Abstract
The study was designed to determine the prevalence of Haemonchus contortus, intestinal helminthes parasite of sheep in the Gharb plain (Morocco).
A total of 1154 sheep were randomly selected from farms, pastures and abattoirs, the samples were examined using a flotation techniques for determination the eggs of Haemonchus contortus. In order to identify Haemonchus contortus among the different gastrointestinal strongyles present in mixed infections, a faecal culture was carried out for each farm and pasture. This study was carried between November 2016 and October 2017. The data were analysed with respect to sex, age, season and body condition of sheep examined. Out of (1154) samples examined, (23.92%) harbored H. contortus. Prevalence among female (30.98%) was higher than that of the males (15.63%), there was significant difference between prevalence of infection and sex (P < 0.05). Based on age group, prevalence of haemonchosis was 6.25% and 43.22% in young and aged animals respectively showing the statistical significance (P < 0.05). Seasonal variation was recorded throughout the year and was highest during spring (36.36%) and autumn (32.37%) against a low rate of 2.7% in the summer with significance difference (P < 0.05). Regarding the relationship between Haemonchosis contortus and body condition, maximum prevalence (80.83%) was recorded in animals with poor body condition, followed by those with moderate body condition (34.26%), while the lowest infestation rate (6.88%) is noted in animals with good body.
Keywords: Prevalence, Haemonchus contortus, Sheep, Gharb plain, Morocco
1. Introduction
The distribution of sheep is global, with high numbers, showing the ability to adapt to various climates and universal interest, it also has a social and cultural very important role in some societies. It is used to accumulate capital and put it in reserve.
In Morocco sheep farming is a reserve of varied and adapted genotypes. It values a natural plant source, free and of good nutritional value that few other animals can exploit. It plays a key role in Moroccan agriculture, in comparison to other ruminant, sheep are produced in every region regardless of agro-ecological and socio-economic conditions. Is economically and socially important in the country. It provides about 35% of the red meat production. This specie contributes to the celebration of the Abraham ceremony, as >4 million heads are sacrificed each year (Boujenane, 2006). However, this type of extensive traditional breeding, which is almost complete in its entirety, suffers from a number of constraints related to its own mode of conduct and which may harm their development, among these constraints, infestations by gastrointestinal nematodes. A literature review of last decade reveals many species of parasites in sheep included: Haemonchus contortus (H.c), Oesophagostomum, Ostertagia, Nematodirus, Trichuris, Moniezia and Fasciola …, the most important of them is H. contortus (H.c), responsible for the parasitic disease: haemonchosis.
H. contortus an important, voracious blood sucking parasite of small ruminants found in abomasum, cause major production losses world-wide and heavy burden of this blood feeding parasite causes anemia, diarrhoea, loss of weight, oedema, recumbency, severe debility and ultimately death (Ejlertsen et al., 2006; Squires et al., 2011; Nabi et al., 2014). H. contortus sucks about 0.05 mL of blood per day by ingestion or liberation from lesions (Qamar and Maqbool, 2012).
Haemonchosis is widespread wherever sheep and goats are raised, but the greatest economic losses occur in temperate and tropical regions (Ijaz et al., 2008; Torres-Acosta and Hoste, 2008; Calvete et al., 2014). The disease has also found in the colder climates and recently been found as far north as the Arctic Circle (Fentahun and Luke, 2012).
There are many risk factors that contribute to the occurrence of haemonchosis, such as, host and environment (agro-ecological conditions, husbandry practices, deworming intervals and pasture management) (Ratanapob et al., 2012). Other risk factors such as host species, sex of the animal, age, body condition and race/genotype (Badaso and Addis, 2015). Parasite species and the intensity of the worm population, have an effect on the development of gastrointestinal parasitic infections (Tariq et al., 2008).
This article investigates the prevalence of H. contortus of sheep raised in Gharb plain according to the risk factors as age of animals, sex, season and body condition.
2. Materials and methods
2.1. Study area
The study was carried out in Gharb plain, which is located in North West of Morocco in the Mediterranean zone. This area profits from a Mediterranean climate, characterized by mild, moderate and rainy in winter and wet and moderate in summer. The location of the region on the Atlantic Ocean confers abundant precipitations to him, which largely exceed the national average. These precipitations are around 500 mm a year at Kenitra (province of the Gharb area) in the last 20 years.
The inequalities of the relief make that the climate presents important local variations and is characterized by an apparent variability (the minimal temperature is of 4 °C and maximal of 40 °C). In summer, the atmosphere is heated appreciably, the more frequent maximum temperatures in July vary between 16 °C and 26 °C. Peaks of 38 °C with 40 °C could be recorded a few days a year, but their frequency remains exceptional.
2.2. Study animals and sampling methods
The present study was conducted from November 2016 to October 2017 to estimate the prevalence of H. contortus in sheep according to some parameters as age, sex, season and body condition of host.
A total of 1154 sheeps were randomly selected from farms, pastures and abattoirs and examined for eggs and worms of H. contortus, (faecal examination: 857; abomasum examination: 297). Faecal samples collected were examined by the modified McMaster technique using saturated solution of sodium chloride. The worms were collected in normal saline and identified based on the characteristics given by Soulsby (1982). The sexes, age and body condition were included in this study to demonstrate the role of host factors in the prevalence of Haemonchosis infection. Collection of data was repeated monthly, with the aim to include more and more flocks in the study and to demonstrate the seasonal differences in the prevalence and level of infection. The body condition score was determined and grouped as good, medium and bad.
2.3. Data analysis
Microsoft excel software was used to store the data and analysis of simple descriptive statistics. Computation of descriptive statistics was conducted using IBM-SPSS version 24.0. Confidence level was held at 95% and statistical analysis for the difference in prevalence of H. contortus among risk factors are considered significant when the P-value was <0.05.
3. Results
During the course of this study, a total of 1154 (531 males and 623 females) sheeps were screened using faecal and postmortem examination. Out of these, 276 were found infected by H. contortus (H.c) (faecal infestation: 209; abomasum infestation: 67) indicating an overall prevalence of 23.92%. The distribution of H.c by sex indicated an overall prevalence in females of 30.98% (faecal prevalence: 31.81%; abomasum prevalence: 28.66%) and 15.63% in males (faecal prevalence: 15.83%; abomasum prevalence: 15.04%) (P < 5%) (Table 1).
Table 1.
Relationship between sex and H. contortus in sheep in Gharb area, Morocco.
| Sex | Faecal examination |
Abomasum examination |
Total of animals examined | Overall prevalence % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Examined | Infected | Prevalence (%) | P value | Examined | Infected | Prevalence (%) | P value | |||
| Male | 398 | 63 | 15.83 | 0.000 (<5%) | 133 | 20 | 15.04 | 0.02 (<5%) | 531 | 15.63 |
| Female | 459 | 146 | 31.81 | 164 | 47 | 28.66 | 623 | 30.98 | ||
| Total | 857 | 209 | 24.39 | 297 | 67 | 22.56 | 1154 | 23.92 | ||
Overall χ2 = 23.10; overall P-value = 0.0000.
The prevalence of parasite according to age, showed in (Table 2), reveal a lower percentage in young sheeps (6.52%) than the old (43.32%) (P = 0.000). The study of the distribution of H. contortus infection according to the seasons revealed a high rate of 36.36% and 32.37% in spring and autumn respectively against a low rate of 2.7% in summer (P = 0.000) (Table 3). Regarding the relationship between haemonchosis and body condition, the prevalence is 6.88% in healthy sheep, 34.26% in moderate body condition, and 80.83% for animals in poor condition with statistical significance variation (P = 0.000) (Fig. 1).
Table 2.
Relationship between age and H. contortus in sheep in Gharb area, Morocco.
| Age | Faecal examination |
Abomasum examination |
Total of animals examined | Overall prevalence % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Examined | Infected | Prevalence (%) | P value | Examined | Infected | Prevalence (%) | P value | |||
| 8 months to 1 year | 170 | 11 | 6.47 | 0.0000 (<5%) | 60 | 4 | 6.67 | 0.004 (<5%) | 230 | 6.52 |
| 1 to 2 years | 151 | 17 | 11.26 | 51 | 5 | 9.8 | 202 | 10.89 | ||
| 2 to 3 years | 178 | 40 | 22.47 | 60 | 12 | 20 | 238 | 21.85 | ||
| 3 to 4 years | 181 | 64 | 35.36 | 67 | 21 | 31.34 | 248 | 34.27 | ||
| >4 years | 177 | 77 | 43.5 | 59 | 25 | 42.37 | 236 | 43.22 | ||
| Total | 857 | 209 | 24.39 | 297 | 67 | 22.56 | 1154 | 23.92% | ||
Overall χ2 = 74.5; overall P-value = 0.0000.
Table 3.
Seasonal dynamics of Haemonchus contortus infections in sheep in Gharb area, Morocco.
| Season | Faecal examination |
Abomasum examination |
Total of animals examined | Overall prevalence % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Examined | Infected | Prevalence (%) | P value | Examined | Infected | Prevalence (%) | P value | |||
| Winter | 224 | 50 | 22.32 | 0.000 (<5%) | 78 | 17 | 21.79 | 0.004 (<5%) | 302 | 22.32 |
| Spring | 209 | 76 | 36.36 | 82 | 27 | 32.93 | 291 | 36.36 | ||
| Autumn | 241 | 78 | 32.37 | 77 | 21 | 27.27 | 318 | 32.37 | ||
| Summer | 183 | 5 | 2.73 | 60 | 2 | 3.33 | 243 | 2.73 | ||
| Total | 857 | 209 | 24.39 | 297 | 67 | 22.56 | 1154 | 23.92 | ||
Overall χ2 = 61.13; overall P-value = 0.0000.
Fig. 1.
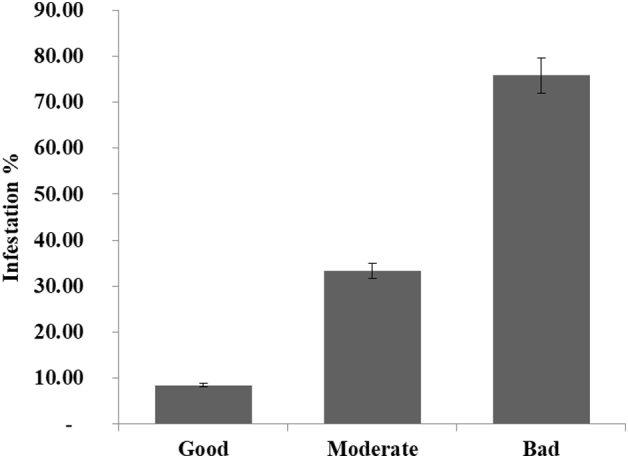
Relationship between body condition and H. contortus in sheeps in Gharb area, Morocco.
4. Discussion
4.1. Overall prevalence of H. contortus infection in sheep
Haemonchosis is a serious health problem that leads to a decline in production due to high morbidity, mortality and cost of treatment and control measures (Qamar and Maqbool, 2012). Examinations of the 1154 (857 faecal and 297 abomasum examinations) for H. contortus resulted in an infestation rate of 23.92% (276/1154) (faecal examination revealed 209 animals infected, examination of abomasum indicated 67 infected animals). The prevalence of ovine haemonchosis in our study is much lower than the prevalence in other studies in different countries such as Sudan 36.4% (Mubarak, 2013), Ethiopia (Sissay, 2007; Abunna et al., 2009; Dagnachew et al., 2011), in Pakistan (Tasawar et al., 2010; Asif et al., 2008; Raza et al., 2009), and in Benin (Attindehou et al., 2012). However, our prevalence is much higher than that found in Iran 9.3% and Egypt 7.9% (Sultan et al., 2010). This difference in prevalence may be due to cold winters and hot summers that destroy the parasite.
4.2. Variation of haemonchosis according to sex of sheep
In the present investigation, statistical analysis of the data on the prevalence of haemonchosis between sexes showed that there was significant difference (P < 0.05) between male (15.63%) and female (30.984%). It is assumed that is a determinant factor influencing prevalence of haemonchosis and females are more susceptible to parasitism due to reproductive stress and decreased immune status (Urquhart et al., 1996). This is coincided with the results of different countries in Pakistan (Raza et al., 2009), in Ethiopia (Dagnachew et al., 2011; Moges et al., 2017), and in Iran (Tehrani et al., 2012), and disagreement with previous findings which were reported in Ethiopia by Tewodros and Girja (2012) as 80.9% and 77.1% in males and females respectively in Gonder town, Zelalem et al. (2014) who reported 73.22% and 64.71% in male and females respectively in and around Finoteselam in Amhara region and Tibeso and Mekonnen (2015) also reported 66.0% in female and 59.7% in male in ArsiNegelle in Oromo region.
4.3. Variation of haemonchosis according to age of sheep
The results obtained showed that H. contortus infection is age-related (P < 0.05) and the highest rate is observed in animals less than one year old (43.22%). The infection is due to the low resistance of these animals that have not been exposed to infection earlier as exposure to nematode infection increases, the immune system of the host animals develops specifically against these parasites and resistance to age is acquired. Therefore, the low level of haemonchosis reported in adult animals, 6.52 in animals over 4 years of age, is due to the development of significant immunity over time. These results of the effect of host age on the prevalence of H. contortus infection are consistent with those found by Sissay (2007) in Ethiopia, Tariq et al. (2008) in India, in Pakistan by Qamar et al. (2009), and Dagnachew et al. (2011), while disagreeing with the findings in Pakistan by Tasawar et al. (2010) and Sudan by Mubarak et al. (2017).
4.4. Variation of haemonchosis according to season of sheep
The seasonal survey showed that the highest rates of infection were observed in spring (36.36%) and in autumn 32.37% and the lowest rate in summer (2.73%), this can be explained by the characteristics of the climate in north-western Morocco which is characterized by a favorable temperature and rainfall (T = 18.5 °C in spring, 17.5 °C in autumn, mean annual rainfall = 570 mm) ensuring the development and survival of pre-parasitic stages and leads to an increased availability of infectious larvae. In contrast, summer is a low-risk season for infestation due to summer heat and lack of moisture leading to the destruction of eggs and larvae. It is only in autumn and at the first rains that infestations on pastures resume (Cabaret, 1983). These results are similar to those found in Algeria, by Bentounsi and Cabaret (2013) who stated that the mild or cool semi-arid climate is the most favorable for protostrongles infestation.
4.5. Variation of haemonchosis according to the body condition of sheep
The relationship between body condition and haemonchosis in sheep was recorded with a statistically significant difference (P = 0.000) between animals with poor, moderate and good status, which means that these animals do not have the same sensitivity to the haemonchosis. In fact, animals in poor condition show a high rate of infection (75.83%) followed by those with a moderate body condition (33.25%) while healthy sheep are less infected (8.44%). Results similar to this study have been reported by Nagasi et al. (2012), Moges and Hebtom (2017), Mesele et al. (2014) and Gonfa et al. (2013) in Ethiopia. This deference in the infestation rate can be explained by the fact that animals in bad body condition have low immunity against parasitism.
5. Conclusion
This study showed that prevalence of H. contortus a major problem in the study areas and could be responsible for the loss of production and mortality, in contrast, the prevalence of haemonchosis is mostly associated with the epidemiological factors such as origin of the animals and host factors such as sex, age and body condition. Climatic factors such as precipitation and temperature, can act in favor of increasing this prevalence which needs great attention when designing the control programs of the parasite.
Conflict of interest
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence their work and there are no professional or other personal interests of any nature or kind in any product service, and/or company that could be construed as influencing the position presented in, or the review of, this paper.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Abunna F., Tsedeke E., Kumsa B., Megersa B., Regassa A., Debela E. Abomasal nematodes: prevalence in small ruminants slaughtered at Bishooftu Town, Ethiopia. J. Vet. Med. 2009;7:1937–8165. [Google Scholar]
- Asif M., Azeem S., Asif S., Nazir S. Prevalence of gastrointestinal parasites of sheep and goats in and around Rawalpindi and Islamabad, Pakistan. J. Vet. Anim. Sci. 2008;1:14–17. [Google Scholar]
- Attindehou S., Salifou S., Félix B.C., Bassa G.O., Adamou N.M., Joseph P.L. Epidemiology of haemonchosis in sheep and goats in Benin. J. Parasitol. Vector Biol. 2012;4:20–24. [Google Scholar]
- Badaso T., Addis M. Small ruminants haemonchosis: prevalence and associated risk factors in ArsiNegelle municipal abattoir, Ethiopia. Glob. Vet. 2015;15(3):315–320. [Google Scholar]
- Bentounsi B., Cabaret J. 2013. Climate and anthelmintic control: influence on protostrongylid infection of sheep in Algeria.http://www.journees3r.fr/IMG/pdf/Texte_14_affiche_sante (Renc. Rech. Ruminants). [Google Scholar]
- Boujenane Ismaıl. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2006. Vol. 1. 2006. Reproduction and production performance of Moroccan sheep breeds. No. 014. [Google Scholar]
- Cabaret J. Caractéristiques des populations de Ostertagiasp Chez les ovins naturellement infestés de la région de Moulay Bouazza (Maroc) Ann. Parasitol. Hum. Comp. 1983;58:377–382. [PubMed] [Google Scholar]
- Calvete C., Ferrer L., Lacasta D., Calavia R., Ramos J., Ruiz-de-Arkaute M., Uriarte J. Variability of the egg hatch assay to survey Benzimidazole resistance in nematodes of small ruminants under field conditions. Vet. Parasitol. 2014;203(1):102–113. doi: 10.1016/j.vetpar.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Dagnachew S., Amamute A., Temesgen W. Epidemiology of gastrointestinal helminthiasis of small ruminants in selected sites of North Gondarzone, Northwest Ethiopia. Ethiop. Vet. J. 2011;15:57–68. [Google Scholar]
- Ejlertsen M., Githigia S.M., Otieno R.O., Thamsborg S.M. Accuracy of an anaemia scoring chart applied on goats in sub-humid Kenya and its potential for control of Haemonchus contortus infections. Vet. Parasitol. 2006;141:291–301. doi: 10.1016/j.vetpar.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Fentahun T., Luke G. Small ruminant Haemonchosis: prevalence and associated determinants in randomly selected restaurants and Hotels of Gondar Town, Ethiopia. Eur. J. Appl. Sci. 2012;4(4):168–172. [Google Scholar]
- Gonfa S., Basaznew B., Achenef M. An abattoir survey on gastrointestinal nematodes in sheep and goats in Hemex-export abattoir DebreZiet, Central Ethiopia. J. Adv. Vet. Res. 2013;3:60–63. [Google Scholar]
- Ijaz M., Khan M.S., Avais M., Ashraf K., Ali M.M., Saima Infection rate and chemotherapy of various helminths in goats in and around Lahore. Pak. Vet. J. 2008;28(4):167–170. [Google Scholar]
- Mesele K., Yisehak T., Nesibu A. Prevalence of Haemonchosis in sheep slaughtered at Abergele Export Abattoir. Mekelle University, College of Veterinary Medicine, P.O. Box 231 Mekelle, Ethiopia. Acta Parasitol. Glob. 2014;5(2):115–119. [Google Scholar]
- Moges S., Hebtom K. Prevalence of Haemonchus contortus of sheep slaughtered at Bahir Dar municipal abattoir, Bahir City, Ethiopia. Glob. Vet. 2017;18(4):269–276. [Google Scholar]
- Mubarak A.M. Sudan University of Science and Technology; Khartoum, Sudan: 2013. Prevalence and Risk Factors of Sheep Haemonchosis in North Kordofan, Sudan. MSc Thesis. [Google Scholar]
- Mubarak A.M. Epidemiological study of Haemonchus contortus among sheep in North Kordufan State, Sudan. Int. J. Vet. Sci. 2017;6(4):209–215. [Google Scholar]
- Nabi H., Saeed K.I., Shah S.R., Rashid M.I., Akbar H., Shehzad W. Epidemiological study of gastrointestinal nematodes of goats in District Swat, Khyber Pakhtunkhwa. Pak. Sci. Int. (Lahore) 2014;26(1):283–286. [Google Scholar]
- Qamar M.F., Maqbool A. Biochemical studies and serodiagnosis of haemonchosis in sheep and goats. J. Anim. Plant Sci. 2012;22(1) [Google Scholar]
- Qamar Epidemiology of Haemonchosis in sheep and goats under different managemental conditions. Vet. World. 2009;2(11):413–417. [Google Scholar]
- Ratanapob N., Arunvipas P., Kasemsuwan S., Phimpraphai W., Panneum S. Prevalence and risk factors for intestinal parasite infection in goats raised in Nakhon Pathom province, Thailand. Trop. Anim. Health Prod. 2012;44(4):741–745. doi: 10.1007/s11250-011-9954-6. [DOI] [PubMed] [Google Scholar]
- Raza M.A., Murtaza S., Bachaya H.A., Dastager G., Hussain A. Point prevalence of Haemonchosis in sheep and goats slaughtered at Multan Abattoir. J Anim Plant Sci. 2009;19:158–159. [Google Scholar]
- Sissay M.M. Swedish University of Agricultural Science; Uppsala, Sweden: 2007. Helminth Parasites of Sheep and Goats in Eastern Ethiopia: Epidemiology and Anthelmintic Resistance and Its Management. PhD Thesis. [Google Scholar]
- Soulsby E.J. 7th ed. Bailleier Tindall and Cassel Ltd.; London, UK: 1982. Helminths, Arthropods and Protozoza of Domestic Animals. [Google Scholar]
- Squires J.M., Ferreira J.F.S., Lindsay D.S., Zajac A.M. Effects of artemisinin and Artemisia extracts on Haemonchus contortus in gerbils (Meriones unguiculatus) Vet. Parasitol. 2011;175:103–108. doi: 10.1016/j.vetpar.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Sultan K., Desoukey A.Y., Elsiefy M.A., Elbahy N.M. An abattoir study on the prevalence of some gastrointestinal helminths of sheep in Gharbia Governorate, Egypt. Glob. Vet. 2010;5:84–87. [Google Scholar]
- Tariq K.A., Chishti M.Z., Ahmad F., Shawl A.S. Epidemiology of gastrointestinal nematodes of sheep managed under traditional husbandry system in Kashmir valley. Vet. Parasitol. 2008;158:138–143. doi: 10.1016/j.vetpar.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Tasawar Z., Ahmad S., Lashari M.H., Hayat C.S. Prevalence of Strongyle spp. in sheep at Research Centre for Conservation of Sahiwal Cattle (RCCSC) Jehangirabad, District Khanewal, Punjab, Pakistan. Pak. J. Zool. 2010;42:735–739. [Google Scholar]
- Tehrani A., Javanbakht J., Jani M., Sasani F., Solati A. Histopathological study of Haemonchus contortus in herrik sheep abomasum. J. Bacteriol. Parasitol. 2012;3:144. [Google Scholar]
- Tewodros F., Girja L. Small ruminant Haemonchosis: determinants in randomly selected restaurants hotels of Gondar Town, Ethiopia. Eur. J. Appl. Sci. 2012;4(4):168–172. [Google Scholar]
- Tibeso B., Mekonnen A. Small ruminants Haemonchosis: prevalence and associated risk factors in ArsiNegelle Municipal Abattoir, Ethiopia. Glob. Vet. 2015;15(3):315–320. [Google Scholar]
- Torres-Acosta J., Hoste H. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Rumin. Res. 2008;77(2):159–173. [Google Scholar]
- Urquhart G., Armour J., Duncan J., Dunn A., Jennings F. 2nd ed. Blackwell Science in Operation With the British Council; 1996. Veterinary Parasitology; pp. 19–164. [Google Scholar]
- Zelalem M., Nigus A., Getachew G., Niraj K. Assessment of small ruminant Haemonchosis and its associated risk factors in and around Finoteselam. J. Agric. Vet. Sci. 2014;4:36–41. [Google Scholar]


