Abstract
Introduction:
Influenza vaccination during pregnancy can offer many benefits to both mother and infant. Despite recommendations from the Advisory Committee on Immunization Practices, vaccine coverage rates among pregnant women during pregnancy are below 40% in the United States. There is a need for a greater understanding of what interventions can improve vaccine uptake among pregnant women.
Areas covered:
This review synthesizes the existing evidence on the effectiveness of interventions to improve maternal influenza vaccine uptake. These interventions are examined within the framework of the three psychological propositions: thoughts and feelings, social processes and changing behavior directly.
Expert commentary:
A number of promising and effective interventions were identified in this review. Nudge-based interventions that build on favorable intentions to vaccinate such as provider prompts and standing orders have demonstrated significant success in improving influenza vaccine uptake. However, substantial gaps in the literature still exist. Provider recommendations are the most important predictor of vaccine receipt among pregnant women, yet few studies evaluated intervening to improve the dialogue between patient and provider. With the potential for even more vaccines to be added to the maternal immunization schedule, it is vitally important to understand how to improve uptake.
Keywords: influenza, interventions, maternal immunization, pregnancy, vaccination, vaccine
1. Introduction
Influenza infection poses a greater risk to pregnant women than to non-pregnant women, especially during pandemics [1, 2]. This risk increases as gestational age increases [3, 4]. In addition to the risks to the mother, influenza infection during pregnancy can lead to lower birthweights [5]. Due to this increased risk of morbidity, seasonal influenza vaccination with inactivated influenza vaccine (IIV) has been recommended by the Advisory Committee on Immunization Practices (ACIP) in the United States since 2004 for pregnant women during any trimester and the World Health Organization (WHO) has identified pregnant women as a priority group for immunization [6, 7]. Despite these recommendations, vaccine coverage is suboptimal.
Seasonal influenza vaccine is recommended as a standard part of prenatal care in most developed countries, but coverage rates in adults rarely exceed 50% [8]. During the 2016–2017 influenza season in the United States, only 37.4% of women reported being vaccinated during pregnancy [9]. Early season estimates for the 2017–2018 influenza season are even lower, with only 35.6% of women reporting receiving the influenza vaccine before or during pregnancy as of November 2017 [10]. Coverage rates have increased since the original ACIP recommendation from approximately 20% during the 2001–2002 influenza season [11] and reached a peak following the 2009–2010 H1N1 pandemic. However, rates have remained below 50% before and during pregnancy since the 2011–2012 influenza season [12] and far below the Healthy People 2020 goal of 80% coverage [13].
Barriers to influenza vaccine uptake among pregnant women have been well characterized. Multiple systematic reviews have identified that a provider recommendation is the single most important predictor of influenza vaccine uptake among pregnant women [14, 15]. In addition to provider recommendation, other barriers include women’s knowledge, attitudes and beliefs about the vaccine, particularly concerns about the safety of the vaccine for both mother and infant, provider knowledge about the recommendation, and logistical barriers such as offering the vaccine on site and vaccine reimbursement from insurance companies [14, 15, 16, 17].
While the barriers to vaccine uptake among pregnant women have been fairly well studied, less work has been done synthesizing evidence from interventions designed to overcome these barriers. A comprehensive review is needed to identify interventions that have an impact on vaccine uptake to inform practice and the direction of future research. In this paper, we share the results of a systematic review of the literature on interventions to improve maternal influenza vaccine uptake within a psychological framework for increasing vaccination proposed by Brewer et al. [18]. Vaccine decisions are moderated by individual level factors, social influences and structural factors. To encompass this, Brewer et al. proposed that interventions to increase vaccine uptake can fall into three general psychological propositions: thoughts/feelings, social processes and changing behavior directly [18]. In the first section, we examine interventions aimed at influencing the thoughts and or feelings of the individual about vaccines, which includes both those of the pregnant woman and of her provider. Second, we examine interventions that aim to influence the social context of vaccination. Last, we examine interventions that attempt to influence the vaccine decision without impacting the individual’s thoughts, feelings or social context. These propositions are further described in the corresponding sections below. Because of the various individual and social factors that influence the vaccine decision making process, approaching the existing literature on interventions to improve maternal influenza vaccine uptake through a psychological framework created in the context of vaccine uptake interventions can offer insights into what interventions are most likely to be effective and why.
2. Systematic Review
We searched databases including PubMed, Medline, CINAHL, EMBASE and the Cochrane Library. The search terms were generated by evaluating other maternal immunization related systematic reviews and selecting those most relevant to the specific aims of this review as well as adding any terms deemed relevant by the authors (Table 1) [14, 15, 19]. Duplicate results were removed and the resulting articles were then scanned by title and abstract. Articles were excluded from the final review if they did not address human influenza vaccine, the content was not related to an intervention to increase maternal influenza vaccine uptake, it was not from a peer-reviewed journal, did not include primary data, was a modelling study, was not published in English or was not published between 2005–2017. If there was any uncertainty about the inclusion of an article a second reviewer was brought in and both reviewers discussed until a consensus was reached.
Table 1.
Keywords used for literature search
| influenza* | AND | vaccin* | AND | pregnancy | AND | knowledge | determinant |
|---|---|---|---|---|---|---|---|
| seasonal influenza* | immuniz* | pregnant | behav* | attitude | |||
| pandemic influenza* | immunis* | women | uptake | trust | |||
| flu | inoculat* | mater* | intervention | distrust | |||
| belief | confidence | ||||||
| emotion* | increas* | ||||||
| mistrust | decreas* | ||||||
| awareness | campaign | ||||||
| acceptance | misconception | ||||||
| doubt* | delay | ||||||
| rejection* | choice | ||||||
| rumor | demand | ||||||
| rumour | refus* | ||||||
| anti-vacc* | intent* | ||||||
| promoter | barrier | ||||||
| dilemma | fear | ||||||
| anxi* | perception |
Twenty-six articles were included in the final full text analysis (Figure 1). The majority of these studies were conducted in the United States (n=21). The remaining five studies were conducted in the United Kingdom, South Korea, Hong Kong and Canada. In addition to information about study setting and design, the articles were also coded by type of intervention (individual-, provider- or practice-level) and what psychological principles were addressed in the intervention (thoughts/feelings, social processes or changing behavior directly). Additional detail on the specific type of intervention (e.g. educational videos, standing orders) and the following sub-categories was collected: risk appraisal, vaccine confidence, motivation, social dyads, social networks, social norms, favorable intentions and shaping behavior.
Figure 1.
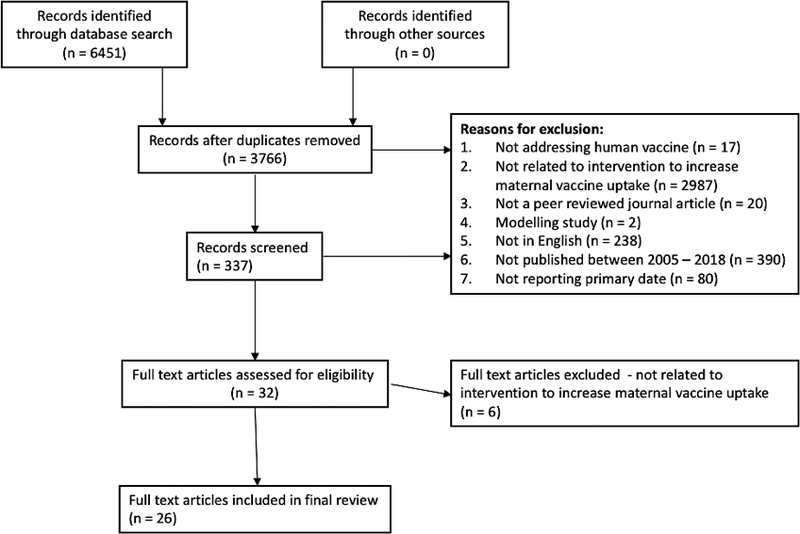
Systematic Review Results
3. What People Think and Feel
The decision to get vaccinated is, ultimately, an individual decision. Therefore, it follows that a significant portion of the research around vaccine decision-making would focus on the thoughts and feelings of the individuals making the decision. Brewer et al. define the thoughts/feeling proposition as including how people view the disease itself (risk appraisal), perceived vaccine effectiveness and concerns about safety (confidence) and what motivates individuals to receive the vaccine (motivation) [18]. For the purposes of this review, we examined interventions aimed at influencing the thoughts and feelings of the individual and those aimed at influencing the thoughts and feelings of providers.
3.1. Risk Appraisal
A common component of many health behavior models, such as the Health Belief Model [20], the Theory of Planned/Reasoned Action [21] and the Protection Motivation Model [22], is the idea of humans as rational actors who make decisions based on perceived risk and perceived reward. Although this is not always true, in the context of vaccination, risk appraisal focuses on the perceived severity of the disease or the perceived susceptibility to the disease [18]. Insufficient knowledge about susceptibility to and morbidity of vaccine-preventable disease and the risks and benefits of vaccination are modifiable barriers to immunization broadly and maternal immunization specifically [23]. Inaccurate beliefs about influenza susceptibility and severity have been associated with reduced maternal influenza uptake [24]. Enhancing pregnant women’s risk perception of influenza disease – perceived susceptibility and severity – may be an important component to maternal vaccine acceptance [23]. This can include both perceived risk to themselves or their infants.
In this review, nearly all of the studies (19 of 26) included some component aimed at altering pregnant women’s or provider’s perceived risk of either the severity of influenza during pregnancy or the perceived susceptibility to influenza during pregnancy. However, many of the studies either combined content on the risk of disease or severity of disease with content about the benefits of the vaccine or included educational materials as part of a larger multi-component intervention, making it difficult to discern the exact effect of altering risk appraisal on vaccine uptake.
Of the 13 studies that included a pregnant woman-centered educational component, half saw an improvement in influenza vaccine uptake among the population in the study [25, 26, 27, 28, 29, 30, 31]. The format of the educational materials varied. In some cases educational material about the risk of disease was written – either delivered via text message along with vaccine reminders [25, 28] or as educational paragraphs or pamphlets [26, 27, 30, 32]. In others the material was deliver orally - in one study educational videos were displayed in the waiting room [31] and in another, the educational component was an in-person session [29]. The exact content of these educational materials or messages varied as well (Table 2). Several of the studies grounded the design of their interventions in various behavioral theories, including the Health Belief Model [25, 33, 34], Prospect Theory [35, 36] and the Elaboration Likelihood Model [37].
Table 2.
Sample messages and descriptions of educational content from patient-centered interventions
| Authors | Year | Intervention | Sample Content or Description of Patient Specific Content | Results |
|---|---|---|---|---|
|
Bushar et al. [25] Jordan et al. [33] |
2017 2015 |
Text4Baby – a free national mobile health service for pregnant women that sends three weekly text messages for the duration of a woman’s pregnancy. In October 2012 Text4baby launched an interactive influenza module | Educational messages were tailored to specific concerns, for example if a woman was concerned about cost, she would get the following message: Low-cost flu shots are available this year. Talk to your doctor, health plan or local drug store. Or call CDC at 800-232-4636 for a location near you. |
Tailored educational messages did not improve the likelihood of receiving the vaccine [33], but women who recalled the messages when surveyed later were 1.44 times more likely to have gotten vaccinated [25] APR = 1.44, 95% CI = 1.30, 1.58 |
|
Chamberlain et al. [42] Chamberlain et al. [43] |
2015 2016 |
Randomized controlled trial testing a multi-component intervention that included interventions on the patient- (interactive iPad-based tutorial, maps to local pharmacies/health departments), provider- (provider-to-patient talking points, peer-to-peer vaccine promotion education) and practice-level (vaccine champions, lapel buttons, posters, brochures) |
“Included text and audio/video content covering the importance of vaccination during pregnancy, dangers of influenza and pertussis to infants, safety of antenatal vaccination, timing of antenatal vaccination and an introduction to childhood vaccination. Videos included obstetric physicians talking about antenatal vaccination as well as two testimonials from mothers whose infants contracted influenza and pertussis” | Overall influenza acceptance: 9.0% Study-adjusted antenatal influenza RD: 3.6%, 95% CI: −4.0, 11.2 |
|
Frew et al. [35] Frew et al. [36] |
2014 2014 |
Randomized controlled trial testing gain-framed messages versus loss-framed messages based in Prospect Theory | Gain-frame messages “…articulate maternal benefits associated with vaccination” Loss-frame messages “…illustrate negative consequences of foregoing immunization” |
Gain vs. Loss: OR = 1.0353, 95% CI = 0.387, 2.767 Gain vs control: OR = 0.5176, 95% CI = 0.203, 1.322) Loss vs. control: OR = 0.5000, 95% CI = 0.192, 1.304 |
| Frew et al. [37] | 2016 | Randomized controlled trial testing affective versus cognitive messaging (a video versus an iBook) based in the Elaboration Likelihood Model | Affective Messaging Intervention: “Pregnant Pause” – a nine-minute video featuring normative and persuasive influences Cognitive Messaging Intervention: “Vaccines for a Healthy Pregnancy” – an information dense, interactive tutorial |
Pregnant Pause vs. Control: RR = 1.10, 95% CI = 0.30, 4.01 Vaccine for a Healthy Pregnancy vs. Control: RR=0.57, 95% CI = 0.11, 2.88 |
| Goodman et al. [93] | 2015 | Randomized controlled trial testing impact of CDC created video Protect Yourself, Protect Your Baby | Short video addressing vaccine health beliefs that are predictive of vaccine behaviors | Intervention vaccine receipt: 28% Control vaccine receipt: 25% (p=0.70) |
| Hoppe et al. [31] | 2011 | Multi-component intervention to improve H1N1 influenza vaccine uptake among pregnant women in an obstetrics clinic | Influenza prevention video in waiting room to increase awareness of the virus. No description of written educational materials given. |
76% of eligible patients received the vaccine (compared to national coverage of about 38%) |
| Jung et al. [26] | 2016 | Women were exposed to four paragraphs about the flu vaccine and intent to vaccinate was measured after each paragraph | The four paragraphs are summarized as follows:
|
56.0% planned to receive vaccine during next pregnancy (overall). Among previously vaccinated women intent to receive after the education paragraphs was as follows: 1. 63.9%, 2. 72.3%, 3. 66.3%, 4. 71.2%. Among unvaccinated women: 1. 16.3%, 2. 30.9%, 3. 23.4%, 4. 36.5% |
| McCarthy et al. [32] | 2012 | Retrospective assessment of a multi-component intervention that included education campaign for patients and providers, | English language patient brochure included information on the benefits, efficacy and safety of influenza vaccination during pregnancy | Influenza coverage increased from 30% to 40% from 2010 to 2011 (p = 0.03) |
| Meharry et al. [27] | 2014 | Randomized controlled trial testing the impact of an educational pamphlet on vaccine uptake and an additional statement on vaccine benefits | Pamphlet had information on the cover and six sections summarized below:
Benefits statement: “If you have the flu shot during pregnancy, you will also help protect your baby against influenza from birth to 6 months” |
66.9% overall were vaccinated; pamphlet-only (72.9%, p < 0.01), pamphlet+statement (86.1%, p<0.001), control (46.9%) |
| Moniz et al. [23] | 2013 | Randomized controlled trial testing impact of a text messaging program containing messages about influenza on top of general preventative healthcare messages | Text messages are described as including information “…addressing the benefits and safety of influenza vaccination during pregnancy.” | General Preventative Messages (31% vaccinated); Flu Messages (33% vaccinated); Percent difference = 1.7 (−11.1, 14.5) p = 0.88 |
| Stockwell et al. [28] | 2014 | Randomized controlled trial evaluating a text-message intervention that included both educational text messages and text message reminders | The three messages that included educational information are summarized below:
|
Intervention (49.3%), Control (46.6%); Percent Difference 2.7% (95% CI: −3.2%, 8.6%); AOR = 1.3 (1.003, 1.69) |
| Wong et al. [29] | 2016 | Randomized controlled trial testing a one-on-one educational session versus a standard educational pamphlet | In person intervention covered the following topics:
|
Intervention (21.1%); Control (10.0%); RD = 11.1 (95% CI: 3.3–19.0, p=0.006) |
| Yudin et al. [34] | 2017 | Randomized controlled trial testing the impact of a text-message educational intervention based in the Health Belief Model | Sample text messages are listed below:
|
Overall vaccination rate 29%; Intervention (31%) vs. Control (27%) p = 0.51 |
| Yudin et al. [30] | 2010 | Pre-/Post-Intervention Assessment | Pamphlet is described as containing information about influenza and vaccine safety during pregnancy and breastfeeding and vaccine recommendations | 2006: 19% 2007: 56% (p < 0.001) |
Abbreviations: APR = adjusted prevalence ratio, CI = confidence interval, RD = risk difference, OR = odds ratio, RR = risk ratio, AOR = adjusted odds ratio
Text-message based interventions had limited impact on uptake. Bushar et al. and Jordan et al. examined women who enrolled in the Text4Baby program, a free national text service that aims to improve health knowledge and behavior by sending three weekly texts to pregnant women and mothers of infants. In addition to reminder messages, participants received messaged tailored specifically to women’s concerns about the vaccine (i.e. “Do not need shot”). The tailored educational text messages did not have an impact on intent to receive the vaccine [33]. Similarly, the other two studies which included primarily text-based interventions did not have a significant or meaningful impact on vaccine uptake [28, 34]. Data were not available on the impact of these text messages on perceived risk.
Yudin et al. and Meharry et al. saw a statistically significant increase in influenza vaccine uptake among pregnant women who received an educational pamphlet compared to women who either received a control pamphlet (Received pamphlet: 72.9% vaccine receipt vs. Control: 46.9% vaccine receipt, p < 0.01) [27] or attended the clinic prior to the implementation of the educational pamphlet (Post-pamphlet: 56% vaccine receipt vs. Pre-pamphlet: 19% vaccine receipt, p <0.001) [30]. In both studies, the pamphlets contained content addressing the risk of influenza during pregnancy as well as the safety of the vaccine, however Yudin et al. did find that 65% of pregnant women who received the pamphlet still believed that they had the same risk of complications from the flu as non-pregnant women [30]. Wong et al. found that a one-on-one in person educational intervention with pregnant women that emphasized the risk of disease along with other topics increased influenza vaccine uptake by about 10% (p = 0.06) [29].
In two separate studies, Frew et al. tested the impact of different approaches to message framing [35, 36, 37]. Interestingly, although one study found that women who had a higher perceived susceptibility to influenza during pregnancy were 2.4 times as likely to be vaccinated during pregnancy (95% Confidence Interval (CI): 1.05, 5.57), loss-frame messages that emphasized the risks of not receiving the vaccine did not have a significant effect on uptake [36]. Similarly, although women who perceived higher effectiveness of the vaccine were more likely to receive the influenza vaccine (Odds Ratio (OR): 7.03, 95% CI: 1.88, 26.3), gain-framed messages emphasizing the benefits of the vaccine did not significantly improve uptake [36]. Additionally, Frew et al. found in a separate study that neither interventions that utilized affective messages nor interventions that utilized cognitive messages had a significant impact on influenza vaccine receipt [37]. These results seem to suggest that message framing may not be effective in isolation.
Many interventions included components aimed at increasing provider knowledge about influenza and vaccine recommendations during pregnancy (Table 3) [31, 32, 38, 39, 40, 41, 42, 43]. Most of these studies did not describe the provider education in detail. With the exception of Chamberlain et al.’s study, all of the studies which included a provider education component had a positive, statistically significant impact on maternal vaccine uptake [31, 32, 38, 40, 41, 44]. However, all of these interventions had multiple components making it difficult to discern the impact of provider education alone. For example, Ogburn et al. found that stocking the vaccine in clinic and provider education sessions only increased influenza vaccination rates in pregnant women from <1% to 3%, but the addition of standing orders to the aforementioned interventions increased uptake to 37% [39]. Mouzoon et al. evaluated immunization rates in pregnant women during six influenza seasons following the implementation of a campaign that included an immunization champion, revised standing orders, and obstetric nurse training to identify eligible patients. Influenza vaccination coverage rates among eligible pregnant women increased from 2.5% at baseline to 37.4% at the end of the study period [38].
Table 3.
Descriptions of provider-centered educational interventions
| Authors | Year | Intervention | Sample Content or Description of provider specific content | Results |
|---|---|---|---|---|
|
Chamberlain et al. [42] Chamberlain et al. [43] |
2015 2016 |
Randomized controlled trial testing a multi-component intervention that included interventions on the patient- (interactive iPad-based tutorial, maps to local pharmacies/health departments), provider- (provider-to-patient talking points, peer-to-peer vaccine promotion education) and practice-level (vaccine champions, lapel buttons, posters, brochures) | Provider-to-patient talking points that emphasized protecting the fetus and newborn A 1-hour peer-to-peer education session that covered the importance of antenatal vaccination and tips for starting an in-house vaccination program |
9.0% received the vaccine overall. Study-adjusted antenatal influenza RD: 3.6%, 95% CI: −4.0, 11.2 [42] |
| Hoppe et al. [31] | 2011 | Multi-component intervention to improve H1N1 influenza vaccine uptake among pregnant women in an obstetrics clinic | Educational sessions for team members in obstetrics clinic covering risk of H1N1 in pregnant women, vaccine recommendations and safety of vaccine | 76% of eligible patients received the vaccine (compared to national coverage of about 38%) |
| McCarthy et al. [32] | 2012 | Retrospective assessment of a multi-component intervention that included education campaign for patients and providers. | Health center staff attended a grand rounds lecture. One of the authors attendant daily clinic meetings to provide additional information. No additional information was provided about the content of these interventions. | Influenza coverage increased from 30% to 40% from 2010 to 2011 (p = 0.03) |
| Mouzoon et al. [38] | 2010 | Retrospective assessment of a multi-component intervention that included education updates for providers. | No specific information is provided about the content of the educational updates. | Rates among pregnant women increased from 2.5% at baseline to 21.1% (2003–2004), 30.6% (2004–2005), 32.5% (2005–2006), 40.5% (2006–2007), 46.5% (2007–2008), 37.4% (2008–2009). P for trend <0.01 |
| Ogburn et al. [39] | 2007 | Retrospective assessment of a multi-component plan to improve vaccine uptake | Educational sessions for provider and clinic personnel. No information about the content of these sessions is provided. | 2002–2003 (<1% vaccinated) 2003–2004 (3% vaccinated) 2004–2005 (37% vaccinated) p<0.001 |
| Panda et al. [40] | 2010 | Pre-/post-intervention assessment of a multi-component intervention to improve vaccine uptake | No information is provided about the content of the provider education sessions. | 2007–2008 (pre-intevention) - 19%; 2008–2009 (post-intervention) - 31% |
| Wallis et al. [41] | 2006 | Pre-/post-intervention assessment of a multi-component intervention to improve vaccine uptake | Brief education sessions on the ACIP recommendations covering the indications, contraindications and side effects of the vaccine. | Proportion discussing before (1.5%), proportion discussing after (21.9%), Difference (20.5%) <0.001 |
Abbreviations: RD = risk difference
3.2. Confidence
The World Health Organization’s Strategic Advisory Group of Experts (SAGE) on Immunization defines vaccine confidence as “Trust in the effectiveness and safety of vaccines and in the system that delivers them, including the reliability and competence of the health services and health professionals and having trust in the motivations of the policy makers who decide which vaccines are needed and when they are needed.” [45]. Improving vaccine confidence or overcoming vaccine hesitancy is one desired impact of vaccine interventions, in addition to the important clinical and public health goal of improving timely vaccination. Even if improving vaccine confidence is secondary to timely uptake as a goal of vaccine communication, a lack of confidence in vaccines leaves the public vulnerable to misinformation and vaccine safety scares.
A component of many education-based interventions is content designed to improve confidence in vaccines. There is evidence that a lack of vaccine confidence is associated with vaccine uptake [46], but limited evidence on whether interventions that increase vaccine confidence can in turn increase vaccine uptake. Educational materials aimed at improving vaccine confidence primarily target two populations – providers and pregnant women. Thirteen of the studies included in this review contained components targeting pregnant women to increase vaccine confidence. These interventions were primarily text message based [23, 25, 28, 33, 34]. The remaining interventions included either written educational materials, in the form of a pamphlet, app or educational paragraphs [26, 27, 30, 32, 36, 42, 43] or some form of in-person education [29].
The majority of these interventions have been described in the previous section (Table 2). There is evidence that interventions that have components aimed to increase vaccine confidence among pregnant women can increase uptake of the vaccine, particularly when messages emphasize the benefits of the vaccine for the infant. Yudin et al. found that the educational pamphlets including information about the benefits and safety of the vaccine for both mother and infant had a positive impact on vaccine confidence, particularly confidence in the benefits to the infant, with 25% more women believing the vaccine was safe during pregnancy (p<0.0001) [30]. Similarly, Meharry et al. found that women who received the pamphlet were significantly more likely to perceive the vaccine as safe compared to women in the control group (p<0.01) [27]. McCarthy et al. included written educational materials for patients as part of a multi-component intervention and reported a 10% increase (p = 0.03) in maternal vaccine uptake in the influenza season after the intervention was implemented; however, as with most multi-component interventions it is unknown whether the impact was due to the educational pamphlets [32]. As described above, information about the safety and benefits of maternal influenza vaccine was frequently paired with content about the risk of the disease, so it is difficult to separate the impact that interventions with content about the risk of disease had compared to content designed to impact vaccine confidence. For both interventions aimed at affecting risk appraisal or vaccine confidence of pregnant women, the results are mixed.
Vaccine confidence among providers is extremely important given that providers are the most frequently used and credible source for vaccine information across populations [47]. Many interventions included a provider-education component [31, 32, 38, 39, 40, 42, 43, 44, 48], however as described above, limited description of the content is provided in most cases. Additionally, educational sessions for providers were always part of multi-component interventions so the direct impact of provider education alone cannot be assessed. Panda et al. surveyed physicians prior to the intervention and found only a small percentage (7.1%) were concerned about vaccine side effects during pregnancy [40]. However, they did not survey physicians after the intervention so it is unknown whether the educational intervention had an impact on these concerns. None of the other studies included in this review measured the impact of the interventions on physician vaccine confidence.
3.3. Motivation
Motivation is conceptualized as wanting to get vaccinated or being open to the idea of being vaccinated [18]. Frequently, intent to get vaccinated is used as a proxy for a behavior [49]. In the case of maternal immunization, however, there is no evidence that intent to get vaccinated is strongly correlated with vaccine receipt.
In addition to information addressing the associated risks of influenza infection during pregnancy and the safety of the vaccine in pregnant women, the gain- and loss-framed messages tested in Frew et al. were tested on their impact on women’s motivation, or intention to be vaccinated. Neither gain-framed nor loss-framed messages had a significant effect on women’s intention to receive the influenza vaccine during pregnancy [35, 36]. Chamberlain et al. measured the impact of the multi-component intervention package on knowledge, attitudes and beliefs about immunizations. This intervention contained components based on the Health Belief Model, including cues to action and self-efficacy which in theory might increase motivation to be vaccinated. However, intent to vaccinate was low across both intervention and control groups and there was no significant difference in the intent to vaccinate of the women enrolled in the study [43]. Both Chamberlain et al. and Frew et al. followed up with these study cohorts longitudinally and neither found a significant effect of their interventions on actual uptake of vaccine [35, 36, 42, 43].
4. Social Processes
Although the decision to get vaccinated is ultimately an individual decision, it is also subject to numerous social factors. The decision to get vaccinated may be influenced by a variety of other individuals and the relationship that an individual has with those around them can shape their decision. Brewer et al. examine three sets of social processes that may influence the vaccine decision making process – social dyads, such as the relationship between provider and patient, social networks and social norms [18]. Interventions that affect these relationships have the potential to influence the individual decision to get vaccinated.
4.1. Social Dyads
A variety of social dyads exist in the context of vaccine decision-making; however, the most influential in maternal immunizations is that of a pregnant woman and her prenatal care provider. There is evidence that a provider recommendation is strongly associated with vaccine receipt [14, 15]. However, there is a significant discrepancy between the proportion of providers who say they recommend the vaccines and the proportion of women who accept the vaccine [17]. As described above, this discrepancy may be due to a lack of provider knowledge about the vaccine [15], provider perceptions of patient attitudinal barriers [17] or other logistical or financial barriers to provision of the vaccine in clinics [17]. Additionally, this discrepancy could be due to the method or content of the provider recommendation. It is unclear if prenatal care providers repeatedly recommend the vaccine or what the effect of the content or the strength of the recommendation is on women’s decision to get vaccinated [17]. Given the importance of a provider recommendation for maternal immunization, there is a surprising dearth of studies intervening in the patient-provider relationship.
As part of a multi-level intervention, Chamberlain et al. included provider-to-patient talking points based on materials produced by the CDC and ACOG. These materials included three primary talking points for each vaccine (both influenza and Tdap) and emphasized the health of the child [42]. Those who received the intervention had a 3.6% higher influenza vaccination rate, however this was not significant [42, 43]. Women in the study who recalled a provider recommendation were much more likely to have been vaccinated and among women who received the intervention, a provider recommendation was the component of the package most strongly associated with vaccine receipt [42].
4.2. Social Networks
It is understood that social networks can play an important role in vaccine decision making [50]. Vaccine accepters tend to cluster with vaccine accepters within their social networks and vaccine refusers tend to cluster with vaccine refusers [51, 52]. It has been demonstrated that these clusters of non-vaccinators can lead to outbreaks of vaccine preventable diseases [51, 52]. However, no studies intervening at the social network level were identified as part of this review. This is as expected given the methodological challenges of designing a controlled network-based intervention.
4.3. Social Norms
Social norms are rules that are implicitly recognized by a group that then can have an impact on an individual’s decisions and behavior [18]. In the context of vaccination, interventions designed to emphasize vaccination as the norm or interventions targeting peers can apply social pressure to perform a certain behavior. This can be applied directly to the vaccine decisions being made by an individual or to other vaccine-related behaviors, such as a provider recommending the vaccine. In the context of maternal influenza immunization, these interventions have been primarily targeted at influencing the social norms in the obstetric care practice setting.
We identified two primarily social-norm based interventions in this review: immunization champions and lapel buttons. Immunization champions or coordinators have been used in pediatric settings and are starting to be recommended in obstetric settings as well [53]. In general, an immunization champion is an individual at the practice level who promotes vaccination and vaccination-related behaviors among the staff. The immunization champions in Mazzoni et al.’s intervention conducted regular chart reviews to prompt providers who had missed opportunities or vaccination [54]. The chief of the obstetrics and gynecology department at the study site for the Mouzoon et al. study served as immunization champion, promoting positive immunization-related behaviors among all staff [38]. Both the Mazzoni et al. and Mouzoon et al. saw significant increases in influenza vaccine uptake among pregnant populations in the years following the implementation of the intervention, increasing from 35.4% pre-intervention to 46.0% post-intervention (p <0.001) in Mazzoni et al. and to a peak of 46.5% from a baseline of 2.5% in Mouzoon et al [38, 54]. However, in both cases the immunizations champions were one portion of a multi-component intervention package again making it difficult to know the impact that the immunization champions had on immunization rates. Chamberlain et al.’s intervention included immunization champions in each clinic and lapel buttons for staff members encouraging immunization, however, as described above the intervention did not have a significant impact on immunization rates [42].
5. Changing Behavior Directly
Intention to receive the influenza vaccine does not always correlate to actual receipt of the vaccine. Additionally, many of the barriers to vaccination identified are separate from the knowledge, attitudes and beliefs about vaccines of pregnant women [14, 15, 17]. The final category of interventions summarized in this review are those aimed at bypassing individual attitudes about vaccines in order to influence vaccination behavior directly. This includes interventions to close the intention-behavior gap (building on favorable intentions and reducing barriers) and those intended to shape behavior without being influenced by individual vaccine attitudes (shaping behavior) [18].
5.1. Favorable Intentions and Reducing Barriers
Not all interventions to enhance uptake of maternal influenza vaccination attempt to change vaccine attitudes. Many such interventions function by attempting to alter an individual’s behavior in a predictable way using ‘nudges’ [55]. Some interventions are aimed specifically at those who already hold favorable vaccination intentions yet for some reason fail to act on these intentions. This phenomenon is known as the intention-behavior gap [56, 57] and is a challenge relevant to vaccination [49, 58]. The two main explanations for this phenomenon in vaccination are forgetting to vaccinate, and barriers to convenient vaccinations [18].
5.1.1. Reminder/Recall Systems and Provider Prompts
The main interventions meant to counteract forgetting to vaccinate are reminder/recall systems and provider prompts. In this context, reminders refer to messages before a patient is due for a vaccine, whereas recall refers to messages when a patient is overdue for a vaccine [18]. Provider prompts notify the provider that a patient is due for the vaccine, usually through an alert in the Electronic Medical Record (EMR). In both cases the reminder serves as a nudge, encouraging the individual to perform the desired behavior (vaccinate in the case of patients or recommend the vaccine in the case of providers). Systematic reviews and meta-analyses have shown such systems to be effective at increasing vaccine coverage in general [59, 60, 61, 62, 63].
Reminder/recall systems for maternal influenza immunization have been primarily text messaged based and have not demonstrated strong effects on vaccine uptake. Three studies showed an increase in vaccine uptake among women receiving text message reminders on maternal influenza vaccination [25, 28, 33]; however, these effects were either marginally statistically significant [28] or not statistically significant [33]. Only one study used immunization records to measure uptake [28]. All three studies were carried out in the United States and examined patient text messaging programs that contained a combination of reminders and educational content. In a randomized controlled trial of 1187 obstetric patients from five community-based clinics in New York City during the 2011–2012 flu season, women who received the intervention (five weekly text messages regarding the influenza vaccine followed by two text message appointment reminders) only increased overall immunization rates only increased by 2% [28]. The other two studies examined the effects of Text4baby [25, 33]. As previously described, the reminder and educational text messages had no significant impact on intent to receive the vaccine at baseline [33].
Seven studies of provider prompts for maternal immunization all demonstrate their effectiveness [31, 32, 38, 41, 64, 65, 66]. These studies were all carried out in the United States with the exception of one study from New Zealand [32]. Five of these studies examined the effect of provider alerts as part of a package along with other interventions such as provider and patient education, standing orders, and use of an immunization champion [31, 32, 38, 41, 64]. Two studies examined the effect of provider alerts on their own [65, 66]. Implementation of automated clinical reminders at two Michigan family medicine teaching clinics increased maternal influenza vaccination (or documented patient decline) from 66% during the baseline period (November 2006 through March 2007) to 79% during the intervention period (November 2007 through March 2008) (p < 0.001) [65]. Implementation of an EMR best-practice alert in a Wisconsin obstetrics and gynecology clinic in October 2008 led to an improvement in the clinic’s influenza vaccination coverage, increasing from 42% in the 2007–2008 season to 61% in the 2008–2009 season (p < 0.001) [66]. However, both of these studies utilized a pre-intervention/post-intervention design and did not adjust for any potential confounding variables.
5.1.2. Standing Orders
Standing orders for vaccination represent a potential strategy to reduce barriers to maternal vaccination uptake. Standing orders have been shown to increase uptake of childhood or maternal vaccination by an average of 24%, and thus are considered among the most effective evidence-based strategies for increasing vaccination uptake [67]. Standing orders can effectively address several identified provider barriers to maternal influenza vaccine uptake, such as lack of provider time to discuss vaccination, lack of provider recommendation, and missed opportunities for vaccination in general [68]. By systematizing influenza vaccination in pregnancy through the use of standing orders, the onus for recommending the vaccine is taken out of the obstetric provider’s hands, as there are many competing demands in obstetrical visits with limited time.
Standing orders also have the potential to overcome patient attitudinal barriers. Depending on how a standing order is implemented, they may be considered as a way to emphasize the social norm of vaccination among providers – maternal flu vaccine as a standard of care. A nurse or medical assistant is empowered to present influenza vaccination as the socially acceptable norm within the clinic, and thus may overcome mild or moderate attitudinal hesitancy about the vaccine. As described above, when presented as the norm, or default, patients are more likely to have a “default” or “status quo” bias, and thus are less likely to resist vaccination [69].
While there have been no randomized controlled trials on the use of standing orders in the obstetrical setting, several studies have examined the use of standing orders as a part of a multimodal intervention to increase influenza vaccination uptake in pregnancy. In a prospective study, Mazzoni et al. reported on the use of standing orders in a safety net system in Colorado as part of an intervention that also included designation of an immunization champion, immunization education for non-provider staff, periodic chart assessment and feedback, and provider prompts [54]. Influenza vaccination rates increased from 35.4% in the two years prior to the intervention to 46.0% in the two years after institution of the intervention (p<0.01). Mouzoon et al. retrospectively examined the impact of standing orders as part of an intervention that included routine chart reviews, designation of an immunization champion, and provider and nurse education [38]. In that study, performed in an obstetrical clinic in Texas, influenza vaccination rates among pregnant women increase steadily from 2.5% at baseline to 37.4% at the end of the study period. As described above, Ogburn et al. saw an increase from 3% during the first year of the intervention to 37% in the season in which standing orders were incorporated [70]. Zakrzewski et al. did not implemental a standard standing order protocol but evaluated the impact of a nurse-driven recommendation protocol compared to a provider-driven recommendation protocol and found limited difference between the two [71].
As of 2015, 66% of obstetricians reported using standing orders for influenza vaccine for their pregnant patients [17]. Barriers to the use of standing orders for maternal immunizations in obstetrics and gynecology practices were explored in a national survey performed in 2016 [72]. In this survey, among 90% of obstetricians who administered vaccines to pregnant women, the most significant barriers to the use of standing orders included provider concern that patients prefer to speak to them first (12% reporting this as a major barrier, 25% somewhat of a barrier), provider belief that they should be the one to recommend vaccines (11% major barrier, 12% somewhat of a barrier), and staff discomfort because of having to answer vaccine-related questions (7% major barrier, 17% somewhat of a barrier). In a qualitative study performed among private and safety net obstetrics clinics in Colorado, investigators reported on facilitators and barriers to implementation of standing orders [73]. Facilitators for adoption of standing orders included process standardization, acceptance of a continual modification process, and training of staff. The most oft-reported barriers included practice and staff competing demands and pregnant women’s preference for medical providers to discuss vaccine information with them.
5.1.3. Vaccine Availability
Implementation of standing orders for influenza vaccination is predicated on stocking and administering the vaccine in the clinic. Even without the use of standing orders, stocking influenza vaccine appears to be important in increasing maternal influenza vaccination uptake. Using data from the 2016–2017 influenza season, CDC reported that among pregnant women who received a recommendation but no offer of vaccination, 43.7% were vaccinated compared to women who received both a recommendation and an offer, of whom 70.5% were vaccinated [74]. Thus, stocking influenza vaccine by obstetric providers appears to be important to increase maternal vaccination uptake.
There have been no randomized trials of stocking influenza vaccine to increase maternal vaccination uptake but two studies did describe providing influenza vaccine in the clinic as part of the intervention, one of which was described above [70]. Panda et al. reported on a prospective intervention in Connecticut that included physician education, posters in the clinic advertising influenza vaccine, and offering vaccination in the clinic for the first time, showing an increase in influenza vaccination uptake of 19% prior to the intervention to 31% after, based on self-reported vaccination status [40].
Another possible intervention to reduce barriers to maternal vaccination uptake is providing transportation to vaccinating clinics. In a multimodal intervention in Washington state during the pandemic H1N1 influenza season (2009) that included educational sessions for staff, provider prompts, patient education, and patient reminders for vaccination, Hoppe and Eckert also offered taxi transportation to the clinic for the purpose of influenza vaccination for pregnant patients >36 weeks, thus reducing a potential access barrier [31]. In that study, 76% of eligible patients received the H1N1 vaccine, higher than the national coverage of 38%. No clinic-specific baseline coverage was reported. Additionally, this study had a small sample size (n=157) and the authors did not report on how many patients actually used the taxi transportation for clinic attendance or influenza vaccination. While offering transportation may hold potential for increasing uptake of maternal vaccinations, this is not a strategy that has been adequately examined.
In summary, based on existing evidence, provider prompts and standing orders for vaccination appear to offer the most potential for reducing barriers to maternal influenza vaccination. Standing orders for influenza vaccination can overcome barriers at the practice-, provider-, and patient-levels. There are recognized surmountable barriers to the use of standing orders. Stocking of vaccine is crucial to both the use of standing orders and increasing maternal influenza vaccination uptake in general.
5.2. Shaping Behavior
Another set of strategies that attempt to increase vaccine uptake without attempting to influence an individual’s specific vaccine beliefs are incentives (or sanctions) and vaccine requirements. Incentives (or sanctions) provide a motivation for individuals to get vaccinated (or in the case of providers, recommend vaccination to their patients) separate from their beliefs about vaccination. Vaccine requirements, on the other hand, restrict individual choice regarding vaccination [18]. No interventions were identified in the literature that included incentives to vaccinate for pregnant women or sanctions for not receiving the vaccine.
6. Conclusion
Pregnant women are particularly susceptible to negative outcomes from influenza infection and immunization during pregnancy can help prevent or mitigate the severity of the disease. In addition to the benefits to the mother, recent research has demonstrated that maternal influenza immunization can also provide health benefits to the infant [75]. There has been an increased focus on how to improve maternal immunization uptake – Ault et al. identified three programmatic issues around maternal influenza immunization including the need for novel strategies to increase vaccine uptake, ongoing professional education for healthcare providers and an emphasis on making influenza immunization a routine part of women’s healthcare [76]. These suggestions have been echoed in practice advisories from the American College of Obstetricians and Gynecologists (ACOG), which has particularly emphasized the role that women’s healthcare providers play in promoting vaccines among their pregnant patient [77, 78]. In this review, we found a number of promising interventions that have demonstrated a substantial impact on maternal influenza vaccine uptake. Despite these advancements, there still exist substantial gaps in our knowledge about how best to improve maternal influenza vaccine uptake.
7. Expert opinion
We summarize the evidence regarding what types of interventions (motivational, peer, reminder/recalls, provider, etc.) have an impact. However, there may be vast variability in the content, quality and impact within these categories. Some motivational or reminder interventions may work much better than others depending on the content and delivery of these interventions.
Individual attitudes and perceptions about vaccines are strongly associated with vaccine receipt. Lack of knowledge about the vaccine and concerns about the safety of the vaccine are common barriers to vaccine receipt among pregnant women [24, 79], even among those who perceive themselves or their infants at risk of the disease [79]. Among mothers of small infants, perceived risk can be a much more important factor in vaccine decision making process than the actual risk of disease [80]. Although individual level attitudes about vaccines are a significant barrier to vaccine uptake, the results of the individual level interventions identified in this review were mixed. Among those that were not part of a multi-component intervention package, only six had a positive impact on vaccine uptake and some of the results were either not significant [26] or small in magnitude [28].
Additionally, care needs to be taken when attempting to change an individual’s attitude about a specific topic [81, 82]. When correcting misconceptions, it is important to replace the existing myth with new information rather than solely debunk an individual’s current belief [83]. More research is needed on both the most important content to include in maternal influenza vaccine messages and the best way to frame these messages. For example, multiple studies have demonstrated that women are frequently very concerned about the impact that medications during pregnancy could have on their infant’s health [79, 84] and that messages focusing on the impact of the disease on the infant may increase likelihood of vaccination [85, 86].
The source and medium of vaccine messages are also important. Provider recommendation is the most significant predictor of vaccine uptake among pregnant women [14, 15] and women most frequently cite their prenatal care provider as their most trusted source of vaccine information [47, 87]. Despite this, there are few studies that focus on intervening to improve either the provision of information between provider and patient or the provision of information to providers. Only one study identified in this review included a component to improve the conversation between patient and provider [42, 43] and although the intervention package did not significantly improve vaccine uptake, a provider recommendation was the component of the package most associated with vaccine uptake [42]. Improving the conversation between provider and pregnant woman about vaccines is an important area for intervention. In pediatric settings, training providers to use presumptive communication [88] with their patients has demonstrated success in improving patient uptake of vaccines [89, 90], but this has yet to be tested in the obstetric care setting.
Greater provider knowledge of the vaccine and the disease has been shown to be associated with vaccine uptake [24] and almost every intervention that included a form of provider education in this review had a positive impact on vaccine uptake. However, in many cases there was limited information available on how the provider education was delivered or the educational content. More information is needed on how public health officials can best support prenatal care providers by providing important vaccine information and communication training.
In our review, the most effective interventions were those that changed vaccine behavior directly without changing attitudes. First, providing the vaccine in clinic eliminates one of the most cited barriers to vaccine receipt. Additionally, both provider prompts and standing orders had substantial impacts on vaccine uptake. Many EMR systems already have the capacity to provide vaccine reminders for providers. Provider prompts may make a provider more likely to recommend a vaccine. Standing orders eliminate a barrier for both providers and patients by improving the ease of delivery and establishing immunization as a routine activity in an obstetric care practice. The barriers to the use of standing orders are surmountable. For example, the assumption that pregnant women prefer to speak with the provider first, or that staff may be uncomfortable answering questions, does not preclude the implementation of standing orders. Patients that prefer to speak with the provider may still do so. Regarding staff discomfort with answering questions, that tends to dissipate once staff implement standing orders, and may lead to better job satisfaction among staff, as they feel more empowered within the clinic [73]. Both provider prompts and standing orders demonstrate opportunities for scalable and cost-effective interventions that can have a substantial impact on vaccine uptake. Additionally, both interventions are in line with current practice advisories from ACOG [77].
8. Five-Year View
Despite the advancements that have been made to overcome barriers to maternal influenza vaccine uptake, there is still a need for additional work in this area. Many studies identified in this review utilized prospective non-randomized intervention studies or historical cohort studies and there is a need for more rigorous, large-scale randomized controlled trials. We also expect to see an increase in the evaluation of multi-component interventions. As demonstrated in this review, there are a variety of direct and indirect factors that influence the vaccine decision making process. With a multicomponent intervention, it is possible to address multiple barriers simultaneously. Additionally, many behavioral and educational interventions are expected to have small effect sizes. By creating multi-component interventions, investigators can expect relatively larger effect sizes. Methods have been developed to help overcome some of the barriers to implementing large trials in the obstetric care setting [91].
Additional investigation is needed into the logistical barriers that providers face in providing vaccines to their patients in the clinic. Although, as discussed above, interventions like standing orders can have a significant impact on vaccine uptake and are recommended as part of standard practice for maternal immunization by ACOG, the majority of prenatal care providers do not report implementing these and other evidence-based strategies to improve uptake [17]. The primary barriers to vaccination reported by physicians are financial barriers to stocking and being reimbursed for the vaccine [16, 17]. In order for any of the interventions identified in this review to have an impact, they must be scalable and sustainable.
Currently the influenza vaccine and the tetanus, diphtheria and acellular pertussis (Tdap) vaccine are the only two vaccines recommended by the ACIP during pregnancy. Although only two studies included in this review evaluated the impact of the included interventions on Tdap uptake [42, 43, 54], many of the interventions described in this review could be applied to Tdap vaccination as well. There is a need for an evaluation of interventions to improve Tdap uptake as well to identify those interventions that may be applicable to maternal immunization broadly. This is increasingly important due to the fact that there are several vaccines in development that may soon be licensed for pregnant women, including ones for respiratory syncytial virus (RSV) and group B streptococcus (GBS) [92]. As the number of vaccines in the maternal immunization schedule increases, there is an even greater need for an effective and comprehensive maternal immunization program. Additionally, as during the 2009 H1N1 influenza pandemic, pregnant women can be a priority vaccination group during a pandemic. The lessons learned from routine maternal immunization can be applied to immunization in pandemic scenarios as well. Lastly, there is evidence that vaccinating during pregnancy is also a predictor of childhood immunization and prenatal visits provide an opportunity to impact childhood vaccine uptake in addition to maternal vaccine uptake [68, 93].
Article highlights.
Interventions that primarily aim to change vaccine attitudes are generally not effective in isolation. However, content that emphasizes the benefits of the vaccine for the infant has demonstrated greater impact.
Despite a provider recommendation being the best predictor of vaccine receipt among pregnant women, few studies have evaluated interventions that focus on improving the provider-patient interaction or the provision of information or communication training to providers from public health officials.
Nudge-based interventions, such as provider prompts and standing orders, that build on favorable intentions to vaccinate without attempting to change attitudes about vaccines have demonstrated substantial success in improving uptake.
Most providers list the primary barriers to providing the vaccine to patients as financial. More work is needed to assist providers in overcoming the logistical barriers to providing vaccine to their pregnant patients, such as navigating reimbursements and stocking the vaccine in clinic.
Acknowledgments
Funding
This work was in part supported by the U.S. National Institutes of Health (grant number:R01AI110482).
Footnotes
Declaration of interest
No conflicts of interest for any of the authors except Dr. Daniel Salmon who received research support and/or consulting funds from Crucell, Pfizer, Walgreens and/or Merck. Dr. Salmon received additional travel funds and honorarium from the National Foundation of Infectious Diseases (NFID).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic Influenza and Pregnant Women. Emerging Infectious Diseases. 2008;14(1):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. The Lancet. 2009;374(9688):451–458. doi: 10.1016/s0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 3.Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007. February 13;176(4):463–8. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuzil KM, Reed GW, Mitchel EF, et al. Impact of Influenza on Acute Cardiopulmonary Hospitalizations in Pregnant Women. American Journal of Epidemiology. 1998;148(11):1094–1102. [DOI] [PubMed] [Google Scholar]
- 5.Mendez-Figueroa H, Raker C, Anderson BL. Neonatal characteristics and outcomes of pregnancies complicated by influenza infection during the 2009 pandemic. Am J Obstet Gynecol. 2011. June;204(6 Suppl 1):S58–63. doi: 10.1016/j.ajog.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2017–2018 Influenza Season. MMWR. 2017;66(2):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(WHO) WHO. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87(47):461–476. [PubMed] [Google Scholar]
- 8.Baguelin M, Flasche S, Camacho A, et al. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013. October;10(10):e1001527. doi: 10.1371/journal.pmed.1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding H, Black CL, Ball S, et al. Influenza Vaccination Coverage Among Pregnant Women - United States, 2016–17 Influenza Season. MMWR. 2017;66(38):1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding H, Black CL, Ball S, et al. Pregnant Women and Flu Vaccination, Internet Panel Survey, United States November 2017: CDC; 2017 [cited 2018 July 5]. Available from: https://www.cdc.gov/flu/fluvaxview/pregnant-women-nov2017.htm
- 11.Kennedy ED, Ahluwalia IB, Ding H, et al. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. Am J Obstet Gynecol. 2012. September;207(3 Suppl):S9–16. doi: 10.1016/j.ajog.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 12.Ding H, Black CL, Ball S, et al. Flu Vaccination Coverage Among Pregnant Women - United States, 2015–2016 Flu Season: CDC; 2016 [cited 2018 July 5]. Available from: https://www.cdc.gov/flu/fluvaxview/pregnant-coverage_1516estimates.htm
- 13.People Health 2020 Topics & Objectives: Immunization and Infectious Disease: Office of Disease Prevention and Health Promotion; [cited 2018 July 5].
- 14.Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014. August 6;32(36):4602–13. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 15.Myers KL. Predictors of maternal vaccination in the United States: An integrative review of the literature. Vaccine. 2016. July 25;34(34):3942–9. doi: 10.1016/j.vaccine.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Power ML, Leddy MA, Anderson BL, et al. Obstetrician-gynecologists’ practices and perceived knowledge regarding immunization. Am J Prev Med. 2009. September;37(3):231–4. doi: 10.1016/j.amepre.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary ST, Riley LE, Lindley MC, et al. Immunization Practices of U.S. Obstetrician/Gynecologists for Pregnant Patients. Am J Prev Med. 2018. February;54(2):205–213. doi: 10.1016/j.amepre.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brewer NT, Chapman GB, Rothman AJ, et al. Increasing Vaccination: Putting Psychological Science Into Action. Psychological Science in the Public Interest. 2017;18(3):149–207. [DOI] [PubMed] [Google Scholar]
- 19.Wong VW, Lok KY, Tarrant M. Interventions to increase the uptake of seasonal influenza vaccination among pregnant women: A systematic review. Vaccine. 2016. January 2;34(1):20–32. doi: 10.1016/j.vaccine.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly. 1984;11:1–47. [DOI] [PubMed] [Google Scholar]
- 21.Fisbein M, Ajzen I. Predicting and changin behavior: the reasoned action approach. New York, NY: Taylor & Francis; 2010. [Google Scholar]
- 22.Rogers RW. A protection motivation theory of fear appeals and attitude change. The Journal of Psychology. 1974;91:93–114. [DOI] [PubMed] [Google Scholar]
- 23.Moniz MH, Hasley S, Meyn LA, et al. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstet Gynecol. 2013. April;121(4):734–40. doi: 10.1097/AOG.0b013e31828642b1. [DOI] [PubMed] [Google Scholar]
- 24.Eppes C, Wu A, Cameron KA, et al. Does obstetrician knowledge regarding influenza increase HINI vaccine acceptance among their pregnant patients? Vaccine. 2012. August 24;30(39):5782–4. doi: 10.1016/j.vaccine.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Bushar JA, Kendrick JS, Ding H, et al. Text4baby Influenza Messaging and Influenza Vaccination Among Pregnant Women. Am J Prev Med. 2017. December;53(6):845–853. doi: 10.1016/j.amepre.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung EJ, Noh JY, Choi WS, et al. Perceptions of influenza vaccination during pregnancy in Korean women of childbearing age. Hum Vaccin Immunother. 2016. August 2;12(8):1997–2002. doi: 10.1080/21645515.2015.1119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meharry PM, Cusson RM, Stiller R, et al. Maternal influenza vaccination: evaluation of a patient-centered pamphlet designed to increase uptake in pregnancy. Matern Child Health J. 2014. July;18(5):1205–14. doi: 10.1007/s10995-013-1352-4. [DOI] [PubMed] [Google Scholar]
- 28.Stockwell MS, Westhoff C, Kharbanda EO, et al. Influenza vaccine text message reminders for urban, low-income pregnant women: a randomized controlled trial. Am J Public Health. 2014. February;104 Suppl 1:e7–12. doi: 10.2105/AJPH.2013.301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong VWY, Fong DYT, Lok KYW, et al. Brief education to promote maternal influenza vaccine uptake: A randomized controlled trial. Vaccine. 2016. October 17;34(44):5243–5250. doi: 10.1016/j.vaccine.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Yudin MH, Salripour M, Sgro M. Impact of Patient Education on Knowledge of Influenza and Vaccine Recommendations Among Pregnant Women. J Obset Gynaecol Can. 2009;32(3):232–237. [DOI] [PubMed] [Google Scholar]
- 31.Hoppe KK, Eckert LO. Achieving high coverage of H1N1 influenza vaccine in an ethnically diverse obstetric population: success of a multifaceted approach. Infect Dis Obstet Gynecol. 2011;2011:746214. doi: 10.1155/2011/746214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy EA, Pollock WE, Nolan T, et al. Improving influenza vaccination coverage in pregnancy in Melbourne 2010–2011. Aust N Z J Obstet Gynaecol. 2012. August;52(4):334–41. doi: 10.1111/j.1479-828X.2012.01428.x. [DOI] [PubMed] [Google Scholar]
- 33.Jordan ET, Bushar JA, Kendrick JS, et al. Encouraging Influenza Vaccination Among Text4baby Pregnant Women and Mothers. Am J Prev Med. 2015. October;49(4):563–72. doi: 10.1016/j.amepre.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yudin MH, Mistry N, De Souza LR, et al. Text messages for influenza vaccination among pregnant women: A randomized controlled trial. Vaccine. 2017. February 1;35(5):842–848. doi: 10.1016/j.vaccine.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Frew PM, Owens LE, Saint-Victor DS, et al. Factors associated with maternal influenza immunization decision-making. Evidence of immunization history and message framing effects. Hum Vaccin Immunother. 2014;10(9):2576–83. doi: 10.4161/hv.32248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frew PM, Saint-Victor DS, Owens LE, et al. Socioecological and message framing factors influencing maternal influenza immunization among minority women. Vaccine. 2014. March 26;32(15):1736–44. doi: 10.1016/j.vaccine.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Frew PM, Kriss JL, Chamberlain AT, et al. A randomized trial of maternal influenza immunization decision-making: A test of persuasive messaging models. Hum Vaccin Immunother. 2016. August 2;12(8):1989–1996. doi: 10.1080/21645515.2016.1199309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouzoon ME, Munoz FM, Greisinger AJ, et al. Improving influenza immunization in pregnant women and healthcare workers. Am J Manag Care. 2010. Mar;16(3):209–16. [PubMed] [Google Scholar]
- 39.Ogburn T, Espey EL, Contreras V, et al. Impact of Clinic Interventions on the Rate of Influenza Vaccination in Pregnant Women. The Journal of Reproductive Medicine. 2007;52:753–756. [PubMed] [Google Scholar]
- 40.Panda B, Stiller R, Panda A. Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Matern Fetal Neonatal Med. 2011. March;24(3):402–6. doi: 10.3109/14767058.2010.497882. [DOI] [PubMed] [Google Scholar]
- 41.Wallis DH, Chin JL, Sur DK, et al. Increasing rates of influenza vaccination during pregnancy: a multisite interventional study. J Am Board Fam Med. 2006. Jul-Aug;19(4):345–9. [DOI] [PubMed] [Google Scholar]
- 42.Chamberlain AT, Seib K, Ault KA, et al. Improving influenza and Tdap vaccination during pregnancy: A cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine. 2015. July 9;33(30):3571–9. doi: 10.1016/j.vaccine.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain AT, Seib K, Ault KA, et al. Impact of a multi-component antenatal vaccine promotion package on improving knowledge, attitudes and beliefs about influenza and Tdap vaccination during pregnancy. Hum Vaccin Immunother. 2016. August 2;12(8):2017–2024. doi: 10.1080/21645515.2015.1127489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baxter D Approaches to the vaccination of pregnant women: experience from Stockport, UK, with prenatal influenza. Hum Vaccin Immunother. 2013. Jun;9(6):1360–3. doi: 10.4161/hv.25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The SAGE Vaccine Hesitancy Working Group. What influences vaccine acceptance: A model of determinants of vaccine hesitancy. 2013.
- 46.Salmon DA, Dudley MZ, Glanz JM, et al. Vaccine hesitancy: Causes, consequences, and a call to action. Vaccine. 2015. November 27;33 Suppl 4:D66–71. doi: 10.1016/j.vaccine.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 47.Healy CM, Rench MA, Montesinos DP, et al. Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine. 2015. October 5;33(41):5445–5451. doi: 10.1016/j.vaccine.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 48.Wallis DH CJ, Sur DKC, Lee MY. Increasing Rates of Influenza Vaccination During Pregnancy: A Multisite Interventional Study. Journal of the American Board of Family Medicine. 2006;19:345–349. [DOI] [PubMed] [Google Scholar]
- 49.DiBonaventura MD, Chapman GB. Moderators of the intention–behavior relationship in influenza vaccinations: Intention stability and unforeseen barriers. Psychology & Health. 2005;20(6):761–774. [Google Scholar]
- 50.Opel DJ, Marcuse EK. Window or mirror: social networks’ role in immunization decisions. Pediatrics. 2013. May;131(5):e1619–20. doi: 10.1542/peds.2013-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 Measles Outbreak in Indiana for Sustained Elimination of Measles in the United States. New England Journal of Medicine. 2006;355(5). [DOI] [PubMed] [Google Scholar]
- 52.Omer SB, Salmon DA, Orenstein WA, et al. Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Disease. New England Journal of Medicine. 2009;360:1981–88. [DOI] [PubMed] [Google Scholar]
- 53.Hainer BL. Vaccine adminstration: making the process more efficient in your practice. Fam Pract Manag. 2007;14(3):48–53. [PubMed] [Google Scholar]
- 54.Mazzoni SE, Brewer SE, Pyrzanowski JL, et al. Effect of a multi-modal intervention on immunization rates in obstetrics and gynecology clinics. Am J Obstet Gynecol. 2016. May;214(5):617 e1–7. doi: 10.1016/j.ajog.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth and Happiness. New York, New York: Penguin Books; 2008. [Google Scholar]
- 56.Sheeran P Intention-Behavior Relations: A Conceptual and Empirical Review. European Review of Social Psychology. 2002;12(1):1–36. [Google Scholar]
- 57.Sheeran P, Webb TL. The Intention-Behavior Gap. Social and Personality Psychology Compass. 2016;10(9):503–518. [Google Scholar]
- 58.Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011. March;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. American journal of preventive medicine. 2000. January;18(1 Suppl):97–140. [DOI] [PubMed] [Google Scholar]
- 60.Groom H, Hopkins DP, Pabst LJ, et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J Public Health Manag Pract. 2015. May-Jun;21(3):227–48. doi: 10.1097/PHH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, et al. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018. January 18;1:CD003941. doi: 10.1002/14651858.CD003941.pub3. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: A review. Jama. 2000. October 11;284(14):1820–7. [DOI] [PubMed] [Google Scholar]
- 63.Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Annals of internal medicine. 2002. May 7;136(9):641–51. [DOI] [PubMed] [Google Scholar]
- 64.Mazzoni SE, Brewer SE, Pyrzanowski JL, et al. Effect of a multi-modal intervention on immunization rates in obstetrics and gynecology clinics. American journal of obstetrics and gynecology. 2016. May;214(5):617.e1–7. doi: 10.1016/j.ajog.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Riley M, Galang S, Green LA. The impact of clinical reminders on prenatal care. Family medicine. 2011. September;43(8):560–5. [PubMed] [Google Scholar]
- 66.Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012. February;119(2 Pt 1):301–5. doi: 10.1097/AOG.0b013e318242032a. [DOI] [PubMed] [Google Scholar]
- 67.Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000. January;18(1 Suppl):92–6. [PubMed] [Google Scholar]
- 68.Link-Gelles R, Chamberlain AT, Schulkin J, et al. Missed opportunities: a national survey of obstetricians about attitudes on maternal and infant immunization. Matern Child Health J. 2012. December;16(9):1743–7. doi: 10.1007/s10995-011-0936-0. [DOI] [PubMed] [Google Scholar]
- 69.Merrill MH, Hollister AC, Gibbens SF, et al. Attitudes of Californians toward poliomyelitis vaccination. Am J Public Health Nations Health. 1958;48(2):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogburn T, Espey EL, Contreras V, et al. Impact of clinic interventions on the rate of influenza vaccination in pregnant women. J Reprod Med. 2007. September;52(9):753–6. [PubMed] [Google Scholar]
- 71.Zakrzewski L, Sur DK, Agrawal N. Staff versus physician vaccine protocols for influenza immunization during pregnancy. J Am Board Fam Med. 2014. Jan-Feb;27(1):56–60. doi: 10.3122/jabfm.2014.01.130002. [DOI] [PubMed] [Google Scholar]
- 72.O’Leary STR L; Lindley MC; Allison MA; Crane L; Hurley LP; Beaty B; Brtnikova M; Albert A; Fisher A; Jiles A; Kempe A Provider Attitudes and Practices Regarding Maternal Vaccination Among Obstetrician-Gynecologists: A National Survey. Open Forum Infectious Diseases. 2017 October 1, 2017;4(Suppl_1):S457. [Google Scholar]
- 73.Barnard JG, Dempsey AF, Brewer SE, et al. Facilitators and Barriers to the Use of Standing Orders for Vaccination in Obstetrics and Gynecology Settings. Am J Obstet Gynecol. 2016. September 26. doi: 10.1016/j.ajog.2016.09.096. . [DOI] [PubMed] [Google Scholar]
- 74.Ding H, Black CL, Ball S, et al. Influenza Vaccination Coverage Among Pregnant Women - United States, 2016–17 Influenza Season. MMWR Morb Mortal Wkly Rep. 2017. September 29;66(38):1016–1022. doi: 10.15585/mmwr.mm6638a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phadke VK, Omer SB. Maternal vaccination for the prevention of influenza: current status and hopes for the future. Expert Rev Vaccines. 2016. October;15(10):1255–80. doi: 10.1080/14760584.2016.1175304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ault KA, Heine RP, Riley LE. Programmatic and research priorities for improving influenza immunization of pregnant women. American Journal of Obstetrics and Gynecology. 2012;207(3):S75–S77. doi: 10.1016/j.ajog.2012.06.078. [DOI] [PubMed] [Google Scholar]
- 77.Practice CoG, Practice CoO, Group IW. Committee Opinion: Integrating Immunizations Into Practice. The American College of Obstetricians and Gynecologists; 2016. [Google Scholar]
- 78.Practice CoO. ACOG Committee Opinion: Influenza Vaccine During Pregnancy. Obstet Gynecol. 2018;131(4):e109–e114. [DOI] [PubMed] [Google Scholar]
- 79.Chamberlain AT, Seib K, Ault K, et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diptheria and Acellular Pertussis (Tdap) Vaccines During Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. PLoS Currents. 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sadique MZ, Devlin N, Edmunds WJ, et al. The effect of perceived risks on the demand for vaccination: results from a discrete choice experiment. PLoS One. 2013;8(2):e54149. doi: 10.1371/journal.pone.0054149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Omer SB, Amin AB, Limaye RJ. Communicating About Vaccines in a Fact-Resistant World. JAMA Pediatr. 2017. October 1;171(10):929–930. doi: 10.1001/jamapediatrics.2017.2219. [DOI] [PubMed] [Google Scholar]
- 82.Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014. April;133(4):e835–42. doi: 10.1542/peds.2013-2365. [DOI] [PubMed] [Google Scholar]
- 83.Cook J, Lewandowsky S. The Debunking Handbook. 2011. [Google Scholar]
- 84.Lynch MM, Squiers LB, Kosa KM, et al. Making Decisions About Medication Use During Pregnancy: Implications for Communication Strategies. Matern Child Health J. 2018. January;22(1):92–100. doi: 10.1007/s10995-017-2358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marsh HA, Malik F, Shapiro E, et al. Message framing strategies to increase influenza immunization uptake among pregnant African American women. Matern Child Health J. 2014. September;18(7):1639–47. doi: 10.1007/s10995-013-1404-9. [DOI] [PubMed] [Google Scholar]
- 86.O’Leary ST, Riley L, Lindley MC, et al. Vaccine Refusal Among Pregnant Women: A National Survey of Obstetrician-Gynecologists. Open Forum Infectious Diseases. 2017;4(suppl_1):S515. [Google Scholar]
- 87.Ellingson MK, Bonk CM, Chamberlain AT. A survey-based study of Zika virus communication preferences among pregnant women in Georgia, United States. BMC Pregnancy Childbirth. 2017. September 26;17(1):325. doi: 10.1186/s12884-017-1516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013. December;132(6):1037–46. doi: 10.1542/peds.2013-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dempsey AF, Pyrznawoski J, Lockhart S, et al. Effect of a Health Care Professional Communication Training Intervention on Adolescent Human Papillomavirus Vaccination: A Cluster Randomized Clinical Trial. JAMA Pediatr. 2018. May 7;172(5):e180016. doi: 10.1001/jamapediatrics.2018.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Opel DJ, Mangione-Smith R, Robinson JD, et al. The Influence of Provider Communication Behaviors on Parental Vaccine Acceptance and Visit Experience. Am J Public Health. 2015. October;105(10):1998–2004. doi: 10.2105/AJPH.2014.302425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Leary ST, Pyrzanowski J, Brewer SE, et al. Evidence-based vaccination strategies in obstetrics and gynecology settings: Current practices and methods for assessment. Hum Vaccin Immunother. 2016. April 2;12(4):866–71. doi: 10.1080/21645515.2015.1130194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swamy GK, Beigi RH. Maternal benefits of immunization during pregnancy. Vaccine. 2015. November 25;33(47):6436–40. doi: 10.1016/j.vaccine.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 93.Navar AM, Halsey NA, Carter TC, et al. Prenatal immunization education the pediatric prenatal visit and routine obstetric care. Am J Prev Med. 2007. September;33(3):211–3. doi: 10.1016/j.amepre.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 94.Goodman KMS, Taksler GB, Emery J, Schramm S, Rothber MB. Impact of video education on influenza vaccination in pregnancy. Journal of Reproductive Medicine. 2015;60(11–12):471–479. [PMC free article] [PubMed] [Google Scholar]


