Abstract
BACKGROUND
Arrhythmias, conduction abnormalities, and intracardiac thrombus are common in patients with cardiac amyloidosis (CA). Outcomes of direct-current cardioversion (DCCV) for atrial arrhythmias in patients with CA are unknown.
OBJECTIVES
This study sought to examine DCCV procedural outcomes in patients with CA.
METHODS
Patients with CA scheduled for DCCV for atrial arrhythmias from January 2000 through December 2012 were identified and matched 2:1 with control patients by age, sex, type of atrial arrhythmia, and date of DCCV.
RESULTS
CA patients (n = 58, mean age 69 ± 9 years, 81% male) were included. CA patients had a significantly higher cardioversion cancellation rate (28% vs. 7%; p < 0.001) compared with control patients, mainly due to intracardiac thrombus identified on transesophageal echocardiogram (13 of 16 [81%] vs. 2 of 8 [25%]; p = 0.02); 4 of 13 of the CA patients (31%) with intracardiac thrombus on transesophageal echocardiogram received adequate anticoagulation ≥3 weeks and another 2 of 13 (15%) had arrhythmia duration <48 h. DCCV success rate (90% vs. 94%; p = 0.4) was not different. Procedural complications were more frequent in CA versus control patients (6 of 42 [14%] vs. 2 of 106 [2%]; p = 0.007); complications in CA included ventricular arrhythmias in 2 and severe bradyarrhythmias requiring pacemaker implantation in 2. The only complication in the control group was self-limited bradyarrhythmias.
CONCLUSIONS
Patients with CA undergoing DCCV had a significantly high cancellation rate mainly due to a high incidence of intracardiac thrombus even among patients who received adequate anticoagulation. Although the success rate of restoring sinus rhythm was high, tachyarrhythmias and bradyarrhythmias complicating DCCV were significantly more frequent in CA patients compared with control patients. (J Am Coll Cardiol 2019;73:589–97)
Keywords: atrial arrhythmia, atrial fibrillation, cardiac amyloidosis, cardioversion, intracardiac thrombus, transesophageal echocardiogram
Amyloidosis is an uncommon disease characterized by extracellular deposition of insoluble protein fibrils in tissues and organs (1–3). It often involves the heart, with fibril deposition occurring in the atria and ventricles (4) leading to a restrictive cardiomyopathy, with deterioration of systolic ventricular function occurring in the late stages. Amyloid infiltration can also involve the conduction system. Cardiac amyloidosis (CA) is often underdiagnosed and tends to be associated with a high mortality due to worsening heart failure, thromboembolic events, and sudden cardiac death (1–4). The 3 main types of CA are immunoglobulin light-chain (AL), wild-type or mutated transthyretin (ATTRwt and ATTRmut, respectively), and less frequently, serum amyloid A (5–7).
Prior studies have shown that conduction disturbances and arrhythmias are frequently observed in this patient population (8–14). The conduction abnormalities are associated with tissue infiltration that can disrupt myocardial conduction either through direct deposition or by promoting adjacent myocardial fibrosis (15–17). Another proposed mechanism of atrial arrhythmia development is increased left ventricular filling pressure leading to atrial wall dilation with resultant myocardial fibrosis (4). Patients with CA have also been found to have an increased risk of intracardiac thrombus, even in sinus rhythm, likely due to loss of atrial mechanical function (18).
A few studies have examined the role of implantable cardioverter-defibrillators in preventing sudden death and catheter ablation for treating atrial arrhythmias in patients with CA (15,19), but the outcomes of directcurrent cardioversion (DCCV) for atrial arrhythmias in this population have not been reported. The loss of atrial contribution to ventricular filling from atrial fibrillation/flutter in the background of diastolic dysfunction often leads to clinical deterioration necessitating DCCV. However, little is known about the safety of DCCV in this population and whether the increased risk of intracardiac thrombus has an impact on planned DCCV. On the basis of the published reports and our own experience, we hypothesized that patients with CA would have inferior outcomes, including a higher cancellation rate, a lower success rate, and a higher complication rate as compared with patients without CA undergoing cardioversion.
METHODS
In this retrospective study, we identified all adults (≥18 years of age) with CA referred for elective DCCV for atrial arrhythmia scheduled at the Mayo Clinic Cardioversion Unit in Rochester, Minnesota, from January 2000 through December 2012. The Mayo Clinic Institutional Review Board approved this study.
The diagnosis of CA was established on the basis of cardiac biopsy or biopsy-proven systemic amyloidosis in conjunction with typical amyloid findings on cardiac imaging (cardiac magnetic resonance or echocardiography) (15,19,20). A control group was selected from patients without a history of CA who were scheduled for DCCV within the same time period. Patients with concomitant congenital heart disease, post-operative atrial arrhythmia, or patients undergoing emergent DCCV for hemodynamic instability were excluded. The CA patients and control patients were matched 2:1 by type of atrial arrhythmia requiring DCCV (i.e., atrial fibrillation, atrial flutter, or atrial tachycardia), age, sex, and date of scheduled DCCV (within 3 months). Two CA patients could only be matched 1:1 (a 56-year-old human with AL amyloidosis and atrial flutter, and a 78-year-old human with ATTRwt amyloidosis and atrial tachycardia). Clinical characteristics of CA patients and control patients were extracted from the electronic medical record.
Procedural success was defined as maintenance of normal sinus rhythm while the patient was in the cardioversion unit (including the post-anesthesia recovery unit), and procedural failure as failure to terminate the arrhythmia or its recurrence while the patient was in the cardioversion unit or postanesthesia recovery unit (20,21). Arrhythmia recurrence was defined as an atrial arrhythmia occurring after the patient left the post-anesthesia recovery unit and documented on electrocardiography (ECG), or Holter monitoring and/or pacemaker/defibrillator interrogation report where applicable.
All patients were at therapeutic levels of anticoagulation at the time of DCCV (international normalized ratio [INR] ≥2.0 for those on warfarin, or activated partial thromboplastin time ≥55 s for those on unfractionated heparin). Transesophageal electrocardiography (TEE) was performed immediately before DCCV according to current guidelines and the referring provider’s clinical judgment (22).
All elective DCCVs were performed using a standardized protocol at the Mayo Clinic Cardioversion Unit (1,2,20,21). Immediate DCCV success was evaluated on a standard 12-lead ECG.
The primary endpoints were procedural success, procedural complications, and arrhythmia recurrence in CA patients and control patients. The secondary endpoint was to compare DCCV outcomes among the different subtypes of CA.
For the analysis of arrhythmia recurrence, we excluded patients with unsuccessful DCCV and reviewed the medical records for the latest dates of documented ECGs, Holter monitor recordings, or device interrogation reports, and considered them as the last follow-up in order to avoid the confounding effect of underreporting for patients lost to follow-up.
STATISTICAL ANALYSIS.
Categorical variables were expressed as the number of patients (proportion of sample), and continuous variables were expressed as mean ± SD or median (interquartile range) for skewed data. Categorical variables were compared with the Fisher exact test; continuous variables were compared with a 2-sided unpaired Student’s t-test or Wilcoxon rank sum test, as appropriate. The Kaplan-Meier method was used to estimate the arrhythmia recurrence rates (CA patients and control patients were censored at the time of death or last follow-up). Those curves were compared between groups using the log-rank test. The p values were 2-sided, and p values < 0.05 were considered significant. All statistical analyses were performed with SAS version 9.4 software (SAS institute, Cary, North Carolina).
RESULTS
BASELINE CHARACTERISTICS.
During the study period, 58 patients with CA and 114 matched control patients were scheduled for DCCV. The baseline clinical characteristics of the study population are summarized in Table 1. The mean age of the overall population was 69 ± 9 years, and 81% were men. Amyloidosis was of the AL type in 29 (50%), ATTRwt type in 25 (43%), and ATTRmut type in 4 (7%). One-half of the patients with CA were diagnosed with amyloidosis <6 months before DCCV. Atrial fibrillation was the presenting atrial arrhythmia in 58% of the patients, whereas atrial flutter and atrial tachycardia were present in 41% and 1% of the patients, respectively; the majority (84%) had the atrial arrhythmia >48 h before DCCV. Compared with control patients, the CA group had more prevalent heart failure on presentation (66% vs. 42%; p = 0.006). Antiarrhythmic drug use was similar (33% vs. 56%; p = 0.16).
TABLE 1.
Clinical Characteristics Of CA Compared With Control Patients
| Cardiac Amyloidosis (n = 58) |
Control Group (n = 114) |
p Value | |
|---|---|---|---|
| Age, yrs | 69 ± 9 | 69 ± 9 | 0.97 |
| Male | 47 (81) | 92 (81) | 1.00 |
| Duration since diagnosis of amyloidosis | |||
| ≤6 months | 29 (50) | ||
| >6 months | 29 (50) | ||
| Atrial fibrillation | 33 (57) | 66 (58) | 1.00 |
| Atrial flutter | 24 (41) | 47 (41) | 1.00 |
| Atrial tachycardia | 1 (2) | 1 (1) | 1.00 |
| Onset of arrhythmia within 48 h | 9 (16) | 41 (36) | 0.007 |
| Current CHF | 38 (66) | 48 (42) | 0.006 |
| NYHA functional class | |||
| I | 6 (10) | 8 (7) | 0.56 |
| II | 14 (25) | 26 (23) | 0.85 |
| III | 17 (29) | 11 (9) | 0.002 |
| IV | 1 (2) | 3 (3) | 1.00 |
| History of CHF | 37 (64) | 25 (31) | <0.001 |
| Hypertension | 29 (50) | 68 (60) | 0.26 |
| Coronary arterial disease | 18 (31) | 48 (42) | 0.19 |
| Implantable cardioverter defibrillator | 4 (7) | 7 (6) | 1.00 |
| Antiplatelet agent | 27 (47) | 65 (57) | 0.26 |
| Warfarin | 41 (71) | 84 (74) | 0.72 |
| Heparin | 25 (43) | 52 (46) | 0.87 |
| Low-molecular-weight heparin | 8 (14) | 14 (12) | 0.82 |
| Antiarrhythmic agent | 19 (33) | 64 (56) | 0.16 |
| Beta-blocker | 31 (53) | 69 (61) | 0.37 |
| Non-dihydropyridine calcium channel blocker |
16 (28) | 31 (27) | 1.00 |
| Diuretic agent | 42 (72) | 51 (45) | <0.001 |
| TEE guidance | 46 (79) | 79 (69) | 0.21 |
| Cardioversion cancelled | 16 (28) | 8 (7) | <0.001 |
Values are mean ± SD or n (%)
CA = cardiac amyloidosis; CHF = congestive heart failure; NYHA = New York Heart Association; TEE = transesophageal echocardiogram.
TRANSTHORACIC ECHOCARDIOGRAM.
Pre-cardio-version transthoracic echocardiogram data are summarized in Table 2. A substantial proportion of both CA patient and control patients had severe left atrial enlargement (47% vs. 42%; p = 0.6). Among the CA patients, severe ventricular septal thickness was present in 55% and increased left ventricular mass index in 86%. The proportion of patients with left ventricular ejection fraction <50% was not different between CA and control patients (36% vs. 35%; p = 0.86).
TABLE 2.
Transthoracic Echocardiogram Data of CA Compared With Control Patients
| Cardiac Amyloidosis | Control Group | p Value | |
|---|---|---|---|
| Normal LA, volume index 16–34 ml/m2 | 6/55 (11) | 16/82 (20) | 0.24 |
| Mildly enlarged LA, volume index 35–41 ml/m2 | 15/55 (27) | 16/82 (20) | 0.30 |
| Moderately enlarged LA, volume index 42–48 ml/m2 | 8/55 (15) | 15/82 (18) | 0.65 |
| Severely enlarged LA, volume index >48 ml/m2 | 26/55 (47) | 35/82 (42) | 0.60 |
| LV septal wall thickness ≤11 mm | 6/58 (10) | 55/83 (66) | <0.001 |
| LV septal wall thickness 12 to 14 mm | 20/58 (35) | 24/83 (29) | 0.58 |
| LV septal wall thickness ≥15 mm | 32/58 (55) | 4/83 (5) | <0.001 |
| LV posterior wall thickness ≤11 mm | 12/58 (21) | 59/83 (71) | <0.001 |
| LV posterior wall thickness 12 to 14 mm | 19/58 (33) | 22/83 (27) | 0.45 |
| LV posterior wall thickness ≥15 mm | 27/58 (46) | 2/83 (2) | 0.02 |
| Abnormal LV mass index, female >88 g/m2, male >102 g/m2 | 49/57 (86) | 49/83 (59) | <0.001 |
| LVEF <50% | 21/58 (36) | 32/92 (35) | 0.86 |
Values are n/N (%).
CA = cardiac amyloidosis; LA = left atrium; LV = left ventricle; LVEF = left ventricular ejection fraction.
TEE FINDINGS.
Pre-cardioversion TEE was performed in 79% of CA patients and 69% of control patients (Table 3). CA patients had significantly lower left atrial appendage (LAA) emptying velocities (20.6 ± 14.1 cm/s vs. 33.9 ± 18.4 cm/s; p < 0.001) and more frequent left atrium/LAA thrombus (13 of 46 [28%] vs. 2 of 79 [2.5%]; p < 0.001) compared with control patients. There were no complications associated with TEE in the CA or control group.
TABLE 3.
TEE Data of CA Compared With Control Patients
| Cardiac Amyloidosis (n = 46) |
Control Group (n = 79) |
p Value | |
|---|---|---|---|
| Spontaneous echocardiogram contrast | 31 (67) | 34 (43) | 0.01 |
| Thrombus identified on echocardiogram | 13 (28) | 2 (2.5) | <0.001 |
| Echo LAA emptying velocity, cm/s | 20.6 ± 14.1 (n = 38) |
33.9 ± 18.4 (n = 65) |
<0.001 |
Values are n (%) or mean ± SD.
LAA = left atrial appendage; other abbreviations as in Table 1.
DCCV IMMEDIATE OUTCOMES.
The CA group had a significantly higher cardioversion cancellation rate (16 of 58 [28%] vs. 8 of 114 [7%]; p < 0.001) compared with control patients (Central Illustration, panel A). The main reasons for cancellation were intracardiac thrombus identified on TEE (13 of 16 [81%] vs. 2 of 8 [25%]; p = 0.02) and spontaneous cardioversion (2 of 16 [13%] vs. 5 of 8 [63%]; p = 0.02) (Central Illustration, panel B). Of the 13 CA patients with intracardiac thrombus, 2 had atrial fibrillation <48 h and 4 had therapeutic INR for ≥3 weeks before; 6 had AL amyloidosis and 7 had ATTRwt amyloidosis (including the 1 patient with atrial tachycardia). There were no differences in cancellation rates among CA patients with atrial fibrillation versus atrial flutter (9 of 33 [27%] vs. 6 of 24 [25%]; p = 1.00).
CENTRAL ILLUSTRATION. Cardioversion in Cardiac Amyloidosis Outcomes.
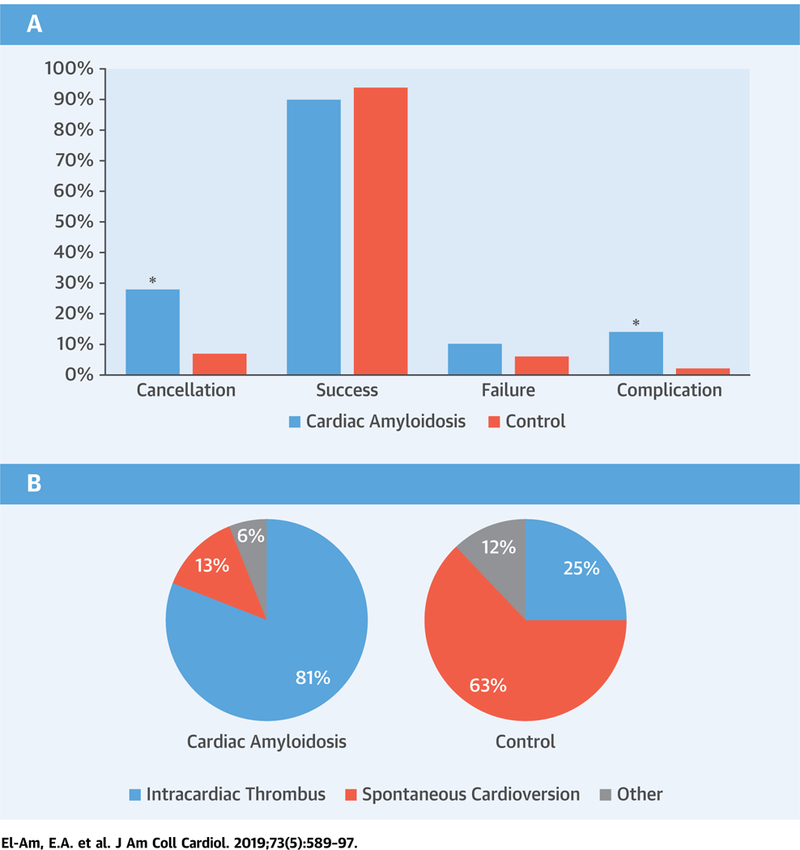
Transesophageal and direct-current cardioversion procedural outcomes (A) and reasons for cancelling planned cardioversion (B) in patients with cardiac amyloidosis (CA) compared with control patients (*p 0.05 cases vs. control patients) (A). This bar graph summarizes direct-current cardioversion procedural outcomes in patients with CA compared with control patients. The CA group had a significantly higher cardioversion cancellation rate (16 of 58 [28%] vs. 8 of 114 [7%]; p < 0.001) compared with control patients. Among the patients who proceeded to DCCV (CA n = 42; control patients n = 106), the success rate (38 of 42 [90%] vs. 100 of 106 [94%]; p = 0.47) was not different between the 2 groups. However, the procedural complication rate was significantly higher in CA versus control patients (6 of 42 [14%] vs. 2 of 106 [2%]; p = 0.007). (B) The 2 pie charts summarize the reasons for cancelling planned cardioversion in the CA cases and control patients. The main reasons for cancellation were intracardiac thrombus identified on transesophageal echocardiogram (CA 13 of 16 [81%] vs. control patients 2 of 8 [25%]; p = 0.02) and spontaneous cardioversion (CA 2 of 16 [13%] vs. control patients 5 of 8 [63%]; p = 0.02). Of the 13 CA patients with intracardiac thrombus, 2 had atrial fibrillation <48 h, and 4 had a therapeutic international normalized ratio for ≥3 weeks before transesophageal echocardiogram.
Among the patients who proceeded to DCCV (CA n = 42; control patients n = 106), success rate (38 of 42 [90%] vs. 100 of 106 [94%]; p 0.47), the proportion of patients requiring >1 shock (14 of 42 [33%] vs. 39 of 106 [37%]; p = 0.85), and the median energy in Joules (110 J vs. 120 J; p = 0.55) were not different between the CA and control groups. Procedural complications rate was significantly higher in CA versus control patients (6 of 42 [14%] vs. 2 of 106 [2%]; p = 0.007) (Central Illustration, panel A).
Among CA patients who underwent DCCV, there were no differences in success rate (22 of 24 [92%] vs. 16 of 18 [89%]; p = 1.00), complications (3 of 24 [13%] vs. 3 of 18 [17%]; p = 1.00), or cancellation rates (9 of 33 [27%] vs. 6 of 24 [25%]; p = 1.00), and the proportion of patients requiring >1 shock (8 of 24 [33%] vs. 6 of 18 [33%]; p = 1.00) when comparing atrial fibrillation versus atrial flutter. However, the median energy in Joules required during DCCV was higher in atrial fibrillation compared with atrial flutter (120 J vs. 63 J; p = 0.04).
Complications in CA included ventricular tachycardia/fibrillation in 2, severe bradyarrhythmia requiring pacemaker implantation in 2, acute hypoxemia in 1, and stroke in 1. The only complication in the control group was self-limited post-cardioversion bradycardia in 2 patients. The details of the CA patients with complications are as follows. 1) One patient with AL amyloidosis and a history of chronic aspiration due to esophageal diverticula (status post-remote surgical repair) developed acute hypoxemia with oxygen saturation reaching the mid-70s while on 2-l nasal cannula oxygen supplementation shortly after leaving the cardioversion suite following successful TEE-guided DCCV and was transferred to the intensive care unit and later found to have aspiration pneumonitis on chest x-ray. This was not considered a complication of TEE per se because the TEE probe was inserted without difficulty, the stomach was not intubated, and the study was focused and lasted 7 min. 2) One patient with AL amyloidosis developed left hemiplegia on the same night following successful DCCV and died 5 days later. This patient had no intracardiac thrombus on pre-DCCV TEE and received adequate anticoagulation; however, his TEE did show moderate spontaneous echo contrast with reduction in LAA emptying velocities. Brain computed tomography imaging without contrast demonstrated low attenuation at the right insular ribbon, which could be consistent with early ischemic change in the right middle cerebral arterial territory; otherwise, there were no interval changes compared with previous brain computed tomography of 5 months earlier. 3) Another patient with AL amyloidosis on metoprolol developed asystole followed by extreme bradycardia following the second shock, thus requiring chest compressions, atropine, epinephrine, and external pacing until a junctional rhythm was achieved. The patient ultimately had a permanent pacemaker implanted the next day. 4) One patient with AL amyloidosis on metoprolol developed ventricular fibrillation during cardioversion despite having synchronized shocks and required cardiopulmonary resuscitation (7 shocks and a dose of lidocaine, and then converted to normal sinus rhythm). 5) A patient with ATTRwt amyloidosis who was on atenolol and had a loading dose of amiodarone developed complete heart block on the same night of successful DCCV and required temporary pacing, and 3 days later, a permanent pacemaker. 6) Another patient with ATTRmut amyloidosis, a pacemaker, and on flecainide developed ventricular tachycardia after leaving the cardioversion suite following a successful DCCV. The patient remained hemodynamically stable and returned to sinus rhythm following overdrive pacing.
CA SUBTYPES.
As shown in Tables 4 and 5, ATTRwt and ATTRmut CA patients were older than AL patients (age 73 ± 9 years vs. 65 ± 8 years; p < 0.001). Of the patients that proceeded with DCCV, the success rate was higher for ATTR compared with AL patients (21 of 21 [100%] vs. 17 of 21 [81%]; p = 0.04). However, the proportion of patients requiring >1 shock (5 of 21 [24%] vs. 9 of 21 [43%]; p = 0.32) was not different. The median energy in Joules (100 J vs. 120 J; p = 0.89) was not different between ATTR and AL patients.
TABLE 4.
Clinical Characteristics of AL Amyloidosis Versus Other Types of Amyloidosis (Attrwt and Attrmut)
| AL Amyloidosis (n = 29) |
Other Amyloidosis (ATTRwt and ATTRmut) (n = 29) |
p Value | |
|---|---|---|---|
| Age, yrs | 65 ± 8 | 73 ± 9 | 0.0004 |
| Male | 22 (76) | 25 (86) | 0.50 |
| Atrial fibrillation | 15 (52) | 18 (62) | 0.60 |
| Atrial flutter | 14 (48) | 10 (35) | 0.42 |
| Atrial tachycardia | 0 (0) | 1 (3) | 1.00 |
| Onset of arrhythmia within 48 h | 3 (12) | 6 (27) | 0.47 |
| Congestive heart failure | 18 (62) | 16 (55) | 0.79 |
| Hypertension | 15 (52) | 14 (48) | 1.00 |
| Coronary arterial disease | 9 (31) | 9 (31) | 1.0 |
| Current CHF | 18 (62) | 20 (69) | 0.78 |
| History of CHF | 18 (62) | 19 (66) | 1.00 |
| Implantable cardioverter-defibrillator | 1 (3) | 3 (10) | 0.61 |
| Antiplatelet agent (aspirin or clopidogrel) | 12 (43) | 15 (56) | 0.60 |
| Warfarin | 20 (69) | 21 (72) | 1.00 |
| Heparin | 10 (35) | 15 (52) | 0.29 |
| Low-molecular-weight heparin | 3 (10) | 5 (17) | 0.71 |
| Antiarrhythmic agent | 7 (24) | 12 (41) | 0.26 |
| Beta-blocker | 17 (59) | 14 (48) | 0.60 |
| Non-dihydropyridine calcium channel blocker | 10 (35) | 6 (21) | 0.38 |
| Diuretic agent | 22 (76) | 20 (69) | 0.77 |
| TEE guidance | 24 (83) | 21 (72) | 0.53 |
| Cardioversion cancelled | 8 (28) | 8 (28) | 1.00 |
Values are mean ± SD or n (%).
AL = immunoglobulin light-chain; ATTRmut = mutant-type transthyretin amyloidosis; ATTRwt = wild-type transthyretin amyloidosis; other abbreviations as in Table 1.
TABLE 5.
TEE Data of AL Amyloidosis Versus Other Types of Amyloidosis (Attrwt and Attrmut)
| AL Amyloidosis (n = 29) |
Other Amyloidosis (ATTRwt and ATTRmut) (n = 29) |
p Value | |
|---|---|---|---|
| Spontaneous echocardiogram contrast | 18 (82) | 13 (65) | 0.52 |
| Thrombus identified on echocardiogram | 6 (25) | 7 (33) | 0.74 |
| Echo LAA emptying velocity, cm/s | 20.7 ± 11.8 | 11.6 ± 17.0 | 0.39 |
ARRHYTHMIA RECURRENCE.
The risk of arrhythmia recurrence during 1-year follow-up was not different between the CA and the control groups (48% vs. 55%; p = 0.75) (Figure 1). During 1-year follow-up, 10 patients died (6 CA and 4 control patients), 68 had a documented recurrence (16 CA and 52 control patients), 54 had no documented recurrences (14 CA and 40 control patients), and the status of arrhythmia recurrence was unknown in 6 patients (2 CA and 4 control patients). The risk of arrhythmia recurrence was not different between AL and ATTR CA (50% vs. 49%; p = 0.81).
FIGURE 1. Rate of Atrial Arrhythmia Recurrence Following Successful DCCV in Patients With CA Compared With Control Patients.
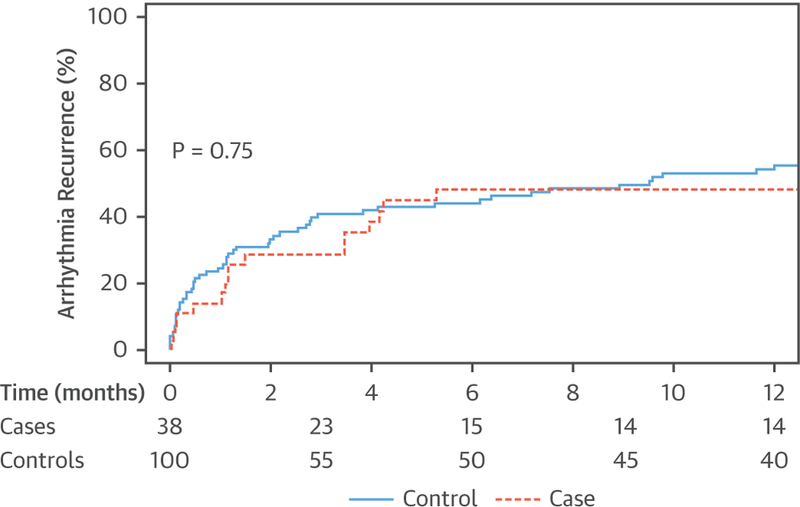
This Kaplan-Meier curve shows that the rate of atrial arrhythmia recurrence at 1 year following successful direct-current cardioversion (DCCV) is not different in patients with cardiac amyloidosis (CA) compared with control patients (48% vs. 55%; p = 0.75). Thirty-eight CA patients underwent successful cardioversion; within 1 year, 16 patients had documented recurrences, 6 died, 14 patients had no documented recurrence, and the status of recurrence was unknown in 2 patients. On the other hand, 100 control patients underwent successful cardioversion; within 1 year, 52 patients had documented recurrences, 4 died, 40 had no documented recurrence, and the status of recurrence was unknown in 4 patients.
DISCUSSION
In this study, we analyzed procedural success, complications, and outcomes of DCCV in patients with CA and matched control patients. Two of the 3 major findings of our study were as expected: 1) intracardiac thrombus was common and detected in 28% of CA patients referred for TEE-guided DCCV, including in patients who met guideline criteria for DCCV without TEE guidance; and 2) the major DCCV procedural complication rate was higher in CA patients than in control patients (14% vs. 0%). The third major finding was more surprising, that is, successful cardioversion rates and atrial arrhythmia recurrence were similar in CA patients and control patients.
CA causes restrictive cardiomyopathy frequently complicated by atrial arrhythmia, which can be poorly tolerated, necessitating DCCV to restore sinus rhythm. The procedural outcomes of CA patients undergoing DCCV for atrial arrhythmias have not been previously reported.
INTRACARDIAC THROMBUS.
Studies have shown that intracardiac thrombosis occurs frequently in patients with CA. In a study from Mayo Clinic on 116 autopsies (18), intracardiac thrombus was detected in 33% of patients with CA compared with none in the control group. The combination of AL amyloidosis and atrial fibrillation was associated with a high risk for thromboembolism (odds ratio: 55.0; 95% confidence interval: 8.1 to 1,131.4) (18). In addition, another retrospective review of 156 patients with CA who underwent TEE at Mayo Clinic (23) revealed the presence of intracardiac thrombus in 27% of patients. AL amyloidosis patients had more frequent intracardiac thrombus compared with the ATTR amyloidosis patients (35% vs. 18%; p = 0.02) despite being younger and having less atrial fibrillation (23). Other studies have also demonstrated an increased incidence of intracardiac thrombus in patients with CA despite the absence of atrial fibrillation/flutter (24–26). This increased risk can be explained by the systolic and diastolic ventricular dysfunction and the chronic amyloid deposition in the atria that leads to atrial enlargement, atrial mechanical dysfunction, and blood stasis (24–26). Our findings are consistent with the previous studies and showed a much higher incidence of intracardiac thrombosis in the CA group, which was also the main reason for cancellation of planned DCCV.
According to the 2014 American Heart Association/ American College of Cardiology/Heart Rhythm Society guidelines for the management of patients with atrial fibrillation, patients considered for DCCV must be at a therapeutic level of anticoagulation with warfarin (or direct oral anticoagulants) for ≥3 weeks before and continuing for ≥4 weeks after cardioversion. It is also mentioned that TEE guidance can be used as an alternative to 3 weeks of anticoagulation before DCCV (22). In our study, 46% of CA patients with intracardiac thrombus at the time of scheduled DCCV either had a therapeutic level of anticoagulation for ≥3 weeksorhad arrhythmia onset <48 h before the planned DCCV. Thus, it might be argued that a TEE is indicated before DCCV in all patients with CA, regardless of duration of arrhythmia or therapeutic level of anticoagulation, given the high incidence of intracardiac thrombosis and thromboembolism in this population of patients (18,23,24,26). The rest of the patients with intracardiac thrombosis (9 CA and 2 control patients) did not have consistently therapeutic INR levels for 3 weeks before DCCV. Some of the CA patients in our cohort were scheduled for non–TEE-guided DCCV (n = 12), although 3 of 12 patients had a TEE within 1 month of the scheduled DCCV that was negative for intracardiac thrombus, and subsequently received adequate anticoagulation. Overall, therefore, of the 58 patients scheduled for DCCV, 49 of 58 (84%) had TEE data available to guide the cardioversion.
CARDIOVERSION PROCEDURAL OUTCOMES.
Several studies have shown that the immediate DCCV success rate is around 90%, with this rate declining as the duration of the arrhythmia increases (27–32). In this cohort, the patients that proceeded with DCCV had similar success rates compared with previously reported studies. Also, the success rate was not different between the CA group and control patients. However, there was a significantly higher procedural complication rate in patients with CA when compared with the control group. The reported incidence of bradyarrhythmia complications following DCCV of persistent atrial fibrillation ranges from 0.8% to 1.5% (33). In a retrospective review of 6,906 DCCV for acute atrial fibrillation (<48 h duration), 0.9% of the cardioversions were followed by a bradyarrhythmia, with 2 patients requiring extrinsic pacing (33). None of the cardioversions were followed by ventricular fibrillation/tachycardia (33). Another retrospective study of 543 elective DCCV procedures for atrial fibrillation reported no thromboembolic or anesthesia-related complications, and no ventricular arrhythmias (34). Ctheir own and 2 requiring pacemaker implantation (34). In our cohort, the incidence of the complication of significant bradyarrhythmia requiring pacemaker implantation was 4.8%, which is higher than previous reports, with 1 occurring many hours after DCCV. Moreover, the incidence of the unexpected complication of ventricular arrhythmia following syncized DCCV was 4.8% and was observed only in the CA group, with 1 occurring after successful cardioversion during observation in the post-anesthesia recovery unit.
On the basis of the current study, DCCV-related complications occur at a higher rate in CA patients than expected, which likely reflects the underlying advanced myopathic and electrical disturbance seen in CA. Whether drugs for rate control or rhythm control contributed to the complications in the subset of patients is speculative.
ARRHYTHMIA RECURRENCE.
Although DCCV is very effective in restoring sinus rhythm, the recurrence rate of atrial arrhythmias is high. In a study on 246 patients undergoing DCCV for chronic atrial fibrillation and flutter, 42% and 36% of patients remained in sinus rhythm after 1 and 2 years of successful DCCV, respectively (35). The recurrence rates just described are similar to those found in our cohort. The recurrence rates were not different between CA patients and the control group, and also not different within the different amyloid groups.
Alternatives to managing atrial arrhythmias are rate control versus catheter ablation of arrhythmia or atrioventricular node. A previous study from the Mayo Clinic examined the efficacy/safety of arrhythmia and atrioventricular node ablation for atrial arrhythmias in patients with CA and showed improvement in symptoms and 1-year and 3-year recurrence-free survival of 75% and 60%, respectively (16).
STUDY LIMITATIONS.
This is a retrospective study subject to limitations of retrospective analyses. Our center is a major referral center for amyloidosis, and there may be a selection bias related to those undergoing cardioversion. Also, despite being a major referral center for amyloidosis, the size of the CA patient cohort scheduled for DCCV for atrial arrhythmias over a 13-year period was small. We may have underestimated the atrial arrhythmia recurrence rate because we relied on ECGs, Holter monitor, or device interrogation documentation in the medical records to determine arrhythmia recurrence.
CONCLUSIONS
DCCV success rate for restoring sinus rhythm is high in patients with CA and atrial arrhythmias. However, TEE guidance of DCCV resulted in a high cancellation rate mainly due to the presence of intracardiac thrombus even among patients who received adequate anticoagulation. Therefore, DCCV in all CA patients should receive TEE guidance, unless the patient is unstable. The complication rate associated with DCCV was higher in amyloid patients compared with control patients, which may be related to cardiac amyloid infiltration. Atrial arrhythmia recurrence is high, but not different from non-CA patients. Additional studies related to optimal management strategies of atrial arrhythmias in patients with CA are needed.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In patients with cardiac amyloidosis and atrial tachyarrhythmias referred for cardioversion, the incidence of atrial appendage thrombus is high even among those who have received anticoagulation agents. The likelihood that direct-current cardioversion will successfully restore sinus rhythm is high, but bradyarrhythmia and tachyarrhythmia complications occur more often than in nonamyloid patients.
TRANSLATIONAL OUTLOOK:
Further studies are needed to determine both the optimum antithrombotic strategy and the safest and most efficacious means of maintaining sinus rhythm in patients with cardiac amyloidosis who have atrial tachyarrhythmias.
Listen to this manuscript’s audio summary by Editor-in-Chief Dr. Valentin Fuster on JACC.org.
Acknowledgments
This work was funded by the Department of Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota.
ABBREVIATIONS AND ACRONYMS
- AL
immunoglobulin light-chain
- ATTRmut
mutant-type transthyretin amyloidosis
- ATTRwt
wild-type transthyretin amyloidosis
- CA
cardiac amyloidosis
- DCCV
direct-current cardioversion
- ECG
electrocardiogram/electrocardiography
- INR
international normalized ratio
- LAA
left atrial appendage
- TEE
transesophageal electrocardiogram/echocardiography
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med 1997;337:898–909. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992;79:1817–22. [PubMed] [Google Scholar]
- 3.Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 2009;120: 1203–12. [DOI] [PubMed] [Google Scholar]
- 4.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005;112: 2047–60. [DOI] [PubMed] [Google Scholar]
- 5.Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med 2007;356:2361–71. [DOI] [PubMed] [Google Scholar]
- 6.Merlini G, Comenzo RL, Seldin DC, Wechalekar A, Gertz MA. Immunoglobulin light chain amyloidosis. Expert Rev Hematol 2014;7: 143–56. [DOI] [PubMed] [Google Scholar]
- 7.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012;126:1286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldrini M, Salinaro F, Mussinelli R, et al. Prevalence and prognostic value of conduction disturbances at the time of diagnosis of cardiac AL amyloidosis. Ann Noninvasive Electrocardiol 2013; 18:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldsmith YB, Liu J, Chou J, Hoffman J, Comenzo RL, Steingart RM. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol 2009;104:990–4. [DOI] [PubMed] [Google Scholar]
- 10.Manuguerra R, Callegari S, Corradi D. Inherited structural heart diseases with potential atrial fibrillation occurrence. J Cardiovasc Electrophysiol 2016;27:242–52. [DOI] [PubMed] [Google Scholar]
- 11.Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol 1997;30:1046–51. [DOI] [PubMed] [Google Scholar]
- 12.Sayed RH, Rogers D, Khan F, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J 2015;36: 1098–105. [DOI] [PubMed] [Google Scholar]
- 13.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol 2005; 95:535–7. [DOI] [PubMed] [Google Scholar]
- 14.Grogan M, Scott CG, Kyle RA, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016;68: 1014–20. [DOI] [PubMed] [Google Scholar]
- 15.Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2013;24:793–8. [DOI] [PubMed] [Google Scholar]
- 16.Ridolfi RL, Bulkley BH, Hutchins GM. The conduction system in cardiac amyloidosis. Clinical and pathologic features of 23 patients. Am J Med 1977;62:677–86. [DOI] [PubMed] [Google Scholar]
- 17.Rocken C, Peters B, Juenemann G, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002;106: 2091–7. [DOI] [PubMed] [Google Scholar]
- 18.Feng D, Edwards WD, Oh JK, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007;116:2420–6. [DOI] [PubMed] [Google Scholar]
- 19.Tan NY, Mohsin Y, Hodge DO, et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2016;27:1167–73. [DOI] [PubMed] [Google Scholar]
- 20.Ammash NM, Phillips SD, Hodge DO, et al. Outcome of direct current cardioversion for atrial arrhythmias in adults with congenital heart disease. Int J Cardiol 2012;154:270–4. [DOI] [PubMed] [Google Scholar]
- 21.Egbe AC, Asirvatham SJ, Connolly HM, et al. Outcomes of direct current cardioversion in adults with congenital heart disease. Am J Cardiol 2017; 119:1468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–76. [DOI] [PubMed] [Google Scholar]
- 23.Feng D, Syed IS, Martinez M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009;119:2490–7. [DOI] [PubMed] [Google Scholar]
- 24.Dubrey S, Pollak A, Skinner M, Falk RH. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J 1995;74:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plehn JF, Southworth J, Cornwell GG 3rd Brief report: atrial systolic failure in primary amyloidosis. N Engl J Med 1992;327:1570–3. [DOI] [PubMed] [Google Scholar]
- 26.Stables RH, Ormerod OJ. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Heart 1996;75:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalzell GW, Anderson J, Adgey AA. Factors determining success and energy requirements for cardioversion of atrial fibrillation. Q J Med 1990; 76:903–13. [PubMed] [Google Scholar]
- 28.Dittrich HC, Erickson JS, Schneiderman T, Blacky AR, Savides T, Nicod PH. Echocardiographic and clinical predictors for outcome of elective cardioversion of atrial fibrillation. Am J Cardiol 1989;63:193–7. [DOI] [PubMed] [Google Scholar]
- 29.Elhendy A, Gentile F, Khandheria BK, et al. Predictors of unsuccessful electrical cardioversion in atrial fibrillation. Am J Cardiol 2002;89: 83–6. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher MM, Guo XH, Poloniecki JD, Guan Yap Y, Ward D, Camm AJ. Initial energy setting, outcome and efficiency in direct current cardioversion of atrial fibrillation and flutter. J Am Coll Cardiol 2001;38:1498–504. [DOI] [PubMed] [Google Scholar]
- 31.Gurevitz OT, Ammash NM, Malouf JF, et al. Comparative efficacy of monophasic and biphasic waveforms for transthoracic cardioversion of atrial fibrillation and atrial flutter. Am Heart J 2005;149: 316–21. [DOI] [PubMed] [Google Scholar]
- 32.Lundstrom T, Ryden L. Chronic atrial fibrillation. Long-term results of direct current conversion. Acta Med Scand 1988;223:53–9. [PubMed] [Google Scholar]
- 33.Gronberg T, Nuotio I, Nikkinen M, et al. Arrhythmic complications after electrical cardioversion of acute atrial fibrillation: the FinCV study. Europace 2013;15:1432–5. [DOI] [PubMed] [Google Scholar]
- 34.Morani G, Cicoira M, Pozzani L, Angheben C, Zanotto G, Vassanelli C. Outpatient electrical cardioversion of atrial fibrillation: 8 years’ experience. Analysis of shock-related arrhythmias. Pacing Clin Electrophysiol 2009;32:1152–8. [DOI] [PubMed] [Google Scholar]
- 35.Van Gelder IC, Crijns HJ, Van Gilst WH, Verwer R, Lie KI. Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct-current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol 1991;68: 41–6. [DOI] [PubMed] [Google Scholar]


