6 However, the therapeutic benefits of whole cannabis are overshadowed by its psychoactive effects,7 which have limited its clinical use. More than 100 phytocannabinoids have been isolated, of which Δ9‐tetrahydrocannabinol, cannabidiol (CBD), and cannabidivarin are considered most relevant in the treatment of epilepsy; however, Δ9‐tetrahydrocannabinol has poor clinical potential due to its psychoactive and potential proconvulsive properties, which may limit chronic use.1, 6 CBD has been shown to have antiseizure activity in several animal models.6, 8, 9, 10 Given its anticonvulsant efficacy in phase 3 clinical trials, the US Food and Drug Administration (FDA) in 2018 approved CBD (Epidiolex; GW Research) as a drug for the treatment of seizures associated with Dravet syndrome or Lennox‐Gastaut syndrome in patients 2 years of age and older.11, 12, 13
Here, we initially tested CBD in a battery of well‐established preclinical seizure and epilepsy models following intraperitoneal administration to define CBD's pharmacological profile and differentiate it from other ASD standards of care. Second, we investigated the efficacy of intravenous (IV) pretreatment with CBD in the rat pilocarpine‐induced status epilepticus (SE) model. SE is one of the most common medical emergencies in patients with epilepsy, and is clinically defined as a seizure lasting >5 minutes or repetitive seizures within this time frame without regaining consciousness.14 The pilocarpine‐induced SE model is typically sensitive to most ASDs when they are administered prior to or commensurate with SE onset; nonetheless, this model is useful to interrogate pharmacological efficacy in a severe seizure model. Finally, there is little information on the potential of CBD to modify epilepsy‐related behavioral comorbidities despite its preclinical and clinical ability to provide acute seizure control.6, 8, 9, 10, 11, 13 Furthermore, no preclinical study has yet shown that sustained exposure to CBD not only reduces seizure burden in a temporal lobe epilepsy (TLE) model but can also attenuate the severity of the associated behavioral comorbidities. Therefore, as a final step we assessed the effects of chronic oral administration of CBD on spontaneous seizures and associated behavioral comorbidities in the newly developed reduced intensity SE–induced spontaneous recurrent seizures (RISE‐SRS) model.15 The RISE‐SRS model exhibits a similar disease progression and pathology as the traditional post‐SE rat models, but with reduced mortality during the immediate post‐SE period. This model provides a platform for the conduct of long‐duration disease modification studies with candidate investigational therapies. Of note, the present study utilized a clinically relevant treatment design, as animals were only enrolled to receive CBD well after the onset of SRS. The results of the present study indicate that CBD exerts acute antiseizure efficacy by multiple routes of administration in several well‐validated preclinical seizure and epilepsy models with a preclinical profile that is different from other prototype ASDs.16, 17 Moreover, this present study provides the first demonstration in a preclinical model of TLE to suggest that CBD may exert potential disease‐modifying effects on SRS and attendant behavioral comorbidities.
3. MATERIALS AND METHODS
All materials and methods are described in online Appendix S1.
4. RESULTS
4.1. CBD demonstrates acute antiseizure efficacy following intraperitoneal administration in a battery of well‐established acute rodent seizure models
CBD (intraperitoneal [IP]) was initially evaluated for acute antiseizure efficacy in a battery of well‐defined rodent seizure and epilepsy models (Table 1). These models have formed the basis for ASD discovery for decades17, 18 and were thus employed to initially define the acute antiseizure efficacy of CBD relative to standard ASDs.17 For the purposes of the present study, we have also included the efficacy data for the ASDs phenobarbital (PB), valproic acid (VPA), and felbamate (FBM) as reported in the National Institute of Neurological Disorders and Stroke public database PANAChE (Table 1). The rotarod test was used to determine the potential for CBD to induce minimal motor impairment in mice and to calculate a median behaviorally impairing dose (TD50). Male mice were found to be impaired in their ability to perform on the rotorod test at a TD50 of 272 mg/kg (95% confidence interval [CI] = 241‐303) when it was administered IP 1 hour prior to testing. In the antiseizure tests in mice, IP administration of CBD prior to electrical stimulation was found to block tonic extension seizures induced by maximal electroshock seizure (MES) in male mice with a median effective dose (ED50) of 80.0 mg/kg (95% CI = 65.5‐96.0), yielding a protective index (PI; TD50/ED50) of 3.4. CBD was also effective in male mice against clonic seizures induced by subcutaneous administration of pentylenetetrazol when administered 1 hour prior to testing (ED50 = 120 mg/kg IP, PI = 2.3). CBD was found to protect male mice against the 6‐Hz partial psychomotor seizure at two different currents following IP administration. With a 32‐mA stimulation delivered 1 hour after drug administration, CBD had an ED50 of 144 mg/kg, yielding a PI for this test of 1.9. Importantly, CBD was also found to be effective and retained its potency at the 44‐mA stimulation current in the 6‐Hz test when administered 1 hour prior to electrical stimulation; ED50 at this current and time point of 173 mg/kg (PI = 1.6). Additionally, in male corneal kindled mice, the ED50 of CBD was determined to be 144 mg/kg (PI = 1.9). Thus, CBD demonstrates broad antiseizure efficacy in several well‐validated mouse models of acute (MES, subcutaneous pentylenetetrazol, 6 Hz) and chronic (corneal kindled) seizures at doses well below the motor‐impairing dose.
Table 1.
Effect of CBD, PB, VPA, and FBM in acute mouse and rat seizure models
| Antiseizure Test | CBD ED50, mg/kg IP (95% CI) PI | CBD TPE, h | PB ED50, mg/kg IP (95% CI)a PI | PB TPE, h | VPA ED50 mg/kg IP (95% CI)a PI | VPA TPE, h | FBM ED50 mg/kg IP (95% CI)a PI | FBM TPE, h |
|---|---|---|---|---|---|---|---|---|
| Maximal electroshock, mouse | 80 (65.5‐96.0) 3.4 | 1 | 11.3 (9.39‐13.7) 4.0 | 2 | 213 (138‐274) 1.8 | 0.25 | 49.3 (39.8‐78.3) 9.1 | 1 |
| Subcutaneous pentylenetetrazol, mouse | 120 (98.5‐146) 2.3 | 1 | 13.9 (12.1‐16.0) 3.3 | 0.5 | 305 (212‐403) 1.3 | 0.25 | 219 (150‐331) 2.1 | 1 |
| 6 Hz, 32 mA, mouse | 144 (102‐194) 1.9 | 1 | 14.8 (8.92‐23.9) 3.1 | 0.5 | 139 (92.4‐197) 2.8 | 0.25 | 72.9 (55.3‐89.6) 6.2 | 1 |
| 6 Hz, 44 mA, mouse | 173 (136‐213) 1.6 | 1 | No public data available | 289 (242‐384) 1.3 | 0.25 | 97.5 (79.3‐122) 4.6 | 1 | |
| Corneal kindled mouse | 115 (77.5‐169) 2.4 | 1 | 9.42 (7.91‐17.0) 4.8 | 0.5 | 174 (135‐208) 2.2 | 0.25 | No public data available | |
| Minimal motor impairment, TD50, mouse rotarod | 272 (241‐303) | 2 | 45.5 (41.8‐49.3) | 0.25 | 390 (382‐396) | 0.25 | 452 (363‐563) | 1 |
| Maximal electroshock, rat | 53.2 (39.1‐67.0) 9.4 | 2 | 2.61 (1.70‐4.04) 15.7 | 2 | 212 (167‐256) 2.2 | 0.25 | 35.0 (21.9‐52.3) 16.4 | 0.5 |
| Minimal motor impairment, TD50, rat open field analysis | > 500 (ND) | ND | 41.2 (37.0‐46.6) | 0.5 | 470 (431‐497) | 0.5 | 573 (285‐886) | 1 |
| Maximal electroshock, formulation vehicle, mice | 0/4 protected | 1 | Methylcellulose; no public data available | Methylcellulose; no public data available | Methylcellulose; no public data available | |||
| 6 Hz, 32 mA, formulation vehicle, mice | 0/4 protected | 1 | Methylcellulose; no public data available | Methylcellulose; no public data available | Methylcellulose; no public data available | |||
| Maximal electroshock, formulation vehicle, rats | 0/4 protected | 1 | Methylcellulose; no public data available | Methylcellulose; no public data available | Methylcellulose; no public data available | |||
CBD, cannabidiol; CI, confidence interval; ED50, median effective dose; FBM, felbamate; IP, intraperitoneal; ND, not done; PB, phenobarbital; PI, protective index; TD50, median behaviorally impairing dose; TPE, time to peak effect; VPA, valproic acid.
Data are from the National Institute of Neurological Disorders and Stroke PANAChE database (https://panache.ninds.nih.gov/ChemDetail.aspx?CHEM_ID=2&TEST_NO=7B#seereport).
In male rats, visual evaluation of minimal motor impairment was scored by a trained investigator to determine the adverse effects of CBD on behavioral performance. Naive male rats were not found to be impaired on this test following administration of CBD doses up to 500 mg/kg; thus, a TD50 was determined to exceed 500 mg/kg (IP). In the antiseizure tests in rats, IP administration of CBD 2 hours prior to electrical stimulation was found to block tonic extension seizures induced by MES with an ED50 of 53.2 mg/kg, yielding a PI of >9.4. Thus, as demonstrated in male mice, CBD was effective in the rat MES test at a dose well below the motor‐impairing dose. Based on this demonstration of efficacy and tolerability in several well‐established acute seizure models in rats and mice, CBD was further evaluated in the pilocarpine‐SE model and an etiologically relevant rat model of post‐SE TLE.
4.2. IV administration of CBD reduces severity of pilocarpine‐induced SE
The pilocarpine‐induced SE model is a well‐established model of severe generalized seizures that is often used in the pursuit of novel ASDs. Based on the acute efficacy of CBD in the rat MES test following IP administration (Table 1), CBD was administered by the IV route 1 hour prior to the onset of SE to determine any potential for effect on maximal SE severity. This dose of CBD was found to significantly attenuate the maximum seizure severity (MSS; P < 0.05) compared to the vehicle‐treated group (Figure 1A). To demonstrate the validity of the protocol and tractability of the model, we also evaluated the efficacy of a supratherapeutic dose of PB (30 mg/kg IV). Administration of PB 30 minutes prior to SE onset significantly reduced the MSS (P < 0.0001) relative to the vehicle‐treated group (Figure 1B). It should be emphasized that the doses of CBD versus PB tested in this model were not pharmacologically equivalent (Table 1), as the IV dose of CBD was threefold lower than the rat MES ED50 (IP), whereas the dose of PB was 15‐fold higher than the rat MES ED50 (IP). Of note, no mortality was observed in any of the experimental animals during the behavioral seizure monitoring period. Thus, CBD pretreatment was found to acutely attenuate MSS in this rat model of SE to a degree consistent with PB.
Figure 1.
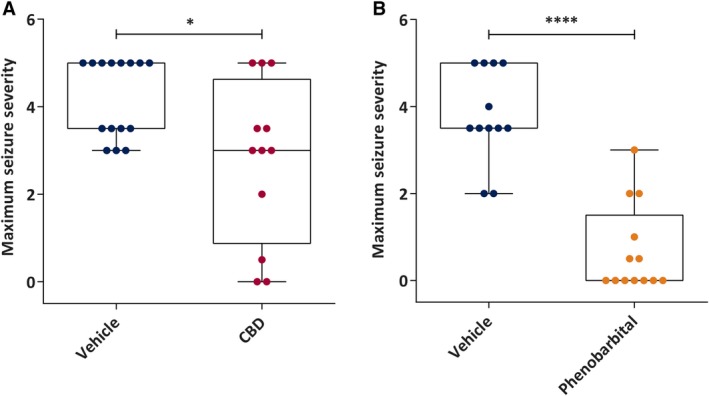
Acute administration of subtherapeutic doses of cannabidiol (CBD) attenuates maximum seizure severity to a similar degree as supratherapeutic doses of the prototype antiseizure drug, phenobarbital (PB), in the pilocarpine‐induced status epilepticus rat model. A, Median maximum seizure severity of vehicle (n = 15) and CBD (n = 12, 10 mg/kg intravenous [IV]). CBD or vehicle was administered to rats 1 hour prior to systemic administration of the chemoconvulsant pilocarpine. CBD‐treated rats demonstrated significantly reduced maximum seizure severity compared to vehicle‐treated rats. B, Median maximum seizure severity of PB (n = 13, 30 mg/kg IV) and its vehicle (n = 12). PB or vehicle was administered to rats 1 hour prior to systemic administration of the chemoconvulsant pilocarpine. PB‐treated rats demonstrated significantly reduced maximum seizure severity compared to vehicle‐treated rats (raw maximum seizure severity data), confirming the therapeutic responsiveness of the model. Data are expressed as median, minimum to maximum, and interquartile range. Data were analyzed by Mann‐Whitney test, *P < 0.05, ****P < 0.0001
4.3. Chronic oral administration of CBD attenuates seizure burden in the RISE‐SRS model of TLE
4.3.1. Chronic oral administration of CBD reduces long‐term seizure burden
To evaluate the effect of chronic CBD administration on disease progression, we examined the effect of treatment upon seizure burden and seizure burden ratio of rats displaying chronic epilepsy. The seizure burden ratio was calculated from the seizure burden in each recording session by using the following formula: seizure burden ratio = (mean seizure burden in final bin) / (mean seizure burden in first bin). A higher seizure burden ratio therefore indicates a worsening of the disease over time (Figure 2B and 2C), as is typical of post‐SE rat models of TLE.15, 19 The seizure burden ratio was significantly greater in vehicle‐treated epileptic animals compared to CBD‐treated epileptic animals (n = 10 per group; Mann‐Whitney test, U = 22, P < 0.05; Figure 2B). Moreover, disease severity was improved (seizure burden ratio < 1) significantly in 70% of the CBD‐treated animals, in contrast to only 10% of vehicle‐treated rats (Fisher's exact test, P < 0.05; Figure 2C). CBD‐treated epileptic rats did not demonstrate a time‐dependent increase in seizure burden (Wilcoxon matched‐pairs test, W = −31, P = 0.13; Figure 2E) observed by a change of median value from 28.50 (interquartile range [IQR] = 26.81‐39.75) to 24.75 (IQR = 19.69‐35.94), whereas vehicle‐treated rats showed a robust increase in seizure burden from a median value of 25.75 (IQR = 18.94‐39.34) to 30.13 (IQR = 21.00‐49.91) in this same time period (Wilcoxon matched‐pairs test, W = 53, P < 0.01; Figure 2D).
Figure 2.
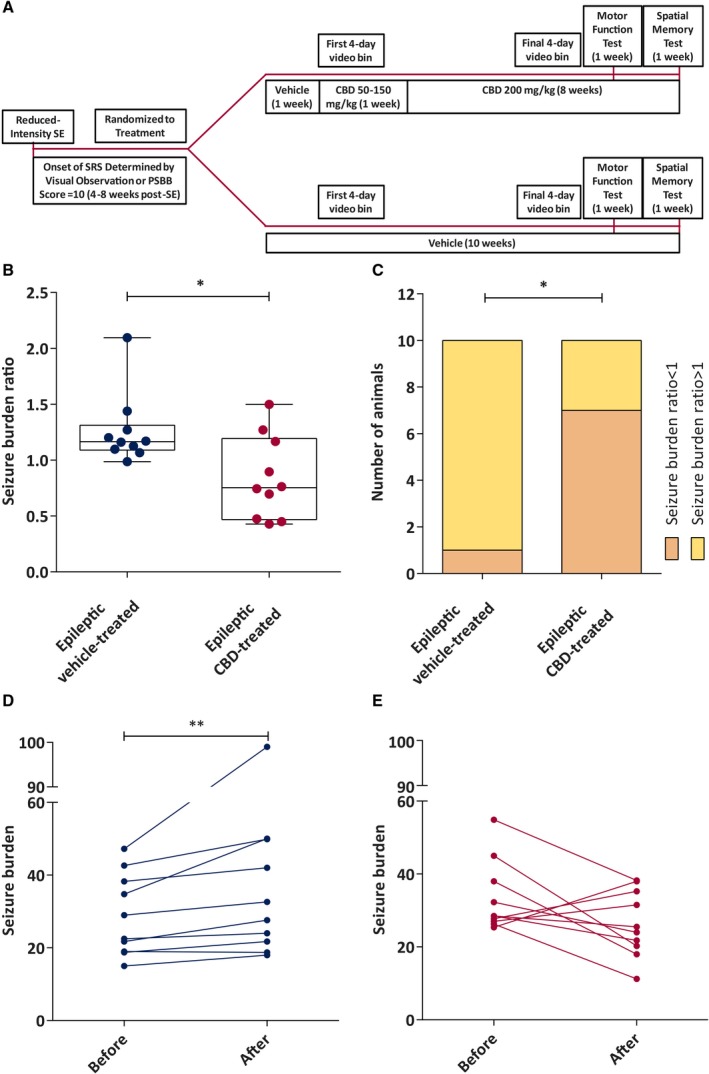
Chronic administration of cannabidiol (CBD) modifies the overall spontaneous seizure burden ratio and seizure burden in the reduced intensity status epilepticus (SE)–induced spontaneous recurrent seizures (SRS) rat model of chronic temporal lobe epilepsy up to 7 weeks postinsult. A, Study time line for the epileptic animals. PSBB, postseizure behavioral battery. B, Median seizure burden ratio of epileptic vehicle‐treated and epileptic CBD‐treated animals recorded during a 7‐week continuous video monitoring period. CBD significantly reduced the seizure burden ratio compared to the vehicle‐treated counterpart (n = 10/group; data were analyzed by Mann‐Whitney test, *P < 0.05). Data are expressed as median, minimum to maximum, and interquartile range. C, Number of epileptic vehicle‐treated and epileptic CBD‐treated animals with seizure burden ratio < or > 1 (data were analyzed by Fisher's exact test, *P < 0.05). D, Median seizure burden before and after vehicle treatment. Seizure burden was increased significantly in this group at the end of the observation period (n = 10; data were analyzed by Wilcoxon matched‐pairs signed‐rank test, **P < 0.01). E, Median seizure burden before and after CBD treatment. CBD‐treated rats showed no change in seizure burden (n = 10; data were analyzed by Wilcoxon matched‐pairs signed‐rank test)
4.3.2. Chronic oral administration of CBD to RISE‐SRS rats attenuates the severity of behavioral comorbidities of TLE
Motor coordination
Motor coordination was tested with the accelerating rotarod test. A significant difference in time spent on the accelerating rod was observed among the groups (one‐way analysis of variance [ANOVA], F 2, 27 = 5.996, P < 0.01). The Holm‐Sidak post hoc test revealed that naive vehicle‐treated (P < 0.05) and epileptic CBD‐treated (P < 0.01) animals spent significantly more time on the rod, compared to the epileptic vehicle‐treated group. This motor test indicates that chronic oral CBD administration attenuated the TLE‐induced motor dysfunction (Figure 3A).
Figure 3.
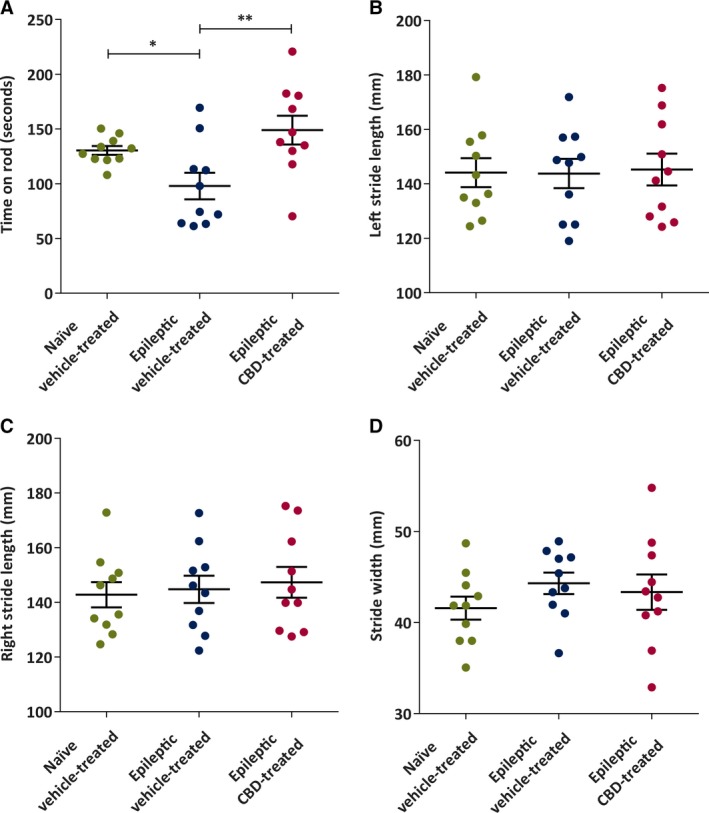
Chronic administration of cannabidiol (CBD) to spontaneously epileptic rats improves some indices of motor coordination 6 weeks after status epilepticus. A, Mean time (in seconds) spent on accelerated rotarod. The naive vehicle‐treated and epileptic CBD‐treated groups spent significantly more time on the accelerating rotarod compared to the epileptic vehicle‐treated group (n = 10/group). B, There were no significant differences in mean left stride length (in millimeters) of naive vehicle‐treated, epileptic vehicle‐treated, and epileptic CBD‐treated rats (n = 10/group). C, There were no significant differences in mean right stride length (in millimeters) between naive vehicle‐treated, epileptic vehicle‐treated, and epileptic CBD‐treated rats (n = 10/group). D, There were no significant differences in mean stride width (in millimeters) of naive vehicle‐treated, epileptic vehicle‐treated, and epileptic CBD‐treated rats (n = 10/group). Data are expressed as mean ± SEM. Data were analyzed by one‐way analysis of variance with Holm‐Sidak post hoc test, *P < 0.05, **P < 0.01
Gait
The gait test was performed to assess left stride length, right stride length, and stride width of the animals. No significant differences among the groups were observed for any of these parameters (one‐way ANOVA; for left stride length, F 2, 27 = 0.019; for right stride length, F 2, 27 = 0.198; for stride width, F 2, 27 = 0.842; Figure 3B‐D). Furthermore, as animals were actively on CBD or vehicle therapy during this motor performance test, this study demonstrates that the dose of CBD necessary to reduce seizure burden was not associated with motor‐impairing effects in rats with SRS.
Cognitive function
To assess the effect of CBD on cognitive performance, epileptic animals were challenged in the hole board test of reference and working memory. In this study, an age‐matched cohort of nonepileptic, naive rats was also included to determine whether chronic CBD administration could normalize behavioral performance. Chronic oral administration of CBD to epileptic rats was found to significantly reduce the number of reference memory errors (RMEs) and working memory errors (Figure 4). A significant difference in RMEs was observed among the groups (one‐way ANOVA, F 2, 42 = 15.06, P < 0.0001). Here, the naive vehicle‐treated (P < 0.0001) and epileptic CBD‐treated (P < 0.05) rats made significantly fewer RMEs (Figure 4A) compared to the epileptic vehicle‐treated rats (Holm‐Sidak post hoc test). However, the CBD‐treated epileptic rats made significantly more RMEs when compared to the naive vehicle‐treated group (Holm‐Sidak post hoc test, P < 0.05). Therefore, CBD failed to completely restore the reference memory in epileptic animals to the extent of their naive, nonepileptic controls. Working memory was similarly affected (one‐way ANOVA, F 2, 42 = 35.72, P < 0.0001; Figure 4B), with the epileptic vehicle‐treated group demonstrating significantly more errors compared to the naive vehicle‐treated group (Holm‐Sidak post hoc test, P < 0.0001). Importantly, chronic CBD‐treated rats demonstrated significantly fewer working memory errors compared to epileptic vehicle‐treated animals (Holm‐Sidak post hoc test, P < 0.0001). Interestingly, working memory performance for CBD‐treated epileptic rats was superior to the naive vehicle‐treated group (Holm‐Sidak post hoc test, P < 0.05).
Figure 4.
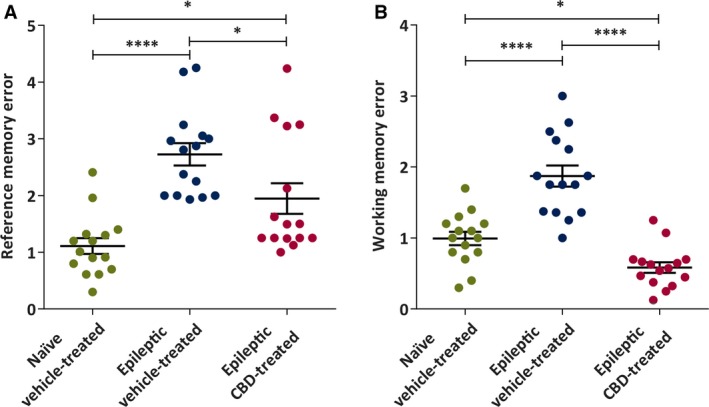
Chronic administration of cannabidiol (CBD) to reduced intensity status epilepticus–induced spontaneous recurrent seizures rats reduces onset of behavioral comorbidities of epilepsy in the hole‐board task of spatial memory. A, Mean reference memory errors in naive vehicle‐treated, epileptic vehicle‐treated, and epileptic CBD‐treated groups (n = 15 mean trial/group). CBD significantly improved reference memory error compared to the vehicle‐treated epileptic group. B, Mean working memory errors. CBD significantly improved working memory error compared to the naive vehicle‐treated and epileptic vehicle‐treated groups. Data are expressed as mean ± SEM. Data were analyzed by one‐way analysis of variance with Holm‐Sidak multiple comparison test, *P < 0.05, ****P < 0.0001
5. DISCUSSION
In the present study, we have demonstrated the acute antiseizure efficacy of CBD in a battery of well‐defined and established acute seizure tests in both mice and rats following IP administration. Our findings are consistent with other reports of the acute antiseizure efficacy of CBD in numerous preclinical seizure models.10, 20 We have also demonstrated that acute IV administration of a subtherapeutic dose of CBD is able to significantly attenuate the MSS in the rat pilocarpine‐induced SE model to a similar degree as a supratherapeutic dose of the prototype ASD, PB. Furthermore, we have demonstrated for the first time that chronic oral administration of CBD improves seizure burden ratio, motor comorbidities, and cognitive function in the RISE‐SRS model of TLE in rats well after the onset of symptomatic seizures. Altogether, the present study further supports a growing body of evidence to demonstrate that CBD is a well‐tolerated and effective therapy for acute and chronic seizures,11 as well as now demonstrating the potential that chronic oral administration of CBD may confer disease‐modifying effects in an etiologically relevant preclinical model of TLE.
All of the FDA‐approved ASDs exhibit acute antiseizure efficacy in one or more of the acute seizure and epilepsy models presently used for the evaluation of CBD.16, 21 However, many of the FDA‐approved ASDs are associated with significant motor adverse effects that may limit clinical utility. In this regard, we presently provide pharmacological data that suggest differentiation between CBD and the FDA‐approved ASD, PB (Table 1). Of note, the activity profile of CBD in these acute seizure models is generally comparable to PB (Table 1). CBD is thus differentiated from numerous ASDs with broad‐spectrum efficacy, including another broad‐spectrum ASD, VPA (Table 1), because of this wide margin separating antiseizure efficacy and minimal motor impairment in numerous preclinical seizure models. The broad‐spectrum efficacy and margin of safety of CBD are more directly comparable to FBM (Table 1), which, like CBD, is also approved for patients with Lennox‐Gastaut syndrome.22 CBD presently demonstrated efficacy in the acute mouse MES, subcutaneous pentylenetetrazol, and 6‐Hz assays at both 32‐ and 44‐mA stimulus intensities. CBD also demonstrated a PI > 1.5 in the 6‐Hz model of pharmacoresistant epilepsy at a 44‐mA stimulus intensity, which substantially differentiates this compound from many other FDA‐approved ASDs, including VPA.23, 24 The activity of FBM is also preserved in the 44‐mA version of the 6‐Hz assay (Table 1); the PI of FBM is also much greater than CBD in this assay (4.6 for FBM vs 1.6 for CBD; Table 1). As the 6‐Hz stimulation intensity increases from 32 to 44 mA, most ASDs lose efficacy or are only effective at motor‐impairing doses16, 17, 23, 25; thus, the finding that CBD retained efficacy and potency at both stimulation intensities in this assay at doses well below the mouse TD50 (272 mg/kg IP) suggests that CBD is highly differentiated from other FDA‐approved ASDs (eg, PB and VPA; Table 1) and suggests potential for efficacy in pharmacoresistant patient populations akin to FBM. Unlike FBM, there have been no reports to date of hepatotoxicity or aplastic anemia with CBD use,26, 27 but whether other clinical adverse events will emerge with greater clinical use of this agent remains to be determined.
The presently reported ED50 values in these acute mouse assays align with work reported by Klein and colleagues using CBD provided by the US National Institute of Drug Abuse10 and by an independent laboratory using the same CF‐1 mouse strain.28 However, we presently report significantly greater potency of CBD in the rat MES test than that reported by Klein and colleagues (ED50 = 88.9 mg/kg [95% CI = 69‐124] IP10), likely due to differences in compound formulation, source, and purity. We also report that CBD exerted dose‐dependent reductions in seizure score in the corneal kindled mouse model of chronic focal seizures, further suggesting that CBD is effective as an antiseizure agent in an epileptic substrate (ie, kindled rodents). Altogether, the presently reported pharmacological profile of CBD supports further evaluation of its broad clinical utility for epilepsy.
IV administration of CBD also reduced MSS in the pilocarpine‐induced SE in rats (Figure 1A), consistent with earlier findings demonstrating efficacy of a preclinical CBD formulation administered via the IP route.9 We believe this is the first demonstration of the efficacy of IV CBD against the onset of SE, with this route of administration most commonly employed in the treatment of clinical SE.6, 9 Although further studies are needed to define whether IV CBD can effectively reduce MSS after the onset of SE (eg, against benzodiazepine‐resistant SE), the present results in the pilocarpine‐SE model further support the acute antiseizure efficacy of CBD in diverse preclinical models of seizure.
TLE is one of the most common forms of acquired epilepsy in humans.29 Therefore, we investigated the effect of a clinically relevant CBD administration protocol in the RISE‐SRS model of TLE.15 We presently demonstrate that CBD is disease‐modifying in this model, where all animals exhibit SRS before being assigned to treatment groups. Specifically, chronic oral administration of CBD significantly decreased the seizure burden ratio of epileptic animals and improved reference memory function. Although CBD did not significantly reduce seizure burden after 7 weeks of treatment, it did markedly modify the natural disease course following SE, as demonstrated by no overall increase in the seizure burden from the first to final seizure monitoring bin (Figure 2). These findings are in stark contrast to the vehicle‐treated post‐SE rats, which demonstrated a notable, time‐dependent increase in disease severity (ie, seizure burden ratio increased). Disease severity, as characterized by a seizure burden ratio < 1, was improved in 70% of the CBD‐treated animals, in contrast to only 10% of vehicle‐treated rats attaining such a ratio (Figure 2). Although several FDA‐approved ASDs have demonstrated disease‐modifying potential in preclinical models of chronic seizure,30, 31 and CBD has been found to be disease‐modifying in a mouse model of Dravet syndrome,32 to our knowledge, this is the first study to demonstrate such an effect with CBD in any preclinical TLE model. The third‐generation ASD topiramate has demonstrated some potential for cognitive sparing and modification of behavioral deficits when administered shortly after SE onset,33, 34 but no study has yet demonstrated such a disease‐modifying effect when treatment is initiated well after the SE insult (8 weeks in present study). Although our study did not include a CBD washout arm, the present results demonstrate that chronic oral administration of CBD is associated with long‐term improvements in disease trajectory in this rat model of epilepsy.
A number of ASDs (eg, PB, VPA, phenytoin) are reported to have an adverse effect on motor function characterized by dyskinesia in people with epilepsy.5, 35, 36, 37 Moreover, patients on phenytoin and VPA treatment sometimes exhibit parkinsonism.5 Although we presently demonstrate that acute administration of CBD is well tolerated at doses up to 500 mg/kg (IP) in naive rats (Table 1), in line with prior reports,9 no studies have yet been conducted to examine the long‐term effects of chronic CBD administration on tolerability and motor function in epileptic animals. Furthermore, no study has yet administered CBD for disease modification purposes after the onset of SRS. In this regard, the present study employed a clinically realistic treatment scenario in a preclinical model of TLE to demonstrate that chronic oral administration of CBD is associated with notable antiseizure efficacy, minimal adverse effects liability, and disease‐modifying potential well after the onset of symptomatic seizures.
It is well known that humans and animals with epilepsy are more sensitive to adverse effects of ASDs38; thus, our present findings that chronic oral administration of CBD in rats with epilepsy was not associated with any adverse effects further supports the potential of this agent for chronic clinical use in epilepsy patient populations. In addition to these drug‐induced adverse effects on motor function, motor deficits are one of the most common comorbidities exhibited by patients with epilepsy.39 Here, we used two well‐validated models of motor function to assess the fine motor control, balance, and gait of animals. Epileptic vehicle‐treated animals fell from the accelerating rotarod sooner than the naive vehicle‐treated ones, indicating that SRS produced significant motor dysfunction related to balance. Similar findings were reported previously in the rotarod40 test, where motor dysfunction was exhibited by epileptic rats. In contrast, CBD‐treated animals remained on the accelerating rotarod for a significantly longer time than the vehicle‐treated epileptic animals. Thus, we show for the first time that CBD is not only well‐tolerated by epileptic animals after prolonged oral administration, but that CBD reduces the severity of motor deficits induced by epilepsy.
Epileptic rats performed comparably to healthy animals in the gait test, which is consistent with gait disorders being rarely reported in adult epileptic patients with TLE. However, ASDs such as VPA and lacosamide have a detrimental effect on gait in human patients.35, 37 It should be emphasized that animals were in the active CBD administration period during this motor test, suggesting that CBD at the dose tested did not confer any adverse effects on motor coordination. Our results demonstrate that chronic oral administration of CBD did not have any adverse effect on gait in rats with epilepsy. Clinical trials conducted on Dravet syndrome and Lennox‐Gastaut syndrome patients also did not report any gait disturbances following CBD administration.11, 13 Whether these side effects may also be absent in the general TLE patient population remains to be further determined.
Cognitive decline is a common comorbidity associated with TLE.41 For example, patients with TLE often exhibit poor executive control and working memory deficits,42 and amnesia or accelerated long‐term loss of memory is also frequently reported in patients with TLE.43 Cognitive function in animals is typically assessed using spatial memory tasks measuring reference memory and working memory errors.32, 33 Reference memory can be defined as long‐term storage of acquired information that remains constant over successive training sessions, whereas working memory is a form of short‐term memory that refers to storage and manipulation of information acquired within a trial session.44 Several spatial memory tasks (eg, radial arm maze, Morris water maze, hole‐board task) have been used to evaluate these two types of memory in rodents.45, 46 Here, we employed the hole‐board task, where epileptic vehicle‐treated rats exhibited impairment of both the working and reference memory aspects of the hole‐board test. These findings are comparable with previous studies of spatial or hippocampus‐dependent memory in rats with TLE, for example, a delayed nonmatching to position task,47 eight arm radial maze,48 and Morris water maze.49 In contrast, CBD‐treated epileptic animals exhibited improved reference memory function compared to epileptic vehicle‐treated animals; however, reference memory function was not restored to levels seen in healthy animals. Interestingly, our study shows that chronic oral administration of CBD improved working memory function compared to both epileptic vehicle‐treated and naive vehicle‐treated animals. However, as we did not include a nonepileptic CBD treated group in our design, we are unable to ascertain whether CBD has memory enhancement properties in healthy animals. A memory‐enhancement effect would be unusual, as previous studies using a radial arm maze have shown that CBD does not improve working memory function in healthy mice.50, 51 Given that the integrity of the blood‐brain barrier is commonly disrupted in epilepsy,52 a limitation of the present study is that we did not determine whether higher CBD concentrations were present in the brains of these epileptic rats. Thus, the dose of CBD required to reach this concentration might not be therapeutically significant in healthy animals. Although SRS are the primary cause of cognitive decline in TLE,53 the improved cognitive function in the CBD‐treated epileptic group might be due to a seizure‐independent mechanism rather than solely by seizure reduction, for example, anti‐inflammatory/neuroprotective action.6 However, further detailed investigation is thus warranted to shed light on the mechanism by which CBD improved the presently tested behavioral comorbidities.
Epidiolex (CBD) was approved by the FDA in 2018 for the treatment of seizures in the catastrophic pediatric encephalopathies Dravet syndrome and Lennox‐Gastaut syndrome. The present study has demonstrated that CBD is also a good potential candidate drug for the treatment of spontaneous symptomatic seizures of TLE. Moreover, chronic oral administration of CBD also reduced severity of motor disorders and cognitive deficits typically associated with TLE, in line with prior demonstrations of disease‐modifying effects in a mouse model of Dravet syndrome.32 Taken altogether, this study further illustrates that CBD not only is a potent and broad‐spectrum ASD, but may also find use to attenuate cognitive deficits associated with TLE well after the onset of SRS.41 More detailed studies are thus required to investigate the potential mechanism of action for CBD as a disease‐modifying agent for the patient with epilepsy.
DISCLOSURE OF CONFLICTS OF INTEREST
GW Research (Cambridge, UK) supplied CBD and financially supported this study. B.J.W., M.B., and N.J. are employees of GW Research. The other authors have no conflicts of interest to report. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
The work described was funded by GW Research and the National Institute of Neurological Disorders and Stroke's Epilepsy Therapy Screening Program. Open‐access fee was Supported by Greenwich Biosciences, Inc. P.H.P. is a postgraduate student sponsored by GW Research (Cambridge, UK) and the University of Reading. M.B.‐H. and H.S.W. were supported by the National Institute of Neurological Disorders and Stroke's Epilepsy Therapy Screening Program contract HHSN 271201100029 C to H.S.W. while at the University of Utah.
Patra PH, Barker‐Haliski M, White HS, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60:303–314. 10.1111/epi.14629
Williams and McNeish contributed equally.
Contributor Information
Claire M. Williams, Email: claire.williams@reading.ac.uk
Alister James McNeish, Email: a.mcneish@reading.ac.uk.
REFERENCES
- 1. Hill AJ, Williams CM, Whalley BJ, et al. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Epilepsy fact sheet. 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed October 10, 2018.
- 3. England MJ, Liverman CT, Schultz AM, et al. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 2012;25:266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. George J, Kulkarni C, Sarma GRK. Antiepileptic drugs and quality of life in patients with epilepsy: a tertiary care hospital‐based study. Value Health Reg Issues. 2015;6:1–6. [DOI] [PubMed] [Google Scholar]
- 5. Zaccara G, Cincotta M, Borgheresi A, et al. Adverse motor effects induced by antiepileptic drugs. Epileptic Disord. 2004;6:153–68. [PubMed] [Google Scholar]
- 6. Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy‐related neuroprotection. Epilepsy Behav. 2017;70:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Do Val‐da Silva RA, Peixoto‐Santos JE, Kandratavicius L, et al. Protective effects of cannabidiol against seizures and neuronal death in a rat model of mesial temporal lobe epilepsy. Front Pharmacol. 2017;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52. [DOI] [PubMed] [Google Scholar]
- 10. Klein BD, Jacobson CA, Metcalf CS, et al. Evaluation of cannabidiol in animal seizure models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42:1939–48. [DOI] [PubMed] [Google Scholar]
- 11. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration . FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy, 2018. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm. Accessed October 10, 2018.
- 13. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 14. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–23. [DOI] [PubMed] [Google Scholar]
- 15. Modebadze T, Morgan NH, Peres IA, et al. A low mortality, high morbidity reduced intensity status epilepticus (RISE) model of epilepsy and epileptogenesis in the rat. PLoS One. 2016;11:e0147265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barker‐Haliski M, Harte‐Hargrove LC, Ravizza T, et al. A companion to the preclinical common data elements for pharmacological studies in animal models of seizures and epilepsy. A report of the TASK3 Pharmacology Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open. 2018;3(Suppl 1):53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barker‐Haliski ML, Johnson K, Billingsley P, et al. Validation of a preclinical drug screening platform for pharmacoresistant epilepsy. Neurochem Res. 2017;42:1904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White HS, Barker‐Haliski M. Antiepileptic drug discovery In: Shorvon SD, Perucca E, Engel J., Jr, eds. The treatment of epilepsy. Chichester, UK: John Wiley & Sons; 2016:52–60. [Google Scholar]
- 19. Hellier JL, Patrylo PR, Buckmaster PS, et al. Recurrent spontaneous motor seizures after repeated low‐dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. [DOI] [PubMed] [Google Scholar]
- 20. Consroe P, Wolkin A. Cannabidiol–antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201:26–32. [PubMed] [Google Scholar]
- 21. Bialer M, Twyman RE, White HS. Correlation analysis between anticonvulsant ED50 values of antiepileptic drugs in mice and rats and their therapeutic doses and plasma levels. Epilepsy Behav. 2004;5:866–72. [DOI] [PubMed] [Google Scholar]
- 22. Montouris GD, Wheless JW, Glauser TA. The efficacy and tolerability of pharmacologic treatment options for Lennox‐Gastaut syndrome. Epilepsia. 2014;55(Suppl 4):10–20. [DOI] [PubMed] [Google Scholar]
- 23. Barton ME, Klein BD, Wolf HH, et al. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–27. [DOI] [PubMed] [Google Scholar]
- 24. Barker‐Haliski ML, Löscher W, White HS, Galanopoulou AS. Neuroinflammation in epileptogenesis: insights and translational perspectives from new models of epilepsies. Epilepsia. 2017;58(Suppl 3):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metcalf CS, West PJ, Thomson KE, et al. Development and pharmacologic characterization of the rat 6 Hz model of partial seizures. Epilepsia. 2017;58:1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–85. [DOI] [PubMed] [Google Scholar]
- 27. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallace MJ, Wiley JL, Martin BR, et al. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428:51–7. [DOI] [PubMed] [Google Scholar]
- 29. Jefferys JGR, Jiruska P. MODELS | The tetanus toxin model of temporal lobe epilepsy In:Schwartzkroin PA, ed. Encyclopedia of basic epilepsy research. Oxford, UK: Academic Press; 2009:804–7. [Google Scholar]
- 30. Potschka H, Soerensen J, Pekcec A, et al. Effect of eslicarbazepine acetate in the corneal kindling progression and the amygdala kindling model of temporal lobe epilepsy. Epilepsy Res. 2014;108:212–22. [DOI] [PubMed] [Google Scholar]
- 31. Brandt C, Gastens AM, Sun MZ, et al. Treatment with valproate after status epilepticus: effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology. 2006;51:789–804. [DOI] [PubMed] [Google Scholar]
- 32. Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114:11229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shatskikh T, Zhao Q, Zhou JL, et al. Effect of topiramate on cognitive function and single units from hippocampal place cells following status epilepticus. Epilepsy Behav. 2009;14:40–7. [DOI] [PubMed] [Google Scholar]
- 34. Frisch C, Kudin AP, Elger CE, et al. Amelioration of water maze performance deficits by topiramate applied during pilocarpine‐induced status epilepticus is negatively dose‐dependent. Epilepsy Res. 2007;73:173–80. [DOI] [PubMed] [Google Scholar]
- 35. Ristić AJ, Vojvodić N, Janković S, et al. The frequency of reversible parkinsonism and cognitive decline associated with valproate treatment: a study of 364 patients with different types of epilepsy. Epilepsia. 2006;47:2183–5. [DOI] [PubMed] [Google Scholar]
- 36. Zaccara G, Perucca P, Loiacono G, et al. The adverse event profile of lacosamide: a systematic review and meta‐analysis of randomized controlled trials. Epilepsia. 2013;54:66–74. [DOI] [PubMed] [Google Scholar]
- 37. Bainbridge J, Backer MD, Eckhardt K, et al. Safety and tolerability of lacosamide monotherapy in the elderly: a subgroup analysis from lacosamide trials in diabetic neuropathic pain. Epilepsia Open. 2017;2:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klitgaard H, Matagne A, Lamberty Y. Use of epileptic animals for adverse effect testing. Epilepsy Res. 2002;50:55–65. [DOI] [PubMed] [Google Scholar]
- 39. Boelen S, Nieuwenhuis S, Steenbeek L, et al. Effect of epilepsy on psychomotor function in children with uncomplicated epilepsy. Dev Med Child Neurol. 2005;47:546–50. [DOI] [PubMed] [Google Scholar]
- 40. Krishnakumar A, Abraham PM, Paul J, et al. Down‐regulation of cerebellar 5‐HT(2C) receptors in pilocarpine‐induced epilepsy in rats: therapeutic role of Bacopa monnieri extract. J Neurol Sci. 2009;284:124–8. [DOI] [PubMed] [Google Scholar]
- 41. Brooks‐Kayal AR, Bath KG, Berg AT, et al. Issues related to symptomatic and disease‐modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013;54(Suppl 4):44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lima EM, Rzezak P, Guimarães CA, et al. The executive profile of children with benign epilepsy of childhood with centrotemporal spikes and temporal lobe epilepsy. Epilepsy Behav. 2017;72:173–7. [DOI] [PubMed] [Google Scholar]
- 43. Miller LA, Mothakunnel A, Flanagan E, et al. Accelerated long term forgetting in patients with focal seizures: incidence rate and contributing factors. Epilepsy Behav. 2017;72:108–13. [DOI] [PubMed] [Google Scholar]
- 44. Olton DS. Mazes, maps, and memory. Am Psychol. 1979;34:583–96. [DOI] [PubMed] [Google Scholar]
- 45. Decker MW. Cognition models and drug discovery In: Levin ED, Buccafusco JJ. eds. Animal models of cognitive impairment. Boca Raton, FL: CRC Press/Taylor & Francis; 2006. Chapter 16. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2526/. Accessed October 1, 2018. [PubMed] [Google Scholar]
- 46. van der Staay FJ. Spatial working memory and reference memory of brown Norway and WAG rats in a holeboard discrimination task. Neurobiol Learn Mem. 1999;71:113–25. [DOI] [PubMed] [Google Scholar]
- 47. Schipper S, Aalbers MW, Rijkers K, et al. Accelerated cognitive decline in a rodent model for temporal lobe epilepsy. Epilepsy Behav. 2016;65:33–41. [DOI] [PubMed] [Google Scholar]
- 48. Wolf DC, Bueno‐Junior LS, Lopes‐Aguiar C, et al. The frequency of spontaneous seizures in rats correlates with alterations in sensorimotor gating, spatial working memory, and parvalbumin expression throughout limbic regions. Neuroscience. 2016;312:86–98. [DOI] [PubMed] [Google Scholar]
- 49. Kalemenev SV, Zubareva OE, Frolova EV, et al. Impairment of exploratory behavior and spatial memory in adolescent rats in lithium‐pilocarpine model of temporal lobe epilepsy. Dokl Biol Sci. 2015;463:175–7. [DOI] [PubMed] [Google Scholar]
- 50. Magen I, Avraham Y, Ackerman Z, et al. Cannabidiol ameliorates cognitive and motor impairments in bile‐duct ligated mice via 5‐HT(1A) receptor activation. Br J Pharmacol. 2010;159:950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avraham Y, Grigoriadis NC, Poutahidis T, et al. Cannabidiol improves brain and liver function in a fulminant hepatic failure‐induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162:1650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marchi N, Granata T, Ghosh C, et al. Blood–brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53:1877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Rijckevorsel K. Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure. 2006;15:227–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


