Abstract
A recent case report described a case of pulmonary arterial hypertension (PAH) associated with use of the Chinese herbal medicine Qing-Dai; however, the clinical course and possible mechanisms have not been characterized. We present the case of a man with ulcerative colitis who was diagnosed with idiopathic PAH. After initiating oral beraprost therapy, the patient showed significant hemodynamic improvements and an unusual course of clinical recovery. In 2016, the Japanese Ministry of Health, Labour, and Welfare issued a warning regarding the possible side effects of Qing-Dai. We learned that our patient had been taking self-purchased Qing-Dai for 2 years. Therefore, we performed an experimental study and determined that Qing-Dai may cause PAH through a mechanism involving nitric oxide synthase inhibition and pulmonary artery endothelial dysfunction.
Keywords: Adverse event, Qing-Dai, Pulmonary arterial hypertension, Indigo
1. Introduction
A number of drugs and toxins can cause pulmonary arterial hypertension (PAH1) as an important adverse event [1]. Herein, we present the case of a man with ulcerative colitis (UC) who was diagnosed with PAH. After initiating oral beraprost therapy, the patient showed significant hemodynamic improvements and an unusual course of clinical recovery. Therefore, we performed an experimental study to investigate the possible mechanism by which Qing-Dai induces PAH.
2. Case report
A 32-year-old man with a 5-year history of UC presented to a community hospital in February of 2014 complaining of dyspnea that worsened upon exertion. Chest x-ray examination did not reveal any significant abnormalities; however, electrocardiogram showed the presence of right ventricular hypertrophy. Pulmonary function tests did not show restrictive and obstructive impairment; however, these tests revealed a reduction in diffusion capacity for carbon monoxide. Contrast-enhanced chest computed tomography excluded possible pulmonary embolism and lung diseases. Transthoracic echocardiography (TTE) revealed the presence of pulmonary hypertension (PH) with typical systolic flattening of the interventricular septum, right ventricle dilation, and severe tricuspid regurgitation (TR) (gradient 72 mmHg). The results of right heart catheterization (RHC) indicated pulmonary artery pressures of 82/34 mmHg (mean 48 mmHg), a pulmonary artery wedge pressure of 8 mmHg, thermodilution cardiac output of 5.3 L/min, a cardiac index of 3.1 L/min/m2, and pulmonary vascular resistance of 7.5 Wood units, consistent with precapillary PH. The findings of a ventilation-perfusion scan were negative for pulmonary embolism. The patient was seen by a rheumatologist who excluded possible underlying collagen tissue disease (laboratory results were positive for anti-nuclear antibodies 1:640, anti-RNP antibody, and anti-SSA antibody, but there was no active joint disease or apparent connective tissue disease). There was no family history of PH. The patient was finally diagnosed with idiopathic pulmonary arterial hypertension (IPAH) and initiated oral beraprost therapy at a dose of 60 μg/day.
In March of 2014, the patient was referred to our department for IPAH. Although beraprost is only weakly recommended by current guidelines due to the lack evidence indicating long-term efficacy [2], the patient showed an improvement in dyspnea from a World Health Organization Functional Class of III to II. Moreover, his TR pressure gradient had decreased from 72 to 37 mmHg on TTE. Given the apparent effectiveness of beraprost, we increased the dose to 120 μg/day in a sustained release form and carefully observed the patient. Three months after treatment initiation, the patient showed remarkable hemodynamic improvements compared to baseline (Table 1); therefore, we maintained the therapy. In 2017 (3 years after initial presentation), it was discovered that the patient had been taking oral Qing-Dai, including natural indigo, for UC at a dose of 3 g/day for 2 years. Qing-Dai is used to treat various inflammatory conditions including UC. Notably, the safety and efficacy of Qing-Dai for UC was evaluated by a Japanese research group [3]; however, the study was terminated due to a case report published in July of 2016 that identified Qing-Dai as a possible cause of PAH [4]. Our patient underwent follow-up RHC and the results showed the continued efficacy of beraprost (Table 1). The patient discontinued Qing-Dai and we decreased the dose of beraprost to 60 μg/day. In November of 2017, the patient stopped taking beraprost and has been without PAH recurrence for 18 months.
Table 1.
Patient characteristics.
| Feb-2014 | June-2014 | Apr-2017 | |
|---|---|---|---|
| WHO-FC | III | II | I |
| Hemodynamics | |||
| PA pressure, mmHg | |||
| Systolic | 82 | 24 | 22 |
| Diastolic | 36 | 10 | 8 |
| Mean | 48 | 17 | 12 |
| PAWP, mmHg | 9 | 3 | 8 |
| CI, L/min/m2 | 3.1 | 2.8 | 2.6 |
| PVR, Wood units | 7.5 | 2.2 | 1.1 |
| RAP, mmHg | 9 | 3 | 3 |
| Blood tests | |||
| BNP, pg/mL | 54 | <5.8 | <5.8 |
| Medication | |||
| PAH drug | No | Beraprost 60mg b.i.d | Beraprost 60mg b.i.d |
| Qing-Dai | 3g a day | 3 g a day | 3 g a day |
BNP = brain natriuretic peptide, CI = cardiac index, PVR = pulmonary vascular resistance.
PA = pulmonary artery, PAH = pulmonary arterial hypertension.
PAWP = pulmonary artery wedge pressure, RAP = right atrial pressure.
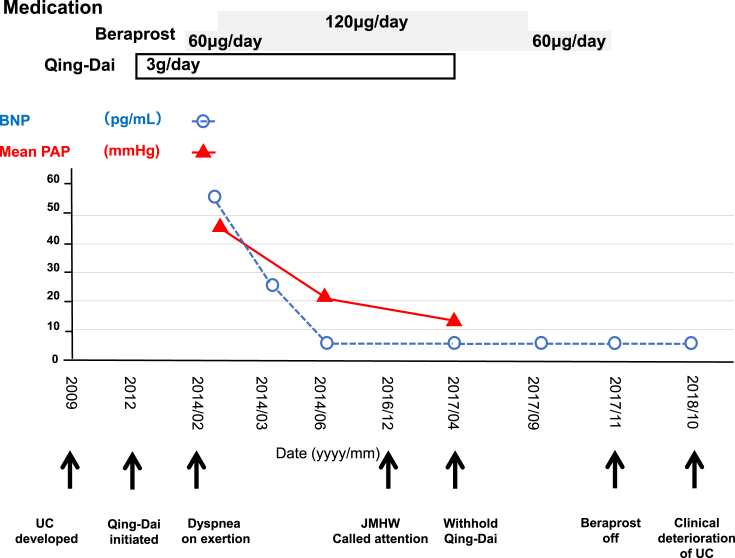
However, the clinical deterioration of UC was observed 6 months after discontinuation of Qing-Dai, requiring an intensive treatment for UC. The patient's clinical course is shown in Fig. 1.
Fig. 1.
Clinical course of the patient including brain natriuretic peptide (BNP) levels (dashed blue line, circles) and mean pulmonary arterial pressure (mPAP; solid red line, triangles) measured by right heart catheterization. The time line on the abscissa is not linear and highlights the development of ulcerative colitis (UC); dyspnea on exertion; the statement issued by the Japanese Ministry of Health, Labour and Welfare regarding Qing-Dai; and the cessation of oral Qing-Dai. Boxes indicate the dose and duration of medication.
3. Discussion
Naganuma and colleagues performed a randomized controlled trial to investigate the safety and efficacy of Qing-Dai in patients with UC [3]. The results indicated that 8 weeks of oral Qing-Dai produced a significant clinical response; however, during the study, the Japanese Ministry of Health, Labour and Welfare issued a statement regarding the possible side effects of Qing-Dai after publication of a case report by Nishio et al. [4]. The clinical trial was terminated although no patients developed PAH during the follow-up period.
Nishio et al. was the first to report the development of PAH in a patient with UC who was taking Qing-Dai [4]. The patient's pulmonary artery pressures were mildly elevated (58/25 mmHg; mean, 36 mmHg); however, the patient's hemodynamics did not improve 1 month after discontinuation of Qing-Dai. In this report, they did not show the effectiveness of PAH specific vasodilators. Recently, Misumi et al. reported a detailed clinical course of a PAH patient taking Qing-Dai [5]. The patient developed PAH at 20 months after the initiation of Qing-Dai treatment for refractory UC. The patient's mean pulmonary artery pressure was 32 mmHg and pulmonary vascular resistance was 7.1 Wood units. The patient recovered from PAH after the withdrawal of Qing-Dai and triple combination therapy including Macitentan, Tadalafil, and Beraprost. They showed that the patient remained free from recurrence of PAH for more than 1 year after the discontinuation of Qing-Dai and PAH drugs.
Our patient exhibited more severe hemodynamics than Misumi's patient; however, the patient responded well to beraprost therapy, regardless of his intake of Qing-Dai, and did not experience PAH recurrence after discontinuing Qing-Dai and beraprost.
We hypothesized that one of the possible mechanisms to trigger the development of Qing-Dai induced PAH may be pulmonary arterial endothelial dysfunction, which induces vasoconstriction. When Qing-Dai-induced PAH is diagnosed in early stages, the patient might show a good response to PAH drugs. However, in the advanced stage, more intensive intervention is required. There are no studies evaluating the effects of Qing-Dai in the context of PAH. Therefore, we examined the effects of Qing-Dai on the rat thoracic aorta (Appendices).
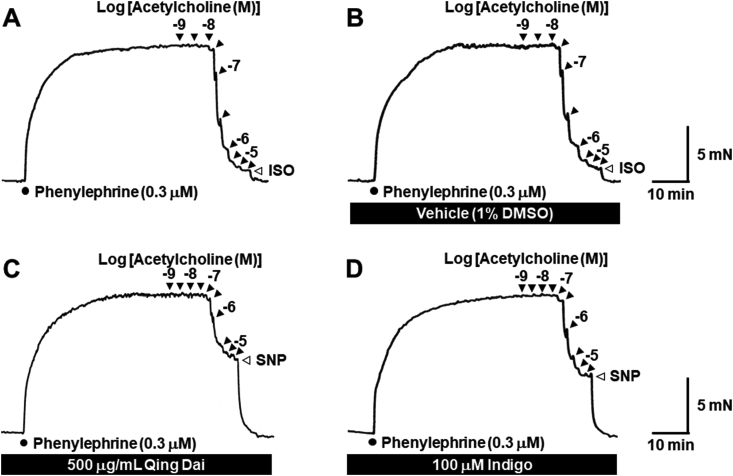
Fig. 2 illustrates typical traces of the relaxant response to acetylcholine in rat thoracic aorta ring preparations precontracted by the stimulation of α1-adrenoceptors with (−)-phenylephrine (0.3 μM). Acetylcholine produced concentration-dependent relaxation (Fig. 2) that was strongly attenuated in the presence of 500 μg/mL Qing-Dai (Fig. 2C and 3A) or 100 μM indigo (Fig. 2D and 3D). The residual components of acetylcholine-insensitive contraction were completely abolished by further administration of sodium nitroprusside (3 μM; Fig. 2C and D), which induces nitric oxide release and guanylate cyclase-mediated vasodilation.
Fig. 2.
Typical traces showing the relaxant effect of acetylcholine in the absence (A) and presence of 1% DMSO (B), 500 μg/mL Qing Dai (C), and 100 μM indigo (D) in rat thoracic aorta ring preparations precontracted with 0.3 μM (−)-phenylephrine. Filled triangles indicate the addition of acetylcholine. Half-log unit increments in acetylcholine concentration were added. Open triangles indicate the addition of 10 μM (−)-isoprenaline (ISO) or 3 μM sodium nitroprusside (SNP).
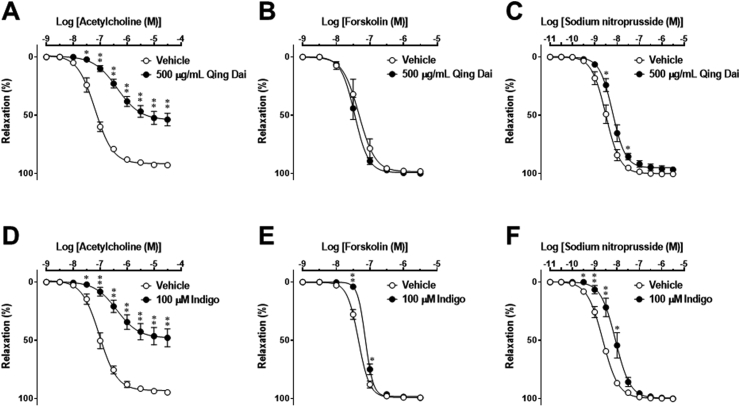
Fig. 3.
Effects of 500 μg/mL Qing Dai (A–C) and 100 μM indigo (D–F) on concentration-response curves for acetylcholine (A and D), forskolin (B and E), and sodium nitroprusside (C and F) in rat thoracic aorta ring preparations precontracted with 0.3 μM (−)-phenylephrine. Acetylcholine (A and D), forskolin (B and E), and sodium nitroprusside (C and F) were applied cumulatively to the bath solution in the presence of vehicle (1% DMSO), 500 μg/mL Qing Dai, or 100 μM indigo. The percentage of smooth muscle relaxation was calculated with respect to basal tension before the application of contracting agents (100% relaxation) and (−)-phenylephrine (0.3 μM) steady-state contractile responses (0% relaxation). Data are presented as the mean ± standard error of the mean from 6 experiments. ∗P < 0.05 and ∗∗P < 0.01 vs. vehicle. When no error bar is shown, the error is smaller than the symbol.
To estimate the direct effects of Qing-Dai and indigo on vascular smooth muscle function, we next examined relaxant responses to forskolin, an activator of adenylate cyclase, and sodium nitroprusside. Forskolin (Fig. 3B and E) and sodium nitroprusside (Fig. 3C and F) produced concentration-dependent relaxation. Unlike acetylcholine, maximum responses to forskolin and sodium nitroprusside were unaffected by 500 μg/mL Qing-Dai (Fig. 3B and C) or 100 μM indigo (Fig. 3E and F); however, 500 μg/mL Qing Dai slightly attenuated the responsiveness of vascular smooth muscle to sodium nitroprusside, and 100 μM indigo diminished the responsiveness of vascular smooth muscle to forskolin and sodium nitroprusside. These results suggested that Qing Dai and indigo attenuate acetylcholine-induced, endothelium-dependent vascular relaxation as a result of endothelial cell dysfunction rather than vascular smooth muscle cell dysfunction.
In conclusion, our case report demonstrates a potential association between oral Qing-Dai and PAH. Oral beraprost monotherapy facilitated hemodynamic normalization even before the cessation of Qing Dai. This clinical course was unusual for a patient with severe PAH. Furthermore, the unique clinical course of our patient and our accompanying experimental study suggested that a possible mechanism to trigger Qing-Dai-induced PAH involves nitric oxide synthase inhibition and endothelial dysfunction in the pulmonary artery. Early diagnosis and intervention can provide good clinical course in UC patients with Qing-Dai induced PAH.
Compliance with ethical standards
There was no funding for this work.
The authors declare that they have no conflict of interest.
Declarations of interest
None.
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Abbreviations: PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RHC, right heart catheterization; TR, tricuspid regurgitation; TTE, transthoracic echocardiography; UC, ulcerative colitis.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.02.007.
Appendices.
1. Materials and Methods
1.1. Drugs
The following drugs were used in the present study: (−)-phenylephrine hydrochloride, forskolin, and sodium nitroprusside dihydrate were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.); (−)-isoprenaline hydrochloride and indigo were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan); acetylcholine chloride was obtained from Nacalai Tesque, Inc. (Kyoto, Japan), and Qing Dai (product name: Seitain) was purchased from Seishin-Syoyakudo (Shinjuku, Japan). All other chemicals were of analytical grade. Qing-Dai and indigo were suspended in dimethyl sulfoxide (DMSO); all other drugs were dissolved in ultra-pure water.
1.2. Animals
Male Wistar/ST rats weighing 250–300 g (Nippon SLC Co., Ltd., Hamamatsu, Japan) were housed under a 12-h light/dark cycle (lights on at 07:00) and had free access to standard laboratory food and tap water. The room temperature and relative air humidity were regulated at 20–26 °C and 40–60%, respectively. The study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Animal Research Committee of Hokkaido University.
1.3. Tissue isolation and preparation
Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg) and sacrificed by exsanguination. The thoracic aorta was immediately isolated and placed in a modified Krebs-Henseleit solution of the following composition (in mM): NaCl, 120.7; KCl, 5.9; MgCl2·6H2O, 1.2; CaCl2·2H2O, 2.0; NaH2PO4·2H2O, 1.2; NaHCO3, 25.5; and D-(+)-glucose, 11.5. The modified Krebs-Henseleit solution was continuously oxygenated with a mixture of 95% O2 and 5% CO2 and maintained at 4 °C (pH = 7.4). The aorta was carefully cleaned of fat and connective tissues and cut into ring segments of approximately 2 mm in length. The arterial rings were mounted using stainless steel hooks under a resting tension of 10 mN in a 5-ml organ bath (UC-5A; UFER Medical Instrument, Kyoto, Japan) containing a continuously oxygenated modified Krebs-Henseleit solution (95% O2 and 5% CO2) that was maintained at 37 °C.
1.4. Measurement of tension changes
Changes in tension were recorded isometrically with a force-displacement transducer (T7-8-240; Orientec, Tokyo, Japan) connected to an amplifier (Polygraph 366 system, NEC Sanei, Tokyo, Japan). Arterial rings were allowed to equilibrate for 30 min before the start of experimental procedures.
1.5. Assessment of the effects of Qing Dai and indigo on acetylcholine, forskolin, and sodium nitroprusside relaxation responses
After a 30-min equilibration period, each arterial ring preparation was contracted with (−)-phenylephrine (0.3 μM). A sustained plateau phase was observed approximately 40 min after the addition of (−)-phenylephrine. Then, we examined the concentration-response relationship for acetylcholine-induced relaxation with 2 applications. Drugs were washed out, preparations were allowed to re-equilibrate for 30 min, and contraction was again induced with (−)-phenylephrine (0.3 μM). Each of vasodilator drugs (acetylcholine, forskolin, and sodium nitroprusside) was cumulatively added to the bath medium in half-log increments until the observation of a maximum relaxant response.
To assess the effects of Qing Dai and indigo on relaxation responses, preparations were pre-incubated with Qing Dai (500 μg/mL), indigo (100 μM), or vehicle (1% DMSO) for 60 min before the administration of (−)-phenylephrine (0.3 μM). Then, concentration-response curves were constructed for each vasodilator drug. At the end of experiments, (−)-isoprenaline (10 μM) or sodium nitroprusside (3 μM) was applied to the bath medium to test relaxant capacity. Our preliminary experiments showed that, for forskolin and sodium nitroprusside, tissue sensitivity and maximum responses were decreased when 2 consecutive relaxant responses were induced in the same muscle segment (data not shown). Therefore, we only obtained 1 concentration-response curve for each drug in each muscle strip, and the effects of Qing Dai, indigo, and vehicle on relaxant responses were compared in different muscle preparations.
1.6. Data analysis
Concentration response curves were constructed for vasodilator drugs (acetylcholine, forskolin, and sodium nitroprusside) and 50% effective-concentration (EC50) values were calculated using GraphPad Prism (version 7.00, GraphPad Software, San Diego, Calif., U.S.A.). EC50 values were converted to negative logarithmic values (pEC50) for analysis. All results are presented as the means ± standard error of the mean, where n refers to the number of experiments. Significant differences between mean values were evaluated in GraphPad Prism (version 7.00) using Student's paired or unpaired t-tests. A P value < 0.05 was statistically significant.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Galie N., Humbert M., Vachiery J.L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., Ghofrani A. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 2.Galie N., Humbert M., Vachiery J.L., Vizza C., Kneussl M., Manes A., Sitbon O., Torbicki A., Delcroix M., Naeije R., Hoeper M. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2002;39:1496–1502. doi: 10.1016/s1062-1458(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 3.Naganuma M., Sugimoto S., Mitsuyama K., Kobayashi T., Yoshimura N., Ohi H., Tanaka S., Andoh A., Ohmiya N., Saigusa K., Yamamoto T. Efficacy of Indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154:935–947. doi: 10.1053/j.gastro.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Nishio M., Hirooka K., Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur. Heart J. 2016;37:1992. doi: 10.1093/eurheartj/ehw090. [DOI] [PubMed] [Google Scholar]
- 5.Misumi K., Ogo T., Ueda J., Tsuji A., Fukui S., Konagai N., Asano R., Yasuda S. Development of pulmonary arterial hypertension in a patient treated with Qing-Dai (Chinese herbal medicine) Intern. Med. 2018 doi: 10.2169/internalmedicine.1523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.