Highlights
-
•
Third-generation cephalosporins are common first line agents to empirically treat typhoid fever.
-
•
An outbreak of extensively-drug resistant (XDR) typhoid has emerged in Pakistan that is resistant to ceftriaxone.
-
•
This is the first Canadian case of XDR typhoid in a child who travelled to Pakistan and was successfully treated with 2 weeks of meropenem.
-
•
Clinicians should consider adapting their empiric management of typhoid for travellers returning from XDR regions.
Keywords: Salmonella Typhi, Typhoid, Pediatric, Extensive drug resistance, Antimicrobial resistance, Canada
Abstract
We report on a three year-old male who contracted enteric fever during a visit to the Sindh province of Pakistan in the summer of 2018. He was diagnosed after returning to Canada and blood cultures isolated Salmonella enterica serovar Typhi which harbored extensive drug-resistance (XDR) to all first-line antibiotics including ceftriaxone. Empiric ceftriaxone was switched to meropenem and he was successfully treated with a two-week course. An outbreak of XDR typhoid is currently emerging from Pakistan and several outbreak-related cases have been identified in the U.K and U.S. Whole genome sequencing confirmed that our child was infected with the XDR outbreak-strain. Current empiric antimicrobial choices will result in treatment failure if an XDR strain is encountered, therefore clinicians must adapt their empiric approach for those returning from high risk regions. This is the first XDR typhoid case in Canada and the first pediatric case to be diagnosed and treated outside of Pakistan. Clinicians must be vigilant of future cases.
Introduction
Enteric fever remains a significant disease that inflicts a great health burden worldwide, with an estimate of 21.6–26.9 million cases and 216 000 deaths each year attributed to Salmonella Typhi [1,2]. The current empiric management of typhoid in many centers is the use of third-generation cephalosporins. In November 2016, a large outbreak of extensively drug-resistant (XDR) typhoid has emerged in Pakistan which demonstrated resistance to all first-line antimicrobials including third-generation cephalosporins, thus creating a new challenge to empiric strategies.
Case report
A healthy three-year-old boy became unwell during a holiday to Karachi, Pakistan in June-July 2018. Before his return to Canada, he developed fever, abdominal pain, diarrhea, and vomiting for two days. He attended a local physician, who prescribed cefixime and advised the family to follow up at the Emergency Department (ED) in Canada. There were no known infective contacts and he stayed with maternal grandparents in Karachi throughout the visit. The family only visited relatives and attended a family wedding in the same area. They consumed locally prepared food and drank bottled water. The family did not seek pre-travel advice and did not receive typhoid vaccine, malaria prophylaxis, or other travel related medication.
The child was seen at the ED at Toronto’s Hospital for Sick Children soon after returning to Canada and was diagnosed with presumed enteric fever, empirically started on ceftriaxone and admitted to a nearby pediatric unit for further management. Stool and blood cultures obtained during the initial ED visit subsequently grew Salmonella enterica serovar Typhi (S. Typhi).
Antimicrobial susceptibility testing of the isolate identified resistance to chloramphenicol, ampicillin, trimethoprim-sulfamethoxazole (TMP-SMX), ciprofloxacin and ceftriaxone but susceptible to meropenem and azithromycin (Table 1). After consultation with the Pediatric Infectious Disease service, the patient was switched to intravenous meropenem at a dose of 20 mg/kg/dose on day two of admission. Blood cultures remained positive twenty-four hours after starting meropenem, but the cultures taken three days later were negative. Despite being treated with appropriate antibiotics, the child continued to have daily high grade fevers thirteen days into meropenem treatment albeit less frequent. Abdominal ultrasound scans only demonstrated mild hepatosplenomegaly which resolved and daily full-systems examinations were unremarkable. He completed fourteen days of meropenem following which he was afebrile and clinically well. Three stool cultures obtained after completion of meropenem therapy were negative.
Table 1.
MIC values of isolated Salmonella Typhi PHL5950.
| Antibiotic | MIC (mg/L) | Susceptibility |
|---|---|---|
| Chloramphenicol | >32 | Resistant |
| Ampicillin | >16 | Resistant |
| TMP-SMX | ≥320 | Resistant |
| Ciprofloxacin | 2 | Resistant |
| Ceftriaxone | >32 | Resistant |
| Meropenem | ≤0.125 | Sensitive |
| Azithromycin | ≤16 | Sensitive |
| Tigecycline | 0.25 | No interpretive guideline |
Abbreviations: MIC (minimal inhibitory concentration); TMP-SMX (Trimethoprim-sulfamethoxazole).
MIC interpretative criteria are based on Clinical and Laboratory Standards Institute M100-S28.
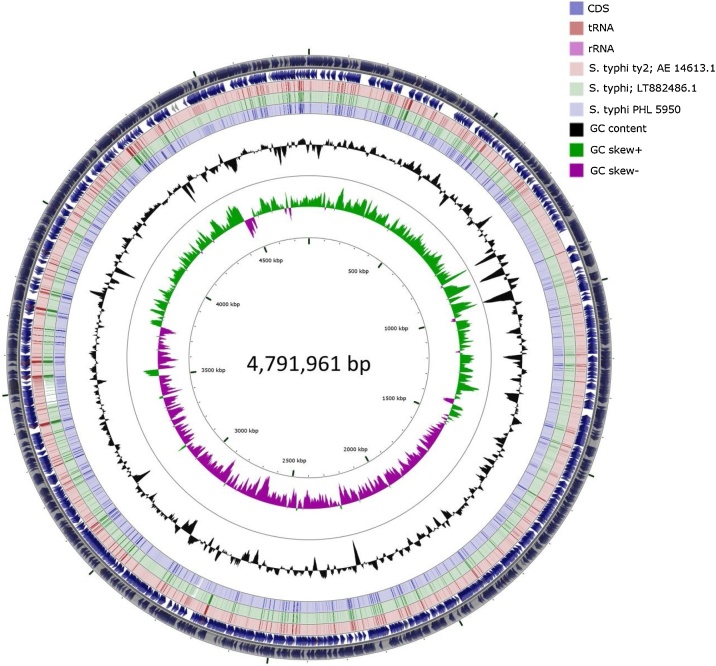
This patient’s S. Typhi isolate (S. Typhi strain PHL5950) underwent whole genome sequencing which was assessed using the ResFinder, SPIFindertools, SeqSero-1.2 Server, and PlasmidFinder [3]. Initially, the raw Illumina reads were trimmed generating 3,625,048 high quality reads corresponding to 866,576,883 detected bases and assembled using de novo assembler in CLC Genomics Workbench version 8.0.1 (CLC bio, Germantown, MD, USA) providing 85 contigs (accession no. RHPM00000000). The closest reference using functional comparison on the RAST server was found to be Salmonella enterica subspecies enterica serovar Typhi Ty2 reference genome NCBI:txid209261 (score, 534). BLAST result of the longest contigs (488765 bp) showed 100% identity to S. enterica subsp. enterica serovar Typhi (accession no. LT882486.1), a recently identified extensively drug-resistant S. Typhi strain from an outbreak in Pakistan, which encodes a chromosomally located resistance region and harbours a plasmid encoding additional resistance elements, including the blaCTX-M-15 extended-spectrum β-lactamase, and carrying the qnrS fluoroquinolone resistance gene (Fig. 1). The ResFinder tool identified multiple resistance genes conferring resistance to various antibiotics (Supplementary Table 1). In addition, mutation at S83 of the gyrA gene was also identified. Based on the PlasmidFinder tool, we identified two plasmids: IncY plasmid, which matched 100% to the plasmid that was isolated in the Pakistan outbreak-strain (accession no. LT906492) [4], and IncQ1 plasmid.
Fig. 1.
Graphical circular map of S. Typhi PHL 5950 de-novo assembly contigs compare to strains LT882486.1 and strain Ty2 drawn with CGView. S. Typhi strain Ty2 used as reference for coding regions.
Discussion
To our knowledge, this is one of the first cases of XDR typhoid in a child outside of Pakistan. Typhoid fever caused by S. Typhi, continues to inflict a significant health burden worldwide with reports estimating 21.6–26.9 million cases and 216 000 typhoid-associated deaths each year [1,2]. The highest incidence of typhoid is in low to middle income countries (LMIC) that have poor sanitation and public health infrastructure; rates are highest in South Asia [5].
The emergence of S. Typhi strains resistant to chloramphenicol first appeared in the early 1970s [6] and resistance to other first line agents including ampicillin and TMP-SMX became increasingly prevalent in the 1980s and early 1990s, leading to the term multidrug resistant (MDR) typhoid. Fluoroquinolones, particularly ciprofloxacin, become first line therapy in the 1990s however, resistance developed shortly thereafter. Phylogenetic studies identified MDR typhoid to be associated with the dominant H58 lineage which has spread throughout the world, namely Asia and Africa [7]. The mode of resistance acquisition is through a combination of plasmid transfer and integrated antimicrobial resistance genes at different chromosomal loci [4,6,7]. Due to the emergence of MDR typhoid, most nations are now reliant upon third-generation cephalosporins like ceftriaxone and azilides like azithromycin, as first-line agents. For LMIC, these antimicrobials have become the only financially feasible options.
International travel is increasing, thus creating a need for clinicians to be vigilant of diseases that are commonly imported from abroad and of the resistance profiles that these infections may harbor. Travelers who visit friends and relatives (VFRs), particularly to the Indian subcontinent, are at the highest risk of contracting enteric fever [8]. In a recent twenty-eight-year review of all children with enteric fever who presented to Toronto’s Hospital for Sick Children, 89% were VFRs and 80% were acquired during travel to Pakistan, India or Bangladesh [8]. All isolates in this study were sensitive to ceftriaxone, which is consistent with the common practice in Ontario to treat suspected typhoid empirically with ceftriaxone.
Over the last two decades, sporadic cases have been reported of XDR typhoid resistant to ceftriaxone [4] and even azithromycin [9]. Since November 2016, Pakistan’s Sindh province has experienced a large outbreak of XDR S. Typhi, predominantly in the Hyderabad and Karachi areas. As of December 2018, more that 5000 cases have been reported and all isolates have been resistant to ampicillin, TMP-SMX, ciprofloxacin and ceftriaxone but remain susceptible to azithromycin [4,10,11]. The Sindh-outbreak strain is associated with the H58 haplotype and holds a chromosomally integrated composite transposon at the yidA locus which carries resistant genes to chloramphenicol (catA1), ampicillin (blaTEM-1), TMP-SMX (dfrA7, sul1, sul2) and ciprofloxacin (chromosomal gyrA mutation S83 F). This strain also includes an IncY plasmid that harbors a resistance gene to ciprofloxacin (qnrS) and an extended-spectrum β-lactamase (ESBL) gene conferring resistance to ceftriaxone (blaCTX-M-15). The MDR H58 haplotype likely transformed to the XDR strain after acquiring the ESBL harboring IncY plasmid from an Escherichia coli in Pakistan [4]. Our strain as well as the isolated plasmid are identical to the Sindh-outbreak strain.
So far in 2018, there have been six Pakistan outbreak-related cases of XDR typhoid in travelers from the United Kingdom and United States [4,6,11]. With the ease of travel, this number will certainly increase and will have implications for the clinicians who look after returning travelers from outbreak areas, namely choice of empiric antibiotics. The current practice of initiating ceftriaxone in these patients will be ineffective if the infecting strain is XDR. A potential empiric strategy is the use of meropenem for the septic child or the combination of ceftriaxone and azithromycin for the clinically stable, with further rationalization once sensitivities become available.
A recent significant advancement in the containment of typhoid is the prequalification of the Vi polysaccharide tetanus-toxoid conjugate vaccine (Typbar-TCV®) issued by the World Health Organization (WHO) in March 2018. The WHO recommends implementation of large scale TCV vaccination programs in endemic areas, which is effective from six months of age [12,13].
We report the first XDR typhoid case in Canada diagnosed in a 3 year old child returning from Pakistan, who was treated successfully with two weeks of meropenem. Clinicians must be vigilant of typhoid resistance in high-risk areas such as Pakistan and develop new strategies for empiric management.
Conflict of interest
No conflict of interest to declare by authors.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Ethical approval
Ethical approval was not required for this work.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Waison Wong: First author with main role in writing the original draft.
Hatem Al Rawahi: Assisted in writing the original draft.
Samir Patel: Assisted in writing and carried out whole genome sequencing.
Yvonne Yau: Assisted in writing (review and editing).
Alireza Eshaghi: Assisted in writing and carried out whole genome sequencing.
Sandra Zittermann: Assisted in writing and carried out whole genome sequencing.
Leah Tattum: Assisted in writing (review and editing).
Shaun Morris: Supervision, writing (review and editing).
Acknowledgements
We would like to pay special acknowledgement to the staff at Public Health Ontario Laboratory for their contributions and for carrying out whole genome sequencing on the isolate.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.idcr.2019.e00492.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Crump J.A., Luby S.P., Mintz E.D. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Buckle G.C., Walker C.L.F., Black R.E. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;(2):010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Genomic Epidemiology. Welcome to the center for genomic epidemiology. http://genomicepidemiology.org/ (Accessed 19 September 2018).
- 4.Klemm E.J., Shakoor S., Page A.J., Qamar F.N., Judge K., Saeed D.K. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. MBio. 2018;9:1–10. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radhakrishnan A., Als D., Mintz E.D., Crum J.A. Introductory article on global burden and epidemiology of typhoid fever. Am J Trop Med Hyg. 2018;99:4–9. doi: 10.4269/ajtmh.18-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine M., Simon R. The gathering storm: is untreatable typhoid fever on the way? MBio. 2018:1–4. doi: 10.1128/mBio.00482-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong V.K., Baker S., Pickard D.J., Parkhill J., Page A.J., Feasey N.A. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter-and intracontinental transmission events. Nat Genet. 2015;47:632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou K., Sauve L.J., Richardson S.E., Lee Ford-Jones E., Morris S.K. Enteric fever in a multicultural Canadian tertiary care pediatric setting: a 28-year review. J Pediatric Infect Dis Soc. 2017;6:98–101. doi: 10.1093/jpids/piw007. [DOI] [PubMed] [Google Scholar]
- 9.Wong M.H.Y., Yan M., Chan E.W.C. Emergence of clinical Salmonella enterica serovar Typhimurium isolates with concurrent resistance to ciprofloxacin, ceftriaxone, and azithromycin. Antimicrob Agents Chemother. 2014;58:3752–3753. doi: 10.1128/AAC.02770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen J. ‘Frightening’ typhoid fever outbreak spreads in Pakistan. Science. 2018;361:214. doi: 10.1126/science.361.6399.214. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . 2018. Typhoid Fever – Islamic Republic of Pakistan.https://www.who.int/csr/don/27-december-2018-typhoid-pakistan/en/ (Accessed 6 January 2018) [Google Scholar]
- 12.Burki T. Typhoid conjugate vaccine gets WHO prequalification. Lancet Infect Dis. 2018;18:258. doi: 10.1016/S1473-3099(18)30088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 2018. Typhoid vaccines: WHO position paper, March 2018 – Recommendations. Vaccine; pp. 4–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.