Abstract
In humans, advanced mast cell (MC) neoplasms are rare malignancies with a poor prognosis. Only a few preclinical models are available, and current treatment options are limited. In dogs, MC neoplasms are the most frequent malignant skin tumours. Unlike low‐grade MC neoplasms, high‐grade MC disorders usually have a poor prognosis with short survival. In both species, neoplastic MCs display activating KIT mutations, which are considered to contribute to disease evolution. Therefore, tyrosine kinase inhibitors against KIT have been developed. Unfortunately, clinical responses are unpredictable and often transient, which remains a clinical challenge in both species. Therefore, current efforts focus on the development of new improved treatment strategies. The field of comparative oncology may assist in these efforts and accelerate human and canine research regarding diagnosis, prognostication, and novel therapies. In this article, we review the current status of comparative oncology approaches and perspectives in the field of MC neoplasms.
Keywords: canine mast cell neoplasm, CD25, CD30, KIT mutations, tryptase
1. INTRODUCTION
Mast cell (MC) neoplasms are haematopoietic disorders characterized by uncontrolled expansion and accumulation of neoplastic mast cells (MCs) in various organ systems.1, 2, 3, 4, 5, 6, 7 In humans, the most frequently affected organs in systemic mastocytosis (SM) are the skin, bone marrow (BM), liver and spleen. Both indolent and aggressive variants of SM have been described.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Indolent variants include patients with cutaneous mastocytosis (CM) and indolent SM (ISM). Advanced forms of SM can be divided into aggressive SM (ASM), SM with an associated hematologic neoplasm (SM‐AHN) and MC leukaemia (MCL).1, 2, 3, 4, 8, 9, 10 MC sarcoma (MCS) is an extremely rare, localized form of advanced mastocytosis in humans. Whereas patients with ISM have an excellent prognosis with normal life‐expectancy,11 patients with advanced SM or MCS have a poor prognosis with short survival times (STs).2, 3, 4, 12, 13 For example, Lim and colleagues reported that patients with ASM have a median ST of 41 months, patients with SM‐AHN have a median ST of 24 months, and MCL patients have a median ST of 2 months.12
In the canine system, cutaneous MC tumours (MCTs) are frequently detected and can be divided into less aggressive and more aggressive variants. The aggressive MCT can involve regional lymph nodes and/or visceral organs.5, 6, 7, 14 It is worth noting that MCTs in dogs are the most frequent malignant skin tumours.5, 6, 7 Whereas histological low‐grade MCTs have a good‐to‐excellent prognosis, metastasized and/or high‐grade canine MCTs have a poor prognosis with short STs.5, 6, 7
In dogs as well as in humans, it is of considerable importance to establish the correct diagnosis and to define whether the patient has an indolent MC neoplasm or an advanced category of the disease.
During the past few decades, the pathogenesis and molecular mechanisms underlying disease evolution and progression of SM have been analysed. In both species, neoplastic MCs display activating KIT mutations that are considered to contribute to disease evolution.1, 2, 3, 4, 15, 16, 17, 18, 19, 20 Based on this concept, various tyrosine kinase inhibitors (TKI) directed against KIT have been developed with the hope that these agents can act as disease‐modifying drugs.21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Unfortunately, clinical responses are unpredictable and often transient in both species, which remains a clinical challenge in daily practice.31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Therefore, current efforts focus on deciphering other molecular pathways involved in the pathogenesis of SM in order to establish new treatment strategies.
Comparative oncology is an emerging field that is based on the assumption that the biochemical processes and pathogenesis contributing to the evolution and progression of spontaneous malignancies in human and animal species are often comparable and that these similarities can be exploited in basic and translational science.41, 42, 43, 44 Based on this assumption, many different projects in the field of comparative oncology have been initiated.44, 45, 46 One emerging example with future potential might be research on human and canine MC neoplasms.47, 48 As mentioned before, these neoplasms have several aspects in common, such as KIT mutations and a poor outcome in advanced stages.
There is hope that the field of comparative oncology can assist in our efforts to accelerate human and canine research on MCs and to improve diagnosis, prognostication and, ultimately, therapy in MC neoplasms. However, a number of questions regarding classification, prognostication and therapy of advanced MC disorders in both species remain. For example, it remains to be explored whether the diagnostic criteria and prognostic parameters that have recently been established in human MC neoplasms, can be employed in a similar, analogous way in the canine system.
In the present article, we review the current status of comparative oncology approaches in the field of MC neoplasms, with special focus on diagnosis, prognostication and standard treatment of patients with MC neoplasm in humans and dogs.
2. CLASSIFICATION OF MC NEOPLASMS AND MINIMAL DIAGNOSTIC CRITERIA
Whereas minimal diagnostic criteria for SM have been established in humans, no such criteria have been validated in canine MCT so far. In human SM, the major diagnostic criterion relates to the multi‐focal dense infiltrates of MCs in the BM and other extracutaneous organs.2, 49, 50, 51, 52, 53, 54 Minor criteria include the abnormal (often spindle‐shaped) morphology of MC, expression of CD2 and/or CD25 in MCs, KIT‐activating mutations at codon 816 of KIT and elevated serum tryptase levels (>20 ng/mL).2, 49, 50, 51, 52, 53, 54 When at least one major and one minor or at least three minor criteria are fulfilled, the diagnosis of SM can be established.2, 49, 50, 51, 52, 53, 54 At present, it remains unknown whether disease‐related parameters that have already been proven to serve as robust diagnostic parameters in human MC neoplasms, such as abnormal expression of CD25 on neoplastic MCs or certain KIT mutations, should undergo validation in dogs with MCTs.55, 56 In addition, it remains unknown whether elevated serum tryptase levels might serve as diagnostic and follow‐up parameters in dogs with MCTs in the same way as in human MC neoplasms. If one or more of these markers were determined to be of diagnostic value, these parameters could be employed as additional diagnostic criteria in dogs in future proposals. For the moment, the diagnostic tools available to classify canine MCTs are morphological assessments, histochemical studies (such as toluidine blue) and immunohistochemical investigations of neoplastic MCs, which may provide a solid basis for forthcoming comparative oncology studies (Figure 1).57, 58, 59, 60
Figure 1.
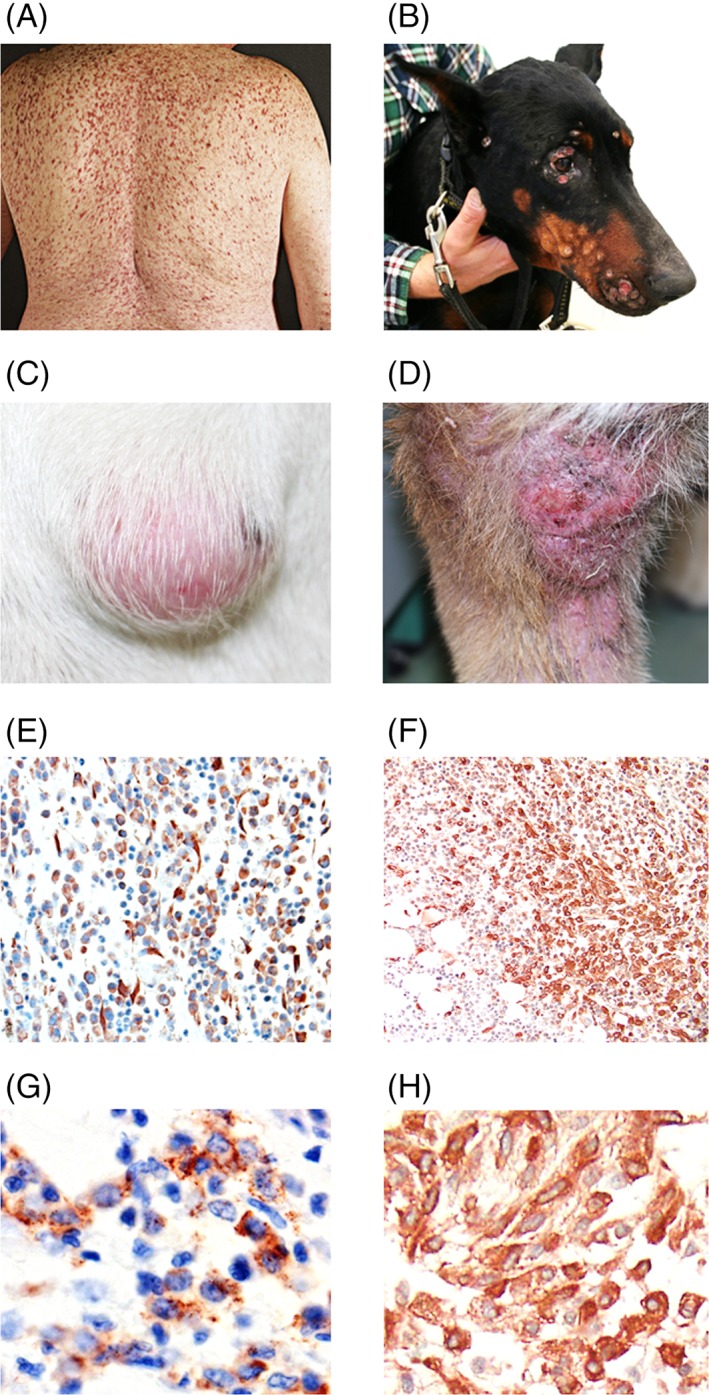
Human and dog mast cell neoplasms. A, Skin involvement in a patient with indolent systemic mastocytosis. B, Disseminated cutaneous lesions of mastocytoma (grade 1 according to the Patnaik Scheme and low‐grade according to the Kiupel Scheme) in a Doberman pinscher. C and D, Localized cutaneous mastocytoma lesions in two different dogs. E, Immunohistochemical detection of neoplastic mast cells in a cutaneous mastocytoma lesion using an antibody against KIT. F, Infiltration of the bone marrow with neoplastic mast cells visualized by tryptase‐staining in a human patient with aggressive systemic mastocytosis. G, KIT‐positive mast cells in a canine patient with multiple cutaneous mastocytoma. H, KIT‐positive mast cells in the bone marrow of human patient with advanced mastocytosis. Original magnifications: E, F: ×200; G, H: ×600 [Colour figure can be viewed at wileyonlinelibrary.com]
While the diagnosis of canine MCT can be made by a fine‐needle aspiration‐based cytology in a majority of the cases, histological grading of MCT requires histological examinations of the primary tumour site. Occasionally, immunohistochemistry may be necessary to confirm the diagnosis, especially when MCT is undifferentiated. Potential diagnostic criteria for canine MCTs and a comparison with diagnostic criteria for human SM are shown in Table 1.
Table 1.
Established and potential diagnostic criteria in human and canine mast cell neoplasms
| Marker/feature | Employed as diagnostic criterion in | |
|---|---|---|
| Human systemic mastocytosis | Canine mast cell tumour | |
| Typical skin infiltrates | –a | –a |
| Histology of mast cell infiltrate | + | + |
| Abnormal morphology of mast cells | + | + |
| Basal serum tryptase level | + | – |
| KIT mutations | + | –b |
| Expression of CD2 or CD25 in mast cells | + | – |
| Expression of CD30 in mast cells | –c | –c |
Typical skin lesions and a positive Darier's sign are diagnostic criteria of human cutaneous mastocytosis and mastocytosis in the skin in those who have systemic mastocytosis. In canines, a positive Darier sign can suggest the presence of a mast cell tumour, but the diagnosis of a mast cell tumour has to be confirmed by cytology and/or histology in all cases.
The presence of KIT mutations (exons 8, 9, and 11) can confirm the presence of a mast cell neoplasm, but is not yet regarded as a disease‐related diagnostic criterion.
CD30 is aberrantly expressed on neoplastic mast cells and is a new emerging disease‐related parameter and potential new criterion of systemic mastocytosis in humans. Whether CD2, CD25, or CD30 can be employed as diagnostic criteria for canine mast cell tumours remains so far unknown. At least in the human system, CD30 is expected to become a new minor criterion for systemic mastocytosis.
3. CLINICAL AND HISTOLOGICAL PRESENTATION OF MC NEOPLASMS
Depending on the affected organ‐system(s), human mastocytosis can be divided into CM, SM, and localized MCTs.2, 49, 50, 51, 52, 53, 54 In the human system, the classification of MC neoplasms proposed by the World Health Organization discriminates between several distinct sub‐variants of CM and SM (Table 2). CM is defined by typical features of cutaneous lesions, a diagnostic skin‐histology, and the absence of criteria sufficient to establish the diagnosis SM.2, 8, 49, 50, 51, 52, 61 A positive Darier's sign supports the conclusion that the patient is suffering from CM. However, identical cutaneous lesions are also seen in SM (Table 1). Therefore, the lesion is described as mastocytosis in the skin (MIS), and only a BM examination can clarify the final diagnosis (CM or SM) in adults.2, 8, 49, 50, 51, 52, 61 It is noteworthy that a minimal infiltration of the BM with neoplastic MCs may be well detected in CM. Even if two minor SM criteria (but no major SM criterion) are found in these patients, the diagnosis remains CM. 2, 8, 49, 50, 51, 52, 61 However, once three minor criteria are documented or at least one major and one minor criteria are found, the final diagnosis is SM.2, 49, 50, 51, 52 SM variants that have a grave prognosis (SM‐AHN, ASM, and MCL) are also referred to as advanced SM. The major criteria for advanced SM are the documented presence of SM‐induced organ damage, also known as C‐findings (Table 3).2, 49, 50, 51, 52 Such C‐findings include marked persistent cytopenia, hepatic disease with elevated liver enzymes and ascites, marked osteolyses (with or without pathologic fractures), or malabsorption with hypalbuminemia and weight loss.2, 49, 50, 51, 52
Table 2.
Classification systems for human mastocytosis according to the WHO criteria
| Variant and sub‐variant(s) | Abbreviation | Minimal diagnostic criteria |
|---|---|---|
| Systemic mastocytosis | SM | SM criteria fulfilleda: at least one major and one minor or at least three minor SM criteriaa (±cutaneous involvement) |
| Indolent SM | ISM | No C‐findingb, <2 B‐findingsb, no AHN, MCs <20% in BM smears |
| BM mastocytosis | BMM | Same as ISM but without skin lesions |
| Smouldering SM | SSM | No C‐findingb, 2 or 3 B‐findingsb, no AHN, MCs <20% in BM smears |
| SM with an AHN | SM‐AHN | Criteria for SM and for an AHN are fulfilledc |
| Aggressive SM | ASM | One or more C‐findingsb and MC <20% on BM smears |
| Classical ASM | ASM | <5% MCs in BM smears |
| ASM in transformation | ASM‐t | 5%‐19% MCs in BM smears |
| MC leukaemia | MCL | ≥20% MCs in BM smears |
| Aleukemic MCL | <10% MCs in peripheral blood | |
| Chronic MCL | No C‐findingb | |
| Acute MCL | One or more C‐findingsb | |
| MC sarcoma | MCS | Local aggressive MC tumour, SM criteria not fulfilled |
| Extracutaneous mastocytomad | Local benign MC tumour, SM criteria not fulfilled |
Abbreviations: AHN, associated hematologic neoplasm; BM, bone marrow; MC, mast cell; WHO, World Health Organization.
The classification presented is based on the WHO proposal of 2001,2, 49 2008,50 and 2017.52, 53, 54 Consensus criteria were first published in 2001,2 and were later confirmed by the same group in 2007 and 2012.51, 61
Minimal criteria to diagnose SM (SM criteria) are shown in Table 3.
B‐findings and C‐findings are listed in Table 3.
The AHN component of disease is classified according to WHO criteria.
Extracutaneous mastocytomas are exceptionally rare.
Table 3.
B‐findings and C‐findings recorded in human patients with SMa
| B‐findings = Indicate a high burden of MCs and expansion of the neoplastic process into multiple haematopoietic lineages without impairment of organ function |
| Mnemonic: B = borderline benign (be watchful) |
| 1. MC infiltration grade in the BM >30% by histology and basal serum tryptase level > 200 ng/mL |
| 2. Hypercellular BM with loss of fat cells, discrete signs of dysmyelopoiesis without substantial cytopenias or WHO criteria for an MDS or MPN |
| 3. Organomegaly: Palpable hepatomegaly, splenomegaly, or lymphadenopathy (on CT or ultrasound: >2 cm) without impaired organ function |
| C‐findings = Indicate organ damage produced by MC infiltration (should be confirmed by biopsy if possible) |
| Mnemonic: C = consider cytoreduction |
| 1. Cytopenia(s): ANC < 1000/μL or Hb < 10 g/dL or PLT < 100 000/μL |
| 2. Hepatomegaly with ascites and impaired liver function |
| 3. Palpable splenomegaly with associated hypersplenism |
| 4. Malabsorption with hypoalbuminemia and weight loss |
| 5. Skeletal lesions: Large‐sized osteolyses with pathologic fractures |
| 6. Life‐threatening organ damage in other organ systems that is caused by local MC infiltration in tissues |
Abbreviations: ANC, absolute neutrophil count; BM, bone marrow; ISM, indolent SM; MC, mast cell(s); MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; PLT, platelets; WHO, World Health Organization.
In SM patients in whom less than two B‐findings and no C‐finding are detected (category A), the diagnosis indolent SM can be established. When two or more B‐findings but no C‐findings are present, the diagnosis is smouldering SM; and when 1 or more C‐findings (+/− B‐findings) are detected, the final diagnosis is either aggressive SM (<20% MCs in BM smears) or MC leukaemia (MCs ≥20% on marrow smears) (see also Table 1B).
In veterinary oncology, the most frequent clinical presentation of MC neoplasms in dogs is a solitary cutaneous nodule (Figure 1).5, 6, 7 MCTs can have a very heterogeneous appearance, not always accompanied by a positive Darier's sign, and the diagnosis of MCT can usually be established by a cytological examination of a fine‐needle aspiration.5, 6, 7 However, fine‐needle biopsy‐derived cytology is not sufficient to determine the grade of the MCT. Therefore, an additional histological examination of the lesion is mandatory to determine the grade of the disease and thus to anticipate the behavior of the MCT in dogs. A number of clinical, molecular, and histopathological variables are considered to be of prognostic significance in canine MCTs (Table S1). The first grading system was established by Patnaik et al57 in 1984 (Table S2). This classification system divides canine cutaneous MCTs into three grades, namely, grade 1 with well‐differentiated morphology, grade 2 with intermediately differentiated cells, and grade 3 with poorly differentiated MCs.57 Although this grading system correlates with the clinical outcome of patients, its practical application showed some inconsistencies because of inter‐observer variations.58, 62, 63 Therefore, a 2‐tier histopathological grading system has been proposed by Kiupel et al58 in 2011 (Table S3), with the aim to improve the Patnaik system regarding prognostication of patients. Nonetheless, it has been shown that approximately 15% to 20% of dogs with Kiupel low‐grade MCTs have metastatic disease at presentation,64 suggesting that there is still a need for better prognostication and an improved grading system in cutaneous MCTs, and for the moment, many centres are using both the Patnaik and Kiupel prognostication model in individual canine MCT patients. In the future, a refined prognostic grading system that includes laboratory and molecular parameters may be developed in canine MCTs. Whether KIT mutations and/or elevated tryptase levels are of prognostic significance in canine MCTs, remains at present unknown.
4. DETECTION OF KIT EXPRESSION AND EVALUATION OF THE KIT MUTATIONAL STATUS
In the human system, KIT is employed as a surface marker to detect normal and neoplastic MCs (KIT+/CD34‐) by flow cytometry.2, 8, 49, 50, 51, 52, 61 In addition, KIT is employed together with tryptase to detect and enumerate neoplastic MCs in the BM of patients with SM by immunohistochemistry.65, 66 In adult patients with SM, the activating KIT mutation D816V is detected in a vast majority of cases. Using highly sensitive polymerase chain reaction techniques, KIT D816V can be detected in the BM and in the peripheral blood of most adult human patients with SM.67, 68, 69 Therefore, KIT D816V not only serves as a disease‐related criterion but can also be employed as a PB screening biomarker for patients with suspected SM.67 However, in a smaller subset of patients with SM, other KIT mutations (in codons other than 816) or no KIT mutations are found.62 In these patients, sequencing studies of the whole KIT structure are sometimes recommended.67 However, these studies are laborious and therefore are not regarded as standard practice.
In dogs, immunohistochemical staining for KIT is of prognostic value, as different staining patterns correlate with recurrence‐rate and survival in MCT.59 In contrast to the human system, a number of different KIT mutations are detectable in dogs with MCT.18, 19, 20 Therefore, a more detailed evaluation of the KIT gene may be clinically helpful. A full sequencing profile of the KIT gene is not standard in daily veterinary practice. However, screening for a limited panel of KIT mutations known to be clinically relevant (activating) and to occur recurrently (in exon 8, 9, and 11 of KIT) in MCTs is recommended when the assay is available.60, 70, 71
5. STAGING INVESTIGATIONS IN PATIENTS WITH MC NEOPLASMS
In the human system, involvement of the BM is always documented by analysing BM biopsy samples by histomorphological and immunohistochemical studies and BM aspirate samples by cytomorphological, flow cytometric, cytogenetic and molecular studies.2, 49, 50, 51, 52, 53, 54, 61, 65, 66, 72 Standard immunohistochemical markers applied for the detection and enumeration of MCs in the BM (or other organs) are KIT (CD117) and tryptase (Figure 1). Other organ systems are usually not examined by histological studies, unless the aetiology of organopathy/organomegaly remains uncertain or the patient is suffering from a sarcoma‐like disease (MC sarcoma). However, in all patients, the size of the liver and spleen is determined by ultrasound or computed tomography (CT).61 In addition, the sizes of the involved lymph nodes, when enlarged, are measured by ultrasound and/or CT. Bone involvement with osteopenia or osteoporosis should be determined by osteodensitometry in all cases with SM.61 Osteopenia is quite frequently detected in human patients with SM, and if not treated appropriately most of these patients progress to osteoporosis that may be complicated by pathologic bone fractures.2, 61 Osteopenia/osteoporosis can occur in any form/variant of SM. By contrast, large osteolyses are rarely seen in SM and are confined to patients with advanced MC disease.2, 61 In case of suspected focal lesions and in advanced SM, an x‐ray of all bones is usually recommended. Finally, the skin is examined in detail by inspection (and by photography if possible) in all patients.11, 61
In the canine system, clinical staging includes a complete blood count with differential counts, serum chemistry and cytological or histological and immunohistochemical studies of the primary organ site and of the secondary (cutaneous or extracutaneous, metastatic) lesion(s) (Figure 1).57, 58 Fine‐needle aspiration biopsies of regional lymph nodes (even if normal in size), abdominal ultrasound (with or without fine‐needle aspiration of liver and spleen regardless of the sonographic appearance) and thoracic radiography are usually performed.64, 73 In the majority of dogs, MCTs disseminate first into the regional lymph nodes, then to the spleen and liver, and finally into other visceral organs, whereas lung involvement is rare.74 In case of major blood count abnormalities and/or visceral involvement, a BM examination, including cytology (in BM smears), histology (morphologic and immunohistochemical studies) is often recommended.75, 76 A detailed investigation of the BM in all dogs with MCTs is unlikely to be clinically helpful as the vast majority of cases are presented with solitary, low to intermediate grade tumours that are locally confined and do not involve the marrow compartment. Whether BM assessment in patients presenting with systemic illness, with negative prognostic indices or for whom blood dyscrasias are present on routine complete blood count can improve the staging system and treatment recommendation, remains to be explored in future investigations. Interestingly, unlike human patients with SM, neither osteopenia/osteoporosis nor osteolyses is detected in canine patients with MCT. Additional parameters, such as immunohistochemical markers (eg, CD25), KIT mutations, and other molecular markers are employed successfully in the diagnosis and prognostication of MC neoplasms in the human system but so far not in the canine system. Whether characterization of such parameters can be helpful in the diagnosis (primary tumour site), grading (primary tumour and other involved organs), staging (investigations of BM, blood and/or other organs), and/or prognostication of canine MCTs remain to be determined in future studies.
6. UTILITY OF TRYPTASE AND OTHER DISEASE PARAMETERS DURING FOLLOW‐UP
In the human system, the basal serum tryptase level is a robust and widely used follow‐up parameter for patients with SM.77, 78, 79, 80 In particular, tryptase is a serine protease that is produced and stored almost exclusively in MCs and is secreted from resting MCs into plasma in a constitutive manner.81, 82 As a result, the basal serum tryptase level reflects the total body burden of MCs in healthy individuals (normal physiologic baseline: 1‐15 ng/mL) and in patients with mastocytosis.82 In contrast, patients with CM generally have basal serum tryptase levels within the normal range, clearly elevated tryptase levels (>20 ng/mL) are almost invariably found in patients with SM and therefore also serve as a minor diagnostic criterion of SM.49 In addition, the tryptase level is an important follow‐up parameter in SM.61 Likewise, whereas patients with ISM have stable tryptase levels, a steadily increasing basal serum tryptase is indicative of advanced SM and/or disease progression. Furthermore, effective therapy is usually accompanied by a decrease in the serum tryptase level.31, 35, 36, 37, 83
In addition, blood counts and (other) serum chemistry parameters, such as alkaline phosphatase levels, are employed in the follow‐up of human patients with SM.61 Furthermore, liver and spleen size (by ultrasound), lymph nodes, and the osteodensitometry (T score) are measured routinely in the follow‐up of patients with SM.61
In dogs, physical examination, in particular investigating the initial location(s) of the MCTs and regional lymph nodes, determination of blood counts and serum chemistry parameters, and abdominal ultrasound, are standard in the follow‐up of MCT patients.75 At present, no MC‐specific serum‐ or plasma markers are available that could be employed as follow‐up parameters for dogs with MCTs. Whether the inclusion of serologic MC‐related follow‐up parameters (like serum tryptase) would be of value for determining the course of canine MCT and for evaluation of responses to conventional or novel therapies remains to be determined in future studies.
6.1. Treatment of Advanced SM and selection of anti‐neoplastic drugs
In human patients with advanced MC neoplasm with slow progression, interferon‐alpha (IFN‐A) or cladribine (2CdA) have been considered as first‐line therapy with response rates ranging between 10% and 30%.83, 84, 85, 86 In patients with rapidly progressing ASM and MCL, more intensive therapy is required. In patients who are fit and eligible, polychemotherapy containing fludarabine or 2CdA, often in combination with cytosine arabinoside (ARA‐C), are recommended, and in case of a sufficient response (clear cytoreduction), haematopoietic stem‐cell transplantation (SCT) should be considered.37, 49, 87, 88 In patients with poor performance status, 2CdA or hydroxyurea can be used as palliate drugs. A more recent approach is to apply targeted drugs directed against KIT D816V.34, 35, 36, 37 Midostaurin has recently been described to exert major disease‐modifying activity in patients with advanced SM including MCL.35, 37 In addition, midostaurin is useful for cytoreducing tumour load in patients with advanced SM prior to SCT.37 In patients with MC sarcoma, the treatment recommendation is similar to that in MCL because most of these patients transform to ASM or MCL within weeks or months.37, 49, 87 In addition to chemotherapy, radiation therapy may be applied in MCS patients.37, 49, 87 However, most patients with MCS die within a short time‐interval despite intensive therapy.
In dogs with resectable MCTs without distant metastasis, surgical intervention is the first‐line therapy, especially for solitary grade I MCTs.7 In cases of incomplete resection (“dirty margins”), re‐excision is recommended whenever possible.89 If re‐excision is considered impossible because of diffuse infiltration or massive tumour expansion, post‐surgical radiation therapy is required, and in high‐grade and/or metastasized MCT, radiation is usually combined with systemic anti‐neoplastic therapy.90, 91 In dogs with high‐grade metastasized or unresectable MCTs, first‐line treatment often consists of TKI therapy using masitinib (EU), toceranib (US, EU) or imatinib (Japan) or cytoreductive chemotherapeutics (eg, vinblastine, lomustine; often in combination with steroids).38, 39, 40 More recently, the combination of toceranib with radiation therapy has been described to be an effective anti‐neoplastic treatment approach for dogs with MCTs, including high‐grade MCTs.92 In case of primary or secondary resistance, different chemotherapy protocols using alternative cytoreductive drugs or combinations with TKI are prescribed.93, 94, 95, 96, 97, 98, 99 However, although remissions can be achieved, responses are usually short‐lived.93, 94, 95, 96, 97, 98, 99 A summary of interventional treatment approaches in advanced SM in the human and canine system is depicted in Table 4.
Table 4.
Standard interventional therapies in advanced MC neoplasms in human and dogs
| Development status and indication in | ||
|---|---|---|
| Drug/therapy | Human patients | Canine patients |
| Imatinib | Approved for the treatment of aggressive SM without D816V KIT mutant or with unknown KIT mutation status | Used off‐label for therapy of advanced MC tumours in Japan |
| Cladribine (2CdA) | Orphan drug approval | n.a. |
| Midostaurin | Approved for the treatment of advanced, including ASM and MCL | n.a. |
| Toceranib | Approved for therapy of canine non resectable Patnaik grade 2/3 MCTs (by EMA and FDA) | |
| Masitinib | Advanced SM without KIT D816V +/− severe mediator symptoms | Approved for therapy of canine non resectable Patnaik grade 2/3 MCTs with KIT mutations (by EMA) |
| Polychemotherapy | CT like in AML | VBL/Pred; CCNU/Pred, also in combination with TKI |
| SCT | Standard in ASM‐t and MCL after debulking in young and fit patients | Not available in daily practice |
Abbreviations: ASM, aggressive systemic mastocytosis; CCNU, chlorethyl‐cyclohexyl‐nitroso‐urea, lomustine; CT like in AML, chemotherapy like in acute myelocytic leukaemia; EMA, European Medicines Agency; FDA, Food and Drug Administration; MC, mast cell; MCL, mast cell leukaemia; n.a., not analysed; Pred., prednisolone; SCT, haematopoietic stem‐cell transplantation; SM, systemic mastocytosis; TKI, tyrosine kinase inhibitor; VBL, vinblastine.
Concomitant treatment with H1‐ and H2‐blockers is recommended to prevent mediator‐related side effects in all patients.100, 101 Whether the use of proton‐pump inhibitors may improve the symptomatic treatment effect in canine MCT patients like in human SM patients remained to be evaluated in future clinical trials.61
6.2. Concluding remarks and future perspectives
Comparative oncology is an emerging field that supports the development of new diagnostic and therapeutic concepts in various tumour models. One example highlighted in this report is comparative research in human and canine MC neoplasms. In both species, indolent and aggressive disease variants have been described and in both species, advanced MC neoplasms are often treated by cytoreductive drugs and KIT‐targeting TKI. However, in both species, there is still a need to develop improved diagnostic criteria, improved prognostication models, and better (curative) drug therapies. We strongly believe that comparative oncology approaches may support these developments. Likewise, based on the higher frequency of canine MCTs, in vivo studies with various drugs and drug combinations may be more feasible in dogs with high grade and/or metastasized MCTs than in human patients with advanced SM. On the other hand, diagnostic and prognostic parameters developed into SM criteria in the human system such as the KIT mutational status, the aberrant expression of surface markers (CD2, CD25, and/or CD30) or the serum tryptase level, should be tested for their diagnostic and/or prognostic value in canine MCT. Whether comparative studies will indeed lead to improved prognostication and therapy in canine and human patients with MC neoplasms remains to be determined in forthcoming investigations. Such comparative strategies require an interdisciplinary dialogue between human and veterinary medicine.
Supporting information
Appendix S1. Supporting information.
Table S1. Prognostic markers for canine mast cell neoplasms.
Table S2. Patnaik morphologic grading classification for canine cutaneous mast cell tumours (1984).1
Table S3. Kiupel two‐tier grading criteria for canine cutaneous mast cell tumours (2011).2
ACKNOWLEDGEMENTS
The authors thank Stéphane Barete for her kind support providing the picture of an ISM patient. This work was supported by the Austrian Science Fund: projects P‐25937‐B13, SFB F4701‐B20, SFB F4704‐B20, SFB F6101, and SFB F6106. M.A. received research grants from Blueprint and from Deciphera, as well as honorarium from Deciphera. P.V. received a research grant from Novartis, from Blueprint, and from Deciphera, received honoraria from Novartis, Celgene, Pfizer, and Deciphera, and served as a consultant in a global Novartis trial evaluating the efficacy of midostaurin in advanced SM. O.H. received research grants from Novartis, Celgene, AB science, and honorarium from AB science.
Willmann M, Hadzijusufovic E, Hermine O, et al. Comparative oncology: The paradigmatic example of canine and human mast cell neoplasms. Vet Comp Oncol. 2019;17:1–10. 10.1111/vco.12440
Funding Information Austrian Science Fund, Grant/Award Number: P‐25937‐B13SFB F4701‐B20SFB F4704‐B20SFB F6101SFB F6106
REFERENCES
- 1. Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Investig Dermatol. 1991;96:2S‐4S. [PubMed] [Google Scholar]
- 2. Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603‐625. [DOI] [PubMed] [Google Scholar]
- 3. Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81:677‐690. [DOI] [PubMed] [Google Scholar]
- 4. Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. London CA, Seguin B. Mast cell tumors in the dog. Vet Clin N Am Small Anim Pract. 2003;33:473‐489. [DOI] [PubMed] [Google Scholar]
- 6. Misdorp W. Mast cells and canine mast cell tumours: A review. Vet Q. 2004;26:156‐169. [DOI] [PubMed] [Google Scholar]
- 7. Sledge DG, Webster J, Kiupel M. Canine cutaneous mast cell tumors: a combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet J. 2016;215:43‐54. [DOI] [PubMed] [Google Scholar]
- 8. Travis WD, Li CY, Hoagland HC, et al. Mast cell leukemia: report of a case and review of the literature. Mayo Clin Proc. 1986;61:957‐966. [DOI] [PubMed] [Google Scholar]
- 9. Georgin‐Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121:1285‐1295. [DOI] [PubMed] [Google Scholar]
- 10. Valent P, Sotlar K, Sperr WR, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25:1691‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European competence network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European academy of Allergology and clinical immunology. J Allergy Clin Immunol. 2016;137:35‐45. [DOI] [PubMed] [Google Scholar]
- 12. Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727‐5736. [DOI] [PubMed] [Google Scholar]
- 13. Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150‐151. [DOI] [PubMed] [Google Scholar]
- 14. Weiss DJ. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996‐2004). J Vet Intern Med. 2006;20:955‐961. [DOI] [PubMed] [Google Scholar]
- 15. Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c‐kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560‐10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c‐KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312‐314. [DOI] [PubMed] [Google Scholar]
- 17. Fritsche‐Polanz R, Jordan JH, Feix A, et al. Mutation analysis of C‐KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357‐364. [DOI] [PubMed] [Google Scholar]
- 18. London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto‐oncogene c‐kit. Exp Hematol. 1999;27:689‐697. [DOI] [PubMed] [Google Scholar]
- 19. Zemke D, Yamini B, Yuzbasiyan‐Gurkan V. Mutations in the juxtamembrane domain of c‐KIT are associated with higher grade mast cell tumors in dogs. Vet Pathol. 2002;39:529‐535. [DOI] [PubMed] [Google Scholar]
- 20. Letard S, Yang Y, Hanssens K, et al. Gain‐of‐function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res. 2008;6:1137‐1145. [DOI] [PubMed] [Google Scholar]
- 21. Gleixner KV, Mayerhofer M, Aichberger KJ, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V‐mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752‐759. [DOI] [PubMed] [Google Scholar]
- 22. Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS‐354825) inhibits KITD816V, an imatinib‐resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286‐291. [DOI] [PubMed] [Google Scholar]
- 23. Gotlib J. KIT mutations in mastocytosis and their potential as therapeutic targets. Immunol Allergy Clin N Am. 2006;26:575‐592. [DOI] [PubMed] [Google Scholar]
- 24. Gleixner KV, Mayerhofer M, Sonneck K, et al. Synergistic growth‐inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V‐mutated oncogenic variant of KIT. Haematologica. 2007;92:1451‐1459. [DOI] [PubMed] [Google Scholar]
- 25. Dubreuil P, Letard S, Ciufolini M, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:e7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35:1143‐1152. [DOI] [PubMed] [Google Scholar]
- 27. Liao AT, Chien MB, Shenoy N, et al. Inhibition of constitutively active forms of mutant kit by multitargeted indolinone tyrosine kinase inhibitors. Blood. 2002;100:585‐593. [DOI] [PubMed] [Google Scholar]
- 28. Gleixner KV, Rebuzzi L, Mayerhofer M, et al. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Exp Hematol. 2007;35:1510‐1521. [DOI] [PubMed] [Google Scholar]
- 29. Hadzijusufovic E, Peter B, Herrmann H, et al. NI‐1: a novel canine mastocytoma model for studying drug resistance and IgER‐dependent mast cell activation. Allergy. 2012;67:858‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halsey CH, Gustafson DL, Rose BJ, et al. Development of an in vitro model of acquired resistance to toceranib phosphate (Palladia) in canine mast cell tumor. BMC Vet Res. 2014;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gotlib J, Berube C, Growney JD, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865‐2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Droogendijk HJ, Kluin‐Nelemans HJ, van Doormaal JJ, Oranje AP, van de Loosdrecht AA, van Daele PL. Imatinib mesylate in the treatment of systemic mastocytosis: a phase II trial. Cancer. 2006;107:345‐351. [DOI] [PubMed] [Google Scholar]
- 33. Hochhaus A, Baccarani M, Giles FJ, et al. Nilotinib in patients with systemic mastocytosis: analysis of the phase 2, open‐label, single‐arm nilotinib registration study. J Cancer Res Clin Oncol. 2015;141:2047‐2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alvarez‐Twose I, Matito A, Morgado JM, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2016;8:68950‐68963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gotlib J, Kluin‐Nelemans HC, George TI, et al. Efficacy and safety of Midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530‐2541. [DOI] [PubMed] [Google Scholar]
- 36. Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo‐controlled, phase 3 study. Lancet. 2017;389:612‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valent P, Akin C, Hartmann K, et al. Midostaurin: a magic bullet that blocks mast cell expansion and activation. Ann Oncol. 2017;28:2367‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Isotani M, Ishida N, Tominaga M, et al. Effect of tyrosine kinase inhibition by imatinib mesylate on mast cell tumors in dogs. J Vet Intern Med. 2008;22:985‐988. [DOI] [PubMed] [Google Scholar]
- 39. Hahn KA, Ogilvie G, Rusk T, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. 2008;22:1301‐1309. [DOI] [PubMed] [Google Scholar]
- 40. London CA, Malpas PB, Wood‐Follis SL, et al. Multi‐center, placebo‐controlled, double‐blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856‐3865. [DOI] [PubMed] [Google Scholar]
- 41. Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Investig. 2000;18:781‐792. [DOI] [PubMed] [Google Scholar]
- 42. Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. 2015;370(1673):20140231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stroud C, Dmitriev I, Kashentseva E, et al. A one health overview, facilitating advances in comparative medicine and translational research. Clin Transl Med. 2016;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LeBlanc AK, Breen M, Choyke P, et al. Perspectives from man's best friend: National Academy of Medicine's Workshop on Comparative Oncology. Sci Transl Med. 2016;8:324ps5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bronzini I, Aresu L, Paganin M, et al. DNA methylation and targeted sequencing of methyltransferases family genes in canine acute myeloid leukaemia, modeling human myeloid leukaemia. Vet Comp Oncol. 2017;15:910‐918. [DOI] [PubMed] [Google Scholar]
- 46. Beer P, Pozzi A, Rohrer Bley C, Bacon N, Pfammatter NS, Venzin C. The role of sentinel lymph node mapping in small animal veterinary medicine: a comparison with current approaches in human medicine. Vet Comp Oncol. 2018;16:178‐187. [DOI] [PubMed] [Google Scholar]
- 47. Khanna C, Gordon I. Catching cancer by the tail: new perspectives on the use of kinase inhibitors. Clin Cancer Res. 2009;15:3645‐3647. [DOI] [PubMed] [Google Scholar]
- 48. Ranieri G, Marech I, Pantaleo M, et al. In vivo model for mastocytosis: a comparative review. Crit Rev Oncol Hematol. 2015;93:159‐169. [DOI] [PubMed] [Google Scholar]
- 49. Valent P, Horny H‐P, Li CY, et al. Mastocytosis In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001:291‐302. [Google Scholar]
- 50. Horny HP, Akin C, Metcalfe DD, et al. Mastocytosis (mast cell disease) In: Swerdlow SH, Campo E, Harris NL, et al., eds. World Health Organization (WHO) Classification of Tumours. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008:54‐63. [Google Scholar]
- 51. Valent P, Akin C, Arock M, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Horny HP, Akin C, Arber D, et al. Mastocytosis In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 3 Lyon, France: IARC Press; 2017:62‐69. [Google Scholar]
- 53. Valent P, Akin C, Metcalfe DD. Mastocytosis 2017: updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mast cell disorders: current status and outlook toward the future. Cancer Res. 2017;77:1261‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meyer A, Gruber AD, Klopfleisch R. CD25 is expressed by canine cutaneous mast cell tumors but not by cutaneous connective tissue mast cells. Vet Pathol. 2012;49:988‐997. [DOI] [PubMed] [Google Scholar]
- 56. Bauer K, Hadzijusufovic E, Cerny‐Reiterer S, et al. IL‐4 downregulates expression of the target receptor CD30 in neoplastic canine mast cells. Vet Comp Oncol. 2017;15:1240‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469‐474. [DOI] [PubMed] [Google Scholar]
- 58. Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2‐tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 2011;48:147‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kiupel M, Webster JD, Kaneene JB, Miller R, Yuzbasiyan‐Gurkan V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 2004;41:371‐377. [DOI] [PubMed] [Google Scholar]
- 60. Marconato L, Zorzan E, Giantin M, Di Palma S, Cancedda S, Dacasto M. Concordance of c‐kit mutational status in matched primary and metastatic cutaneous canine mast cell tumors at baseline. J Vet Intern Med. 2014;28:547‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Investig. 2007;37:435‐453. [DOI] [PubMed] [Google Scholar]
- 62. Northrup NC, Harmon BG, Gieger TL, et al. Variation among pathologists in histologic grading of canine cutaneous mast cell tumors. J Vet Diagn Investig. 2005;17:245‐248. [DOI] [PubMed] [Google Scholar]
- 63. Northrup NC, Howerth EW, Harmon BG, et al. Variation among pathologists in the histologic grading of canine cutaneous mast cell tumors with uniform use of a single grading reference. J Vet Diagn Investig. 2005;17:561‐564. [DOI] [PubMed] [Google Scholar]
- 64. Stefanello D, Buracco P, Sabattini S, et al. Comparison of 2‐ and 3‐category histologic grading systems for predicting the presence of metastasis at the time of initial evaluation in dogs with cutaneous mast cell tumors: 386 cases (2009‐2014). J Am Vet Med Assoc. 2015;246:765‐769. [DOI] [PubMed] [Google Scholar]
- 65. Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001;25:543‐551. [DOI] [PubMed] [Google Scholar]
- 66. Horny HP, Sotlar K, Sperr WR, Valent P. Systemic mastocytosis with associated clonal haematological non‐mast cell lineage diseases: a histopathological challenge. J Clin Pathol. 2004;57:604‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kristensen T, Vestergaard H, Bindslev‐Jensen C, Møller MB, Broesby‐Olsen S. Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89:493‐498. [DOI] [PubMed] [Google Scholar]
- 68. Hoermann G, Gleixner KV, Dinu GE, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arock M, Sotlar K, Akin C, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European competence network on Mastocytosis. Leukemia. 2015;29:1223‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zavodovskaja R, Chien MB, London CA. Use of kit internal tandem duplications to establish mast cell tumor clonality in 2 dogs. J Vet Intern Med. 2004;18:915‐917. [DOI] [PubMed] [Google Scholar]
- 71. Amagai Y, Tanaka A, Matsuda A, et al. Heterogeneity of internal tandem duplications in the c‐kit of dogs with multiple mast cell tumours. J Small Anim Pract. 2013;54:377‐380. [DOI] [PubMed] [Google Scholar]
- 72. Escribano L, Diaz‐Agustin B, López A, et al. Spanish network on Mastocytosis (REMA). Immunophenotypic analysis of mast cells in mastocytosis: when and how to do it. Proposals of the Spanish network on Mastocytosis (REMA). Cytometry B Clin Cytom. 2004;58:1‐8. [DOI] [PubMed] [Google Scholar]
- 73. Stefanello D, Valenti P, Faverzani S, et al. Ultrasound‐guided cytology of spleen and liver: a prognostic tool in canine cutaneous mast cell tumor. J Vet Intern Med. 2009;23:1051‐1057. [DOI] [PubMed] [Google Scholar]
- 74. Thamm DH, Vail DM. Mast‐cell tumors In: Withrow SJ, Vail DM, eds. Withrow and MacEwen's Small Animal Clinical Oncology. 4th ed., St Louis, Saunders Elsevier; 2007:402‐424. [Google Scholar]
- 75. Marconato L, Bettini G, Giacoboni C, et al. Clinicopathological features and outcome for dogs with mast cell tumors and bone marrow involvement. J Vet Intern Med. 2008;22:1001‐1007. [DOI] [PubMed] [Google Scholar]
- 76. Pizzoni S, Sabattini S, Stefanello D, et al. Features and prognostic impact of distant metastases in 45 dogs with de novo stage IV cutaneous mast celltumours: a prospective study. Vet Comp Oncol. 2018;16:28‐36. [DOI] [PubMed] [Google Scholar]
- 77. Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast‐cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622‐1626. [DOI] [PubMed] [Google Scholar]
- 78. Schwartz LB. Clinical utility of tryptase levels in systemic mastocytosis and associated hematologic disorders. Leuk Res. 2001;25:553‐562. [DOI] [PubMed] [Google Scholar]
- 79. Sperr WR, Jordan JH, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136‐141. [DOI] [PubMed] [Google Scholar]
- 80. Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin N Am. 2006;26:451‐463. [DOI] [PubMed] [Google Scholar]
- 81. Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611‐2615. [PubMed] [Google Scholar]
- 82. Schwartz LB, Sakai K, Bradford TR, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Investig. 1995;96:2702‐2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hauswirth AW, Simonitsch‐Klupp I, Uffmann M, et al. Response to therapy with interferon alpha‐2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;8:249‐257. [DOI] [PubMed] [Google Scholar]
- 84. Kluin‐Nelemans HC, Jansen JH, Breukelman H, et al. Response to interferon alfa‐2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326:619‐623. [DOI] [PubMed] [Google Scholar]
- 85. Casassus P, Caillat‐Vigneron N, Martin A, et al. Treatment of adult systemic mastocytosis with interferon‐alpha: results of a multicentre phase II trial on 20 patients. Br J Haematol. 2002;119:1090‐1097. [DOI] [PubMed] [Google Scholar]
- 86. Kluin‐Nelemans HC, Oldhoff JM, Van Doormaal JJ, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102:4270‐4276. [DOI] [PubMed] [Google Scholar]
- 87. Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116:5812‐5817. [DOI] [PubMed] [Google Scholar]
- 88. Ustun C, Reiter A, Scott BL, et al. Hematopoietic stem‐cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264‐3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kry KL, Boston SE. Additional local therapy with primary re‐excision or radiation therapy improves survival and local control after incomplete or close surgical excision of mast cell tumors in dogs. Vet Surg. 2014;43:182‐189. [DOI] [PubMed] [Google Scholar]
- 90. Frimberger AE, Moore AS, LaRue SM, Gliatto JM, Bengtson AE. Radiotherapy of incompletely resected, moderately differentiated mast cell tumors in the dog: 37 cases (1989‐1993). J Am Anim Hosp Assoc. 1997;33:320‐324. [DOI] [PubMed] [Google Scholar]
- 91. Hahn KA, King GK, Carreras JK. Efficacy of radiation therapy for incompletely resected grade‐III mast cell tumors in dogs: 31 cases (1987‐1998). J Am Vet Med Assoc. 2004;224:79‐82. [DOI] [PubMed] [Google Scholar]
- 92. Carlsten KS, London CA, Haney S, Burnett R, Avery AC, Thamm DH. Multicenter prospective trial of hypofractionated radiation treatment, toceranib, and prednisone for measurable canine mast cell tumors. J Vet Intern Med. 2012;26:135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thamm DH, Mauldin EA, Vail DM. Prednisone and vinblastine chemotherapy for canine mast cell tumor—41 cases (1992‐1997). J Vet Intern Med. 1999;13:491‐497. [DOI] [PubMed] [Google Scholar]
- 94. Camps‐Palau MA, Leibman NF, Elmslie R, et al. Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997‐2004). Vet Comp Oncol. 2007;5:156‐167. [DOI] [PubMed] [Google Scholar]
- 95. Cooper M, Tsai X, Bennett P. Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Vet Comp Oncol. 2009;7:196‐206. [DOI] [PubMed] [Google Scholar]
- 96. Rassnick KM, Bailey DB, Russell DS, et al. A phase II study to evaluate the toxicity and efficacy of alternating CCNU and high‐dose vinblastine and prednisone (CVP) for treatment of dogs with high‐grade, metastatic or nonresectable mast cell tumours. Vet Comp Oncol. 2010;8:138‐152. [DOI] [PubMed] [Google Scholar]
- 97. Rivera P, Akerlund‐Denneberg N, Bergvall K, et al. Clinical efficacy and safety of a water‐soluble micellar paclitaxel (Paccal vet) in canine mastocytomas. J Small Anim Pract. 2013;54:20‐27. [DOI] [PubMed] [Google Scholar]
- 98. Burton JH, Venable RO, Vail DM, et al. Pulse‐administered Toceranib phosphate plus Lomustine for treatment of Unresectable mast cell tumors in dogs. J Vet Intern Med. 2015;29:1098‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Miller RL, Van Lelyveld S, Warland J, Dobson JM, Foale RD. A retrospective review of treatment and response of high‐risk mast cell tumours in dogs. Vet Comp Oncol. 2016;14:361‐370. [DOI] [PubMed] [Google Scholar]
- 100. Welle MM, Bley CR, Howard J, Rüfenacht S. Canine mast cell tumours: a review of the pathogenesis, clinical features, pathology and treatment. Vet Dermatol. 2008;19:321‐339. [DOI] [PubMed] [Google Scholar]
- 101. Robat C, London C, Bunting L, et al. Safety evaluation of combination vinblastine and toceranib phosphate (Palladia) in dogs: a phase I dose‐finding study. Vet Comp Oncol. 2012;10:174‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Table S1. Prognostic markers for canine mast cell neoplasms.
Table S2. Patnaik morphologic grading classification for canine cutaneous mast cell tumours (1984).1
Table S3. Kiupel two‐tier grading criteria for canine cutaneous mast cell tumours (2011).2


