Abstract
Advanced therapy medicinal products (ATMPs) require evaluation by the European Medicines Agency’s Committee for Advanced Therapies prior to being placed on the European market, subject to a Marketing Authorisation granted by the European Commission. In common with other medicinal products, various regulatory pathways are available for taking ATMPs through clinical trials to market authorisation, and the regulatory pathway taken will depend on a product’s characteristics and the target patient population. With the industry poised to deliver more late-stage clinical and commercial ATMPs for serious diseases with high unmet medical need (e.g., T cell immunotherapies for cancer), bringing medicines to patients through optimized regulatory strategies and expedited pathways is assuming greater importance. The European Medicines Agency’s priority medicines (PRIME) scheme was introduced in 2016 specifically to enable this, and eligibility has been granted to 19 ATMPs as of the fourth quarter (Q4) 2018. Furthermore, two chimeric antigen receptor (CAR) T cell therapies, Yescarta and Kymriah, have recently completed their journeys through the scheme to Marketing Authorisation. This review discusses how the regulatory pathway for any particular ATMP, with or without PRIME designation, is determined and navigated.
Keywords: Marketing Authorisation, advanced therapy medicinal product, cell therapy, gene therapy, regulation, conditional approval, accelerated assessment, PRIME, RMAT designation, Sakigake designation
Main Text
In the European Union (EU), an established legal framework is in place that governs the regulation of all medicinal products for human use, including advanced therapy medicinal products (ATMPs), i.e., medicinal products comprised of cells, genes, or tissues. In essence, this framework ensures the quality, safety, and efficacy of medicines placed on the market in the EU. The regulatory framework is established principally in Directive 2001/83/EC, and a number of other Directives and Regulations (e.g., on clinical trials, manufacturing, orphan medicinal products, pediatric research, and ATMPs) establish its principles (Table S1). To quote the European Commission (EC) from their website, “the EU legal framework for human medicines sets standards to ensure a high level of public health protection and the quality, safety and efficacy of authorised medicines. In addition, it promotes the functioning of the internal market, with measures to encourage innovation. It is based on the principle that a medicinal product requires a Marketing Authorisation by the competent authorities before being placed on the market.”
The Marketing Authorisation Application (MAA) procedure, therefore, ensures the quality, safety, and efficacy of all medicinal products for human use, by requiring regulatory review of quality, safety, and efficacy data generated during clinical development prior to Marketing Authorisation (i.e., commercial licensing). In turn, the clinical development activities and product manufacturing must comply with the particular standards and requirements within the legislation and the principles of good clinical practice and Good Manufacturing Practice to ensure that the data presented in the MAA are complete, accurate, and satisfactory.
The medicinal product regulatory framework established by the EC is implemented by the European Medicines Agency (EMA) together with the national regulatory agencies in the EU member states. A key focus of the EMA in recent years, as well as regulatory agencies in the United States and Japan, has been to develop and implement schemes to expedite clinical development and enable new medicines to reach the market, and patients, as early as possible. In the EU, the EMA introduced the priority medicines (PRIME) scheme in 2016 for this particular purpose.
PRIME uses tools already existing in the EU regulatory framework—such as scientific advice, conditional approval, and accelerated assessment—to define and optimize the development pathway for priority medicines addressing high unmet medical need and/or demonstrating therapeutic innovation (discussed subsequently). Scientific advice is formal dialogue with the EMA in which feedback on, and endorsement of, development programs can be obtained to ensure that the appropriate data needed for the MAA are generated. Conditional approval and accelerated assessment are regulatory procedures that can be used within a regulatory strategy, which can enable an MAA to be submitted, reviewed, and approved as early and as quickly as possible, helping to ensure timely provision of novel medicines to patients. How the EU regulatory framework applies to ATMPs in general (Figure 1), and is leveraged for ATMPs designated as priority medicines, is discussed further in this review.
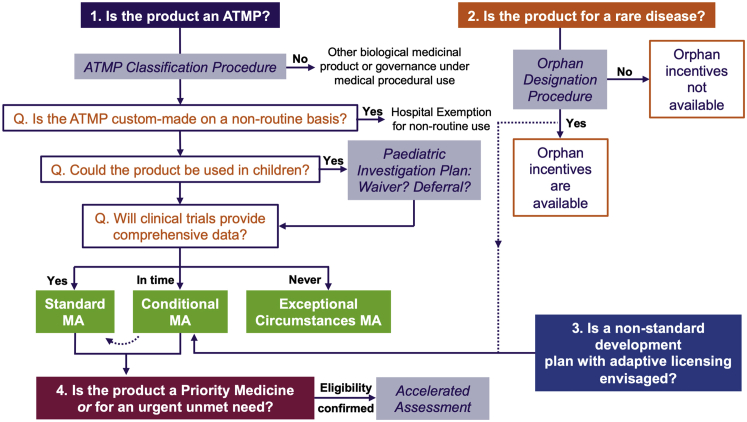
Figure 1.
Regulatory Pathways to Marketing Authorisation for ATMPs
Under the EU regulatory framework for medicinal products, a Marketing Authorisation (MA) is required to place medicinal products on the pharmaceutical market. To determine whether a cell- or gene-based therapy is classified as a medicinal product, the EMA offers an ATMP classification procedure administered by the CAT. If a product is classified as an ATMP, it must undergo clinical trials to demonstrate safety and efficacy before a MA application (MAA) can be submitted. If the ATMP will be used on a non-routine basis within a hospital environment in an individual member state, the hospital exemption scheme may be followed. An MA may be granted in three ways: standard MA, conditional MA, or MA under exceptional circumstances. The type of MA applied for depends on the extent of clinical data obtained during development and/or whether the medicine addresses an unmet medical need. Clinical development must include pediatric studies if the medicine is intended to be used in children. Medicines for which comprehensive clinical data, relative to the patient population, can be provided at the time of MAA will go through the standard MA procedure. Medicines for which comprehensive clinical data are never expected to be obtained will go through the MA under the exceptional circumstance procedure. Medicines that qualify as orphan medicinal products (based on the rarity of the therapeutic indication), and medicines under an accelerated development program, may go through the conditional MA (CMA) procedure initially until the MA can be converted to a standard MA at a later stage. An initial CMA may also be sought for medicines for which a standard development program is not achievable and for which an adaptive licensing route is appropriate. Finally, accelerated assessment (expedited review) of standard and CMAAs may be possible for priority medicines (in the PRIME scheme) or other medicines addressing an urgent unmet need. All principles outlined in the figure are discussed fully in the text.
Regulation of ATMPs as Medicinal Products
A key aspect of medicinal product legislation is that it defines what a medicinal product is. Directive 2001/83/EC defines a medicinal product as (1) any substance or combination of substances presented as having properties for treating or preventing disease in human beings; or (2) any substance or combination of substances that may be used in or administered to human beings, either with a view to restoring, correcting, or modifying physiological functions by exerting a pharmacological, immunological, or metabolic action or to making a medical diagnosis.
The 1990s witnessed a breakthrough in the development of experimental therapies based on human genes and/or cells within university hospital environments; for example, gene-based therapies for severe combined immunodeficiencies (adenosine deaminase [ADA]-SCID and X-linked [X]-SCID) and hemophilia and cell-based therapies for cornea and cartilage repair. Recognizing the fact that these pioneering investigational therapies met the criteria for medicinal products, and, therefore, to ensure that their clinical use was conducted with quality, safety, and efficacy in mind, the EC introduced cell- and gene-based therapies into European medicinal product legislation via Directive 2003/63/EC, amending Directive 2001/83/EC, in June 2003 as a new category of biological medicinal products, termed ATMPs.
Next, in late 2008, Directive 2001/83/EC and Regulation (EC) No. 726/2004 (on procedures for human medicinal product authorisation and supervision within the EU and EEA) were amended by a specific Regulation on ATMPs: Regulation (EC) No. 1394/2007. This regulation (which is known as the ATMP regulation) defines ATMPs as three specific types of medicinal products, including gene therapy medicinal products (GTMPs), somatic cell therapy medicinal products (SCTMPs), and tissue-engineered products (TEPs), all of which meet one of the definitions of medicinal products described above. In addition, combined ATMPs are those that contain a medical device, as an integral part of a viable cell- or tissue-containing product, or that contain non-viable cells or tissues, which are liable to act upon the body with action that can be considered primary to the device element. The ATMP regulation entered into force to ensure that products defined as ATMPs are subject to appropriate regulatory evaluation, according to the regulatory framework for human medicinal products, prior to clinical and commercial use in a consistent way across the European community. Central to this was the formation of an expert committee within the EMA, the Committee for Advanced Therapies (CAT), to perform the primary evaluation of ATMP MAAs, contribute to other ATMP-specific activities of the EMA, and follow scientific developments in the field.
The introduction of the ATMP regulatory framework has now led to the growth of an industry around cell and gene therapy development, with many clinical trials now taking place worldwide.1, 2, 3 The number of ATMPs with a current Marketing Authorisation (MA) as of early 2019 is nine (Table 1; including products for SCID, cartilage disease, and corneal disease that evolved from the pioneering experimental medicines of the 1990s), while four previously authorised ATMPs are no longer available for various reasons. The number of authorised ATMPs is expected to increase significantly over the next few years, particularly given that cell and gene therapy development is also blossoming in other global territories, and, importantly, in the other ICH (International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, previously the International Conference on Harmonization) regions (see later) of the US and Japan (Table 1). Indeed, three ATMPs are currently in MAA procedures (Table 1), and they can reasonably be expected to be granted MAs in the coming months.
Table 1.
Cell and Gene Therapies to Have Been Granted an MA in Various Jurisdictions Worldwide, as of the Fourth Quarter 2018
| European Union | ||||
| Product | Class | Disease Area | Year | Company |
| Luxtuma | non-cell-based GTMP | retinal disease | 2018 | Spark Therapeutics Ireland |
| Yescarta | cell-based GTMP | relapsed or refractory DLBCL and PMBCL | 2018 | Kite, a Gilead Company |
| Kymriah | cell-based GTMP | relapsed or refractory DLBCL | 2018 | Novartis Europharm |
| B cell precursor ALL | ||||
| Alofisel | SCTMP | rectal fistula | 2018 | TiGenix NV/Takeda Pharmaceutical Company |
| Spherox | TEP | cartilage diseases | 2017 | CO.DON |
| Zalmoxis | SCTMP | HSCT adjunctive treatment | 2016 | MolMed |
| Strimvelis | cell-based GTMP | SCID | 2016 | Orchard Therapeuticsa |
| Imlygic | non-cell-based GTMP | melanoma | 2015 | Amgen |
| Holoclar | TEP | corneal diseases | 2015 | Chiesi Farmaceutici |
| Provengeb | SCTMP | prostatic neoplasms | 2013 | Dendreon Pharmaceuticals |
| Macib | combined ATMP | fractures, cartilage | 2013 | Vericel |
| Glyberab | non-cell-based GTMP | hyperlipoproteinemia type I | 2012 | uniQure |
| Chondrocelectb | TEP | cartilage diseases | 2009 | TiGenix |
| ATMPs Currently under MAA Review by the CAT or CHMP | ||||
| Active Substance/INN | Class | Disease Area | Regulatory Status | Company |
| ATIR101 | SCTMP | HSCT adjunctive treatment | MAA day 180: May 2018 | Kiadis Pharma |
| orphan medicinal product | ||||
| Axalimogene filolisbac | GTMP | cervical cancer | CMA submission: February 2018 | Advaxis |
| LentiGlobin BB305 | cell-based GTMP | transfusion-dependent beta-thalassemia | MAA submission under accelerated assessment: September 2018 | bluebird bio France |
| priority medicine | ||||
| adaptive pathways | ||||
| United States | ||||
| Product | Class | Disease Area | Year | Company |
| Kymriah | cell-based gene therapy | relapsed and refractory DLBCL | 2018 | Novartis Pharmaceuticals |
| B cell precursor ALL | 2017 | |||
| Luxturna | non-cell-based gene therapy | retinal disease | 2017 | Spark Therapeutics |
| Yescarta | cell-based gene therapy | large B cell lymphoma | 2017 | Kite, a Gilead Company |
| Maci | tissue-engineered producr | cartilage diseases | 2016 | Vericel |
| Imlygic | non-cell-based gene therapy | melanoma | 2015 | Amgen |
| Carticel | cell therapy | cartilage diseases | 2007 | Sanofi Biosurgery |
| Gintuit | tissue-engineered product | mucogingival conditions | 2012 | Organogenesis |
| Provenge | cell therapy | prostatic neoplasms | 2010 | Dendreon Pharmaceuticals |
| Japan | ||||
| Product | Class | Disease Area | Year | Company |
| Jace | tissue-engineered product | severe burns | 2016 | Japan Tissue Engineering |
| HeartSheet | cell therapy | cardiovascular disease | 2015 | Terumo |
| Temcellc | cell therapy | GvHD | 2015 | JCR Pharmaceuticals |
| Jacc | tissue-engineered product | cartilage diseases | 2012 | Japan Tissue Engineering |
ALL, acute lymphoblastic leukemia; DLBCL, diffuse large B cell lymphoma; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; SCID, severe combined immunodeficiency; PMBCL, primary mediastinal large B cell lymphoma.
The MA was transferred from GlaxoSmithKline to Orchard Therapeutics on August 23, 2018.
These products are no longer authorised or are suspended.
TEMCELL is equivalent to Prochymal, a product manufactured by Osiris Therapeutics, which is licensed in Canada, Australia, and New Zealand. Japan Tissue Engineering utilizes the technology in-licensed from Osiris Therapeutics in 2003.
Key Aspects of the ATMP Regulatory Framework: The Centralised Procedure, Directive 2009/120/EC, and the ATMP Classification Procedure
The introduction of the ATMP regulation required ATMPs to be evaluated under the centralised procedure described in Regulation (EC) No. 726/2004, with the evaluation being performed primarily by the CAT. The centralised procedure requires that MAAs for certain medicinal products are evaluated by the appropriate EMA committee rather than by a national regulatory agency as part of a mutual recognition or decentralised procedure, thus making a product available throughout the EEA on the basis of a single MA granted to the MA holder (MAH), the legal entity with responsibility for placing and maintaining the ATMP on the market.
According to the EMA, most new active substances marketed in the EU now go through the centralised procedure, but it is mandatory for ATMPs, medicines derived from biotechnology processes, orphan medicinal products (see later), and medicines for the treatments of certain diseases such as cancer and HIV. This is because the evaluation of these products is considered to require broad and diverse scientific expertise from across the European community. In addition, the scientific and technical requirements needed to demonstrate the quality, safety, and efficacy of ATMPs in MAAs being evaluated via the centralised procedure are specific to this class of medicines in many respects, and this was addressed through the introduction of Directive 2009/120/EC in late 2009 to replace Part IV of Annex I to Directive 2001/83/EC. In recognition of the unique challenges associated with the clinical development of ATMPs, Directive 2009/120/EC further introduced the risk-based approach (RBA) for these products, providing the scope to justify, on specific aspects of the product and patient population, the type and extent of quality, safety, and efficacy data included in the MAA. Together with the medicines directive and the ATMP regulation, the centralised procedure (as set out in Regulation [EC] No. 726/2004), with primary evaluation performed by the CAT according to the technical requirements of Directive 2009/120/EC, is key to the ATMP regulatory framework.
Directive 2009/120/EC additionally provides updated definitions of ATMPs relative to the ATMP regulation (Table S2). According to Directive 2009/120/EC, GTMPs comprise a category of ATMPs containing an active substance, which contains or consists of a recombinant nucleic acid and whose mechanism of action involves regulating, repairing, replacing, adding, or deleting a genetic sequence that mediates a therapeutic, diagnostic, or prophylactic effect either directly or indirectly through a protein it expresses (not including vaccines against infectious diseases). They are manufactured through processes that involve the generation of genetic constructs and the amplification of these constructs (often as viral vectors) in cell lines, following which they are either purified for direct administration (non-cell-based, or in vivo, gene therapies) or used for the transduction of therapeutic cells (cell-based, or ex vivo, gene therapies). Products containing cells or tissues that are not genetically modified are defined as ATMPs (either SCTMPs or TEPs) if they are manufactured using a process that involves substantial manipulation of the starting materials (cells or tissues), which differentiates them from cells or tissues used in medical procedures that undergo processing via minimal manipulation (and are, therefore, not considered starting materials for product manufacture). Minimal manipulation may simply involve cell purification (without culture) and/or washing before infusion into a patient (hematopoietic stem cell transplantation being the classic example), and so any processing that is inherent to the modification of their biological characteristics, physiological functions, or structural properties would be considered substantial manipulation (e.g., in vitro cell culture).
Importantly, cell-based therapies are also considered to work through a pharmaceutical, immunological, or metabolic mode of action, consistent with the definitions of medicinal products. Tissue-engineered products are further defined (in the ATMP regulation) as products that contain or consist of engineered cells or tissues and act through the repair, replacement, or regeneration of damaged or diseased tissues and organs, and this is generally achieved in combination with a scaffold. Therefore, SCTMPs, GTMPs, and TEPs are regulated as medicinal products because their mode of action is typical of other medicinal products, their mode of action is mediated by a genetic sequence, and/or their production involves substantial manipulation and industrial manufacturing processes. The exception to this rule is when cells are only manipulated minimally but are used for a purpose not reflecting the same essential function of the cells in the recipient as in the donor; in this case, such non-homologous therapeutic use of cells means that they are regulated as a medicinal product, i.e., an ATMP.
To determine whether a therapeutic product based on human cells or tissues meets the criteria that define ATMPs, developers can apply for an ATMP classification from the EMA, as established in article 17 of the ATMP regulation. After submission of the application, the EMA should deliver its recommendation after consultation with the EC and within 60 days of receiving the request. The ATMP classification procedure was established to address questions on borderline classifications, including whether products may be classified as combined ATMPs if they contain a medical device. In this respect, the ATMP classification procedure is helpful not only for determining whether a putative product is an ATMP or not (e.g., substantially manipulated versus minimally manipulated cells) and would, therefore, be subject to regulation under the medicinal product framework but also to determine what type of ATMP a product is (thus informing certain aspects of the development program, e.g., the specific dossier requirements and quality guidance to be followed4).
This latter concept can be illustrated using two retroviral vector-transduced blood cell-based ATMPs that have successfully gone through the MAA procedure in the EU, MolMed’s Zalmoxis, a T cell-based product, and GlaxoSmithKline’s Strimvelis,5 a hematopoeitic stem cell (HSC) product (now owned by Orchard Therapeutics). In both products, the autologous cells are transduced with a retroviral vector before being transplanted into the patient. In Strimvelis, the genetic modification introduces a functional ADA gene into the HSC genome, leading to a gain of ADA enzyme function in ADA-SCID patients in which this enzyme is defective. Strimvelis is, therefore, a GTMP because the genetic modification contributes to the mechanism of action (gain of ADA function) in the transduced HSCs. In Zalmoxis, the retroviral vector encodes a truncated form of the human low-affinity nerve growth factor receptor (ΔLNGFR; which enables identification of transduced cells) and the herpes simplex I virus thymidine kinase (HSV-TK Mut2; a suicide gene). Zalmoxis is used as an adjunctive treatment to HSC transplantation to reconstitute the patient’s immune system; however, complications linked to its use (e.g., graft-versus-host disease [GvHD]) can occur in some patients. The genetic modification with the HSV-TK Mut2 suicide gene makes the cells in Zalmoxis susceptible to ganciclovir and valganciclovir, such that if a patient develops GvHD, ganciclovir or valganciclovir is given to kill the administered T cells, thereby treating the complication and preventing its further development. Therefore, the genetic modification in Zalmoxis does not contribute to the mechanism of action of its therapeutic indication, and the product is classified as an SCTMP. Note that therapies based on genes or cells may also be classified as biological medicinal products if they do not fulfill all of the criteria defining ATMPs.
ATMPs May Also Be Orphan Medicinal Products
Many ATMPs in development in the EU are for rare diseases and conditions. If population analysis can demonstrate that a therapeutic indication for which any medicinal product is being developed is rare, as well as meeting certain other criteria, the medicinal product is likely to be eligible for orphan medicinal product (OMP) designation. The EMA’s orphan designation procedure was introduced in 2000 with the implementation of Regulation (EC) No. 141/2000 on orphan medicinal products (together with Regulation [EC] No. 847/2000 [as amended], which sets out definitions and rules for implementation). The full set of criteria that a medicinal product must meet to qualify for EU orphan designation includes the following: (1) intention to treat, prevent, or diagnose a disease that is life threatening or chronically debilitating; (2) a prevalence of the condition in the EU of not more than 5 individuals in every 10,000 members of the population, or an unlikeliness that marketing of the medicine would generate sufficient returns to justify the investment needed for its development; and (3) no satisfactory method of diagnosis, prevention, or treatment of the condition concerned can be authorised, or, if such a method exists, the medicine must be of significant benefit to those affected by the condition.
Further guidance is provided in the EC publications ENTR/6283/006 and 2016/C 424/03.7 According to Regulation (EU) No. 536/2014 on clinical trials, ultra-rare diseases are generally considered to be those that affect less than 1 in 50,000 members of the EU population. Ultra-rare disease designation is used more by health technology assessment (HTA) bodies for making reimbursement decisions rather than by the EMA for orphan medicinal product designation, which simply requires that rare disease status is confirmed (HTA bodies are regional or national organizations that provide recommendations or guidance on medicines and other health technologies that can be financed or reimbursed by the healthcare system in a particular member state or region on the basis of a value assessment).
The concept of significant benefit is of key importance to orphan designation for medicines in development that target diseases with current treatment options. In simplistic terms, significant benefit means that a medicine produces a clinically relevant advantage or provides a major contribution to patient care, compared with existing treatments. Furthermore, significant benefit may mean that an orphan medicine is suitable for patients for whom current treatments do not work, it is likely to improve patient outcomes in combination with a current treatment, or it works as well as other treatments but is significantly easier or more convenient to use.
Obtaining an orphan designation brings with it certain advantages aimed at incentivizing the development of new medicines for rare diseases. The very nature of rare diseases means that the development of medicines intended for small patient populations may have limited commercial value, because a return on the investment into the development of the medicine may not be obtained. Incentives provided to developers of EU orphan medicinal products include market exclusivity for the product in the protected indication for 10 years following the granting of the MA, and protocol assistance, a type of scientific advice tailored to orphan medicinal products that is charged at a reduced rate compared with standard scientific advice (some other procedural fees are also reduced).8
Market exclusivity is protected by a requirement for applicants submitting an MAA to indicate in the application if any medicinal product has been designated as an orphan medicinal product for a condition relating to the proposed therapeutic indication. If it has and that orphan medicinal product is still under market exclusivity, the applicant is further required to submit a report on the similarity of the active substances, with significant differences being needed to demonstrate non-similarity and allow the competitor product to be authorised and marketed. In addition, even if two products are determined to be similar, an MA can still be granted for the second product in the protected indication if the second applicant can show that their product is safer, more effective, or otherwise clinically superior (or if the first MA holder gives their consent or is unable to supply sufficient quantities of their orphan medicinal product).
Definitions of medicinal product similarity were initially established in EC Regulation No. 847/2000, but these definitions have recently been reviewed given the progress in medicinal product development, including the increase in ATMP development, in the intervening years. On May 29, 2018, EC Regulation (EU) 2018/781 was published to amend Regulation (EC) No. 847/2000 as regards the definition of the concept similar medicinal product. The definitions of non-similarity for ATMPs are presented in Table S4.
The Development of Medicines for Pediatric Use Requires Special Considerations
Traditional clinical trials, with products other than ATMPs, start with first time-in-human studies in which initial safety is generally tested in healthy adult volunteers (although oncology medicines, for example, would be used directly in patients), and later studies may enroll adults only. However, medicines developed in this way may also be suitable for diseases or conditions that affect children. In recognition of the fact that clinical protocols need to be tailored to pediatric patients rather than them being treated according to an adult trial protocol, the pediatric regulation (Regulation [EC] No. 1902/2006) was introduced in the EU in early 2007 with the aim of increasing the availability of medicines for children that have been demonstrated to have safety and efficacy in the pediatric population.
The implementation of the pediatric regulation led to the creation of the pediatric investigation plan (PIP), which defines the clinical studies to be conducted in children, including details of the timing of studies relative to adult studies and the measures proposed to show medicinal product safety and efficacy in all subsets of the pediatric population. The PIP requirement is applicable to all new medicinal products for human use (and, in some situations, products that were authorised before the regulation became applicable), including ATMPs. However, deferrals can be granted when there is sufficient information to demonstrate safety and efficacy in adults and that development in children could delay the MAA submission, and waivers can be granted when development in children is not appropriate (e.g., if a disease or condition only affects the adult population, the product is likely to be ineffective or unsafe in the pediatric population, or the product does not represent a significant therapeutic benefit over existing treatments). The PIP application (including deferral and waiver requests) should be submitted before completion of the human pharmacokinetic studies (phase I or first time-in-human clinical trials), and it cannot be submitted after the initiation of the pivotal study or registration, i.e., the clinical trial performed to generate the main dataset to be used to support the MAA (except in duly justified circumstances).
The EMA published a 10-year report in October 2017 containing an analysis of the data collected since the implementation of the pediatric regulation.9 The report describes an increase in medicines available for children over this period, particularly in rheumatology and infectious diseases but also in diseases that only affect children or where the disease shows biological differences between adults and children (e.g., rare diseases). It has been concluded that the implementation of the pediatric regulation has generally had a positive effect on medicine development for children (albeit that certain issues remain). As a result, the EMA together with the EC recently published a joint action plan to further support the development of pediatric medicines.10
A number of incentives are available for developers of pediatric medicines, including an additional 2 years market exclusivity for orphan medicinal product indication (i.e., 12 years in total) or an additional 6 months duration of a supplementary protection certificate protecting the product and no fees for scientific advice and protocol assistance.
Regulatory Agency Roles and Responsibilities in the Evaluation of ATMPs
The requirement for ATMP MAAs to be evaluated according to the centralised procedure by the CAT emphasizes the role of the EMA not only in the evaluation of the MAA but also in scientific advice during the development phase, in addition to the other MA-enabling procedures that are the sole remit of the EMA (e.g., orphan medicinal product designations and PIPs). Approval of clinical trial authorisation (CTA) applications, however, occurs at a national level independently (usually, unless the voluntary harmonization procedure is followed) within each member state in which the CTA is submitted. Regulatory agencies in the EU member states are known as national competent authorities (NCAs), and while they have authority to approve MAAs in their own member state for medicines not subject to the centralised procedure (as part of a mutual recognition, decentralised, or national procedure), their main role during ATMP development, other than the evaluation of CTA applications, is scientific advice on clinical trial designs. EMA scientific advice can be obtained in addition to NCA scientific advice during clinical development to ensure that the requirements for the MAA are being met and that the appropriate regulatory pathways are being followed. EMA also offers parallel scientific advice together with the European Network for Health Technology Assessment (EUnetHTA), allowing medicine developers to obtain feedback from regulators and HTA bodies on their evidence generation plans, to support decision-making on MA and reimbursement of new medicines at the same time.
The EMA itself is organized into seven committees, including the CAT, and several working parties. They all contribute to the development of medicines through scientific advice and the publication of scientific guidelines and other guidance documents (publications intended to help developers of medicinal products understand how to comply with the regulations; Table S3). The committees and working parties comprise members of the NCAs together with dedicated EMA experts in some instances, and sometimes certain other stakeholders may also be involved (e.g., patient representatives or members of the public).
The key committee within EMA is the Committee for Medicinal Products for Human Use (CHMP), which is responsible for the scientific evaluation of (most, other than herbal medicinal products) human medicines to determine their quality, safety, efficacy, and benefit-risk balance. This is done via the evaluation of MAAs and post-authorisation variations to approved MAs (e.g., to introduce new indications or changes to manufacturing processes). In addition, the CHMP together with its working parties contributes to scientific advice meetings and prepares scientific guidelines to provide support to medicine developers on the requirements of a development plan prior to the MAA. For ATMPs, the CAT, rather than the CHMP, prepares the initial draft opinion on an ATMP-related submission (i.e., an MAA or a variation), with the CHMP responsible for final adoption of the CAT opinion. In addition to its primary roles in the evaluation of ATMP MAAs and execution of ATMP classification procedures, the CAT also contributes to other ATMP-related activities, including the provision of support to scientific advice procedures, advice on pharmacovigilance or risk management systems, evaluation of post-authorisation variation submissions, and preparation of scientific guidelines.
The CAT’s activities in pharmacovigilance and risk management are in support of another key EMA committee, the Pharmacovigilance Risk Assessment Committee (PRAC). The PRAC is responsible for assessing and monitoring the safety of human medicines, and it was established in line with the pharmacovigilance legislation (Directive 2012/26/EC) that updated Directive 2001/83/EC and came into effect in 2012 to help strengthen the safety monitoring of medicines across the EU. The PRAC is primarily responsible for assessing risk management plans (RMPs) submitted with MAAs and for evaluating post-authorisation safety studies (discussed later), among other responsibilities.
With respect to the designation of orphan medicinal products and evaluation of PIPs as discussed earlier, these activities are the responsibility of the Committee for Orphan Medicinal Products (COMP) and Pediatric Committee (PDCO), respectively. The two other EMA committees, the Committee on Herbal Medicinal Products (HMPC) and Committee for Medicinal Products for Veterinary Use (CVMP), are not involved in the regulation of ATMPs for human use.
A number of working parties and related groups support the EMA’s scientific committees on scientific issues relating to their particular field of expertise. For ATMPs, the key working party is the Scientific Advice Working Party (SAWP), which is composed of NCA members and executes scientific advice and protocol assistance procedures. Another EMA initiative with a key role in early development of ATMPs for human use is the Innovation Task Force (ITF), a forum for early dialogue with industrial and academic organizations on innovative aspects of medicine development, which recruits experts from relevant committees and working parties as needed. Unlike scientific advice and protocol assistance, which provide to medicine developers formal, documented feedback and recommendations that are intended to be MAA enabling, ITF meetings are for the informal exchange of information through which the EMA can (1) clarify at an early stage the route to market for innovative medicines, and (2) maintain an awareness of current developments on innovative medicines in preparation for their assessment.
Innovative medicines are defined by EMA as those medicines that contain an active substance or combination of active substances that has not been authorised before. ITF meetings are provided free of charge as an incentive to developers of innovative medicines—which include ATMPs—to engage in early dialogue with the EMA. Other incentives provided specifically for the development of ATMPs include the following: a 65% fee reduction for a scientific advice request (or 90% for organizations registered with the EMA as micro-, small-, and medium-sized enterprises [SMEs]); and, also for SMEs, a 90% fee reduction for the ATMP certification procedure, which involves the scientific evaluation by the CAT of non-clinical and quality data generated at any stage during the ATMP development process to identify any potential issues early such that they can be addressed prior to MAA submission.
The MA Procedure Ensures the Quality, Safety, and Efficacy of ATMPs through Clinical Development
Defining Quality, Safety, and Efficacy: The Role of Clinical Development
In accordance with the EU regulatory framework, medicinal products must be demonstrated to be safe, efficacious, and of suitable quality for use in humans. During clinical development (Figure 2), medicinal product safety (i.e., the tolerability of the product and the minimization and/or management of adverse events caused by it) and efficacy (i.e., the ability of the product to induce the desired therapeutic response) are addressed in progressive non-clinical studies (e.g., in animal models of disease) and human clinical trials. Concurrently, medicinal product quality is established through the development of a defined manufacturing process and its associated analytical and stability testing procedures, i.e., the so-called chemistry, manufacturing, and control (CMC) studies. The output of these CMC studies can be considered to be a controlled, commercial-scale process that enables routine production of a characterized product defined by a set of quality attributes that correlate with safe and efficacious use in patients.4
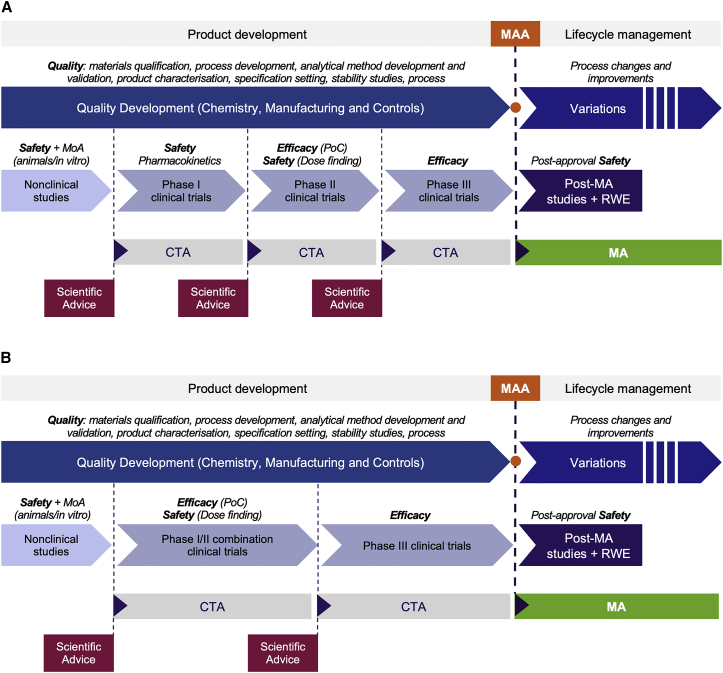
Figure 2.
Medicinal Product Development Pathway, from Clinical Trials to MA
The development of a medicinal product involves a number of discrete stages designed to demonstrate the product’s quality, safety, and efficacy. Medicinal product quality is established through CMC development, while safety and efficacy are demonstrated in non-clinical studies and progressive clinical trials. A clinical trial authorisation (CTA) application must be approved before each trial can start, and scientific advice may be sought prior to each CTA application to gain regulatory agency endorsement of the development plan. (A) A typical development route for a medicinal product for which initial healthy volunteer studies can be performed may involve the initial assessment of general safety in phase I (first time-in-human [FTIH]) trials, followed by the assessment of dose-related safety and proof of concept (PoC) of the therapeutic mechanism (initial efficacy) in phase II trials, and finally confirmation of efficacy in phase III (pivotal) trials. (B) Many ATMPs are not amenable to healthy volunteer studies for ethical reasons, and the FTIH trials therefore enroll patients into a phase I/II combination trial to evaluate safety and initial efficacy. Confirmation of efficacy is then confirmed in a subsequent phase III or pivotal trial. Data from pivotal clinical trials are used to support an MAA to the EMA. If an MA is granted, post-authorisation safety data may need to be obtained through post-authorisation studies and/or real-world evidence (RWE) to maintain the MA.
At the time of MAA, the safety and efficacy data generated during clinical development are reviewed with the intention of concluding on the benefit-risk balance of the product. A medicinal product may only be authorised if the benefit-risk balance is positive, i.e., the benefits outweigh the risks. The benefit-risk assessment may be quantitative or qualitative, depending on the therapeutic context and clinical study design, but the benefits are related to the key favorable effects based on the primary and most important secondary clinical endpoints, while the risks describe the incidence, severity, duration, reversibility, and dose-response relationship of unfavorable effects of the medicine, including adverse events. Benefits and risks also have limitations and uncertainties that are taken into consideration when concluding on the benefit-risk balance, e.g., sample size, representativeness of the target patient population, statistical modeling, and adequacy of monitoring. In all cases, the way in which a conclusion on the benefit-risk balance of a medicine is made is described in the assessment reports generated during the MAA review.
Clinical Trials with ATMPs
The clinical development of a medicinal product involves a number of discrete stages designed to generate the data on the product’s safety and efficacy needed to demonstrate the benefit-risk balance. Typically, clinical development begins upon completion of pre-clinical proof-of-concept (PoC) studies in in vitro or in vivo models of disease (the discovery phase). The developmental medicinal product is then progressed into non-clinical studies to demonstrate safety and provide an initial indication of mode of action supportive of clinical trials in humans. The non-clinical data are then used to support a CTA application. If the CTA application is approved on the basis of the non-clinical data, the investigational medicinal product (IMP) is next progressed into clinical studies. In an idealized phase I-II-III approach (Figure 2A), the IMP would first be tested for general safety in phase I (first time-in-human) trials, next for dose-related safety and PoC of the therapeutic mechanism (initial efficacy) in phase II trials (which may be delineated as phase IIa for short-term safety and as phase IIb for dose finding), and then for confirmation of efficacy in phase III (pivotal) trials. Data primarily from pivotal clinical trials are used to support the MAA. If an MA is granted, post-authorisation studies may need to be performed to provide ongoing evidence of the positive benefit-risk balance to maintain the MA, together with data from real-world use of the commercial product in patients.
For small molecule or biotechnology-derived drugs, phase I studies are usually performed in healthy volunteers. However, for many ATMPs, e.g., autologous products, phase I trials are conducted in a small target patient population for ethical reasons, and the evaluation of safety is often combined with an early evaluation of efficacy in a phase I-II transitional study type design (Figure 2B). Subsequent phase (II and) III trials continue to gather safety data and also study a number of efficacy endpoints designed to show that the ATMP has a beneficial therapeutic effect in increasing patient numbers.
ATMPs are being studied in a range of disease indications, and the clinical efficacy data required to achieve a MA for an ATMP in a particular indication is dependent on the rarity of the indication, the urgency of the unmet medical need, and the magnitude of benefit observed with the ATMP. How the safety and efficacy endpoints are staged and the size and demographics of the patient population in which they are tested need to be agreed upon with the competent authority responsible for approving the trial for each individual ATMP. This is acknowledged in EMA guidance on ATMPs. For example, the CHMP Guideline on Cell-Based Medicinal Products (Table S3) states, “Special problems might be associated with the clinical development of human cell-based medicinal products. Guidance is therefore provided on the conduct of pharmacodynamic/pharmacokinetic studies, dose finding, and clinical efficacy and safety studies. The guideline describes the special consideration that should be given to pharmacovigilance aspects and the RMP for these products.” For any particular ATMP, the clinical development strategy will, therefore, be product and patient specific, and early and ongoing scientific advice with NCAs and the EMA is recommended to ensure that trial designs are appropriate to support an MAA and enable the correct authorisation route to be identified. Furthermore, Directive 2009/120/EC describes additional non-clinical and clinical requirements needed for the development of ATMPs, highlighting the potential need for additional studies to demonstrate comparability, particularly when changes to the quality development strategy are implemented.
The concepts of pharmacovigilance and risk management highlighted above are important for establishing the benefit-risk balance both pre-authorisation in clinical studies and post-authorisation in clinical studies and/or real-world use. Pharmacovigilance is defined by the EMA as “science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem,” while an RMP describes how these activities will be performed once a medicinal product is marketed (discussed later). Given that many ATMPs are lifelong treatments for some patients, this is an important consideration for the RMP that needs to build on monitoring procedures initiated during clinical studies.
Good Clinical Practice
Clinical trials worldwide are required to be performed in compliance with good clinical practice (GCP). Clinical trials and GCP in the EU are currently governed by Directive 2001/20/EC (due to be replaced by Regulation (EU) No. 536/2014 in 2019) and Directive 2005/28/EC (which will be replaced by an implementing regulation for trials submitted under the new regulation). The aim of GCP is to protect the safety and dignity of human volunteers who enter into a clinical trial by defining ethical and scientific quality standards for the design, conduct, and recording of the studies. In this respect, obtaining informed consent and independent ethical approval are key elements underlying the authorisation of clinical trials.
GCP was initially introduced via ICH guidelines, but it has been elaborated on by specific legislation in certain jurisdictions, and this legislation supersedes the ICH guidance where available. ICH was established to standardize, where possible, the approach to medicinal product development, such that the requirements for registration in different countries are common. This reduces the need to perform multiple, different studies for medicinal products that will be marketed in different jurisdictions, thus improving patient access to the treatments they need. ICH publishes guidelines that provide detailed information on how key aspects of the quality, safety, and efficacy of a medicinal product should be demonstrated. These guidelines are officially adopted by the United States, the EU, and Japan (the founding members and signatories of ICH); by the European Free Trade Association (EFTA) states of Iceland, Lichtenstein, Norway, and Switzerland; and also by Canada. They are broadly used in other countries too. It should be noted that none of the ICH guidelines are specific to cell and gene therapies but are generically applicable to the key elements of the product development process for all medicinal products.
In the EU, specific guidelines have been developed by the EC on GCP in the context of clinical trials conducted with ATMPs. These guidelines make recommendations to sponsors, manufacturers, and clinical sites on critical aspects relevant to ATMPs, including, among others, the legal obligations toward donors, procurement of starting materials from tissue and blood establishments, animal facility management for xenogenic cell-based medicinal products, testing of the investigational medicinal product, and traceability of starting materials. These guidelines are compiled in chapter V (Additional Information) of EudraLex11 volume 10 according to Directive 2001/20/EC, and they are currently being revised for alignment with Regulation (EU) No. 536/2014 as part of a broader action plan on ATMPs implemented by the EC and EMA.12 The key points being addressed in the new guidelines cover all of the main aspects of clinical trials, including design, application dossier, investigational medicinal product quality, administration procedure, traceability, sample retention, protection of clinical trial subjects, and safety reporting and monitoring. The intent of the EC is that the revised guidelines will further adapt GCP to ATMPs by focusing on ATMP specificities only, while the EU (established via Directive 2005/28/EC) and ICH guidelines will remain valid for the more generic aspects. Revision of the EC guidelines is in progress following the conclusion of an open consultation with stakeholders,13 and the finalized guidelines will likely be published in late 2019.
The Unusual Case of Holoclar
Clinical trials should be prospectively planned and appropriately controlled to ensure that GCP-compliant data that demonstrate the safety and efficacy of an investigational medicinal product can be generated in support of an MAA. However, before the ATMP regulation came into force, some cell-based therapies were already being used on a named-patient basis in hospital environments. One such therapy developed by Professors Michele De Luca and Graziella Pellegrini of Holostem in Italy, comprising autologous tissue grafts grown from limbal epithelial stem cells to repair corneal damage caused by chemical burns, was successfully used in a few hundred patients between 1998 and 2007.14, 15 Treatment of these patients was performed under the appropriate human tissues for therapeutic use regulations. However, with the introduction of the ATMP regulation, the Italian medicines agency (Agenzia Italiana del Farmaco [AIFA]) stipulated that the therapy should be licensed as an ATMP for continued use beyond December 2012. As a result, the therapy was commercially developed by Holostem and Chiesi Farmaceutici S.p.A. as an ATMP now known as Holoclar,16, 17 and it received a conditional MA in 2015.
To support the Holoclar MAA, Chiesi Farmaceutici S.p.A. used retrospective data from the named-patient treatments rather than performing prospective clinical trials in newly enrolled patients.16 Evidence from 119 treatments (106 patients) was provided from a long-term efficacy and safety study and from 29 patients who received a single treatment in an observational study aimed at assessing long-term safety of the product. Both studies were non-randomized, non-controlled, multicenter trials performed as retrospective independent analyses of ocular photographs to provide an objective assessment of clinical efficacy. The primary endpoint was a composite endpoint of the rate of patients with a successful transplantation at 12 months post-intervention, based on the co-presence of clinical signs.
As discussed later, the conditions of the MA required the MAH to perform additional studies to confirm safety and efficacy post-authorisation, but the route to market for Holoclar is remarkable in that authorisation based on retrospective clinical data is unprecedented, and this is testament to the therapeutic effect of the product and the quality of the named-patient treatment programs.
Compassionate Use
A medicinal product used in a clinical trial prior to MA is referred to as an IMP to indicate its developmental, unauthorised status. Use of an unauthorised product outside of a clinical trial typically means that the data generated cannot be used to support an MAA (the aim of clinical trials is research, while the aim of unauthorised product use outside of a clinical trial is treatment). However, in some situations, patients who would benefit from an unauthorised medicine may not be able to enter clinical trials, for example, because enrollment has ended, the trial has been completed, or the patient does not meet all inclusion criteria. In such cases, for patients with life-threatening, long-lasting, or seriously debilitating diseases, the use of unauthorised medicines outside of clinical trials is possible either under a compassionate use scheme, which is provided for pursuant to article 5 of Directive 2001/83/EC (as amended) and article 83 of Regulation (EC) No. 726/2004 (as amended), or on a named-patient basis (as set out in national legislation), including in some cases on a cohort (group) scheme basis (depending on the national legislation).
Compassionate use is performed under regulatory agency oversight to enable patients who cannot enter clinical trials to benefit from treatment with products in development when suitable authorised therapies are not available. For products that are within the scope of articles 3(1) and 3(2) of Regulation (EC) No. 726/2004, article 83 clarifies that such products may be made available on a compassionate use basis to a group of patients. The EMA provides recommendations through the CHMP for such compassionate use of an unauthorised medicine, which must be undergoing clinical trials or have entered the MAA procedure, in patients meeting certain criteria. However, the actual compassionate use programs would be implemented on a member state basis according to procedures defined by the NCAs (where provided for in national law). The NCAs must inform the EMA that they have approved a compassionate use program for such a product. While early clinical studies will generally have been completed for a product approved for compassionate use, its full safety profile and dosage guidelines may not be fully established. Schemes similar to EU compassionate use are also operative in other global jurisdictions, including the United States and Japan (expanded access), Canada (special access programme), Australia (special access scheme), and Korea (treatment use of an investigational new drug).
Treatment on a named-patient basis is also possible, through which medical practitioners obtain medicines directly from manufacturers, prior to authorisation, for an individual patient. This is done under the direct responsibility of the medical practitioner, is subject to the regulations implemented at a national level governing named-patient supply, and would not involve the EMA. EU member state national laws may also cover cohort program compassionate use and/or named-patient supply for products that would not fall within the scope of the centralised procedure.
The MAA and the Centralised Procedure
At the end of a successful clinical development program through which product safety and efficacy in a subset of the target patient population are demonstrated, provision of the ATMP to the wider patient population via commercialization on the EU pharmaceutical market requires a central MA to be obtained under the centralised procedure through submission of an MAA to the EMA. MAAs for ATMPs must be submitted to the EMA for evaluation under the centralised procedure, which results in one MA with one product name that is valid in all member states as well as in the EEA countries of Iceland, Liechtenstein, and Norway, and is based on one scientific opinion issued by the CHMP rather than individual member state opinions.
Submission of an MAA must be carefully planned and managed by both the applicant and the EMA. The applicant is responsible for preparing the MAA in accordance with regulatory, scientific, and procedural guidelines, while the EMA must review the submission according to all applicable aspects of the legal framework. The submission is made in electronic common technical document (eCTD) format, which presents the quality, safety, and efficacy data, together with administrative content, as a dossier for regulatory review. Guidance to applicants is provided in EC EudraLex Notice to Applicants Volume 2B.18 Prior to submission of the MAA, the applicant is obliged to submit an eligibility request for review under the centralised procedure and, subsequently, a notification of the intention to apply 7 months prior to submission (submissions should be made according to a timetable published by the EMA). Upon acceptance of the submission, the EMA will appoint a rapporteur and a co-rapporteur to conduct the scientific evaluation of the dossier. A rapporteur is a member of an EMA committee or working party who leads the evaluation of an application by a team that they appoint. For ATMPs, the rapporteur and co-rapporteur are appointed from the CAT, whereas they would be appointed from the CHMP for other human medicinal products. A PRAC rapporteur is also appointed to evaluate the RMP, while a PRAC co-rapporteur is appointed to support the CAT rapporteur. Following their appointments, the (co-)rapporteurs hold a pre-submission meeting with the applicant to discuss the regulatory aspects of the upcoming application and to clarify any application-specific issues, following which the MAA can be submitted.
Within the EMA, the CAT is responsible for reviewing the data on quality, safety, and efficacy of an ATMP submitted in the MAA, and this is the role of the rapporteur, co-rapporteur, and their teams. However, the CAT itself does not grant an MA for an ATMP but rather makes a recommendation to the CHMP, which may then issue a positive opinion to the EC, which, provided that it accepts the positive opinion, would then issue the MA. Because the CAT and CHMP comprise members representing all NCAs, the centralised procedure involves all EU member states in the decision-making process.
The aim of the centralised procedure is to enable rapid, EU-wide authorisation of medicinal products, including ATMPs. The procedure itself involves a number of discrete steps that follow a specific timetable to achieve a CHMP opinion in 210 procedural days (not including clock-stops), followed by an EC decision within 67 days in the event that the CHMP issues a positive opinion at day 210. An idealized MAA procedure should, therefore, take 277 procedural days from submission to EC decision (Figure 3A; note that the accelerated assessment procedure shown in Figure 3B is discussed subsequently). Based on experience during the first decade of the ATMP regulation, the EMA has recently published procedural advice (Table S3) on how the evaluation of ATMPs should be executed to ensure efficiency and full collaboration among the CAT, CHMP, PRAC, working parties, and scientific advisory groups, thus enabling timely opinions.
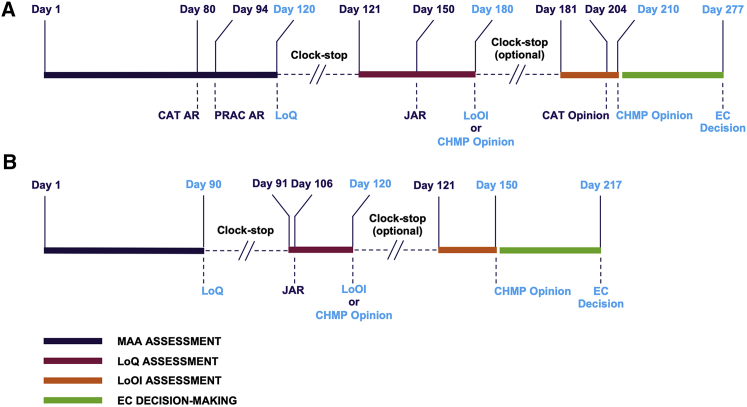
Figure 3.
Review of MAAs according to the Centralised Procedure
The evaluation of ATMP MAAs is performed according to the EMA’s centralised procedure. Two timetables are possible: standard assessment and accelerated assessment. (A) The standard procedure for ATMPs involves an initial assessment period in which the CAT and PRAC (co)-rapporteurs generate independent assessment reports (ARs), which are sent to the applicant initially for information only on day 80 or 94, and which are then integrated with the CHMP comments into a consolidated list of questions (the day 120 LoQ) to be formally addressed by the applicant. A 90-day clock-stop is then implemented for the applicant to respond to the LoQ. Upon submission of the applicant’s responses, the review clock starts again on day 121. The CAT then generates a joint AR (JAR), which is circulated to CHMP coordinators; to PRAC, CAT, and CHMP members; and to the applicant, again for information only, on day 150. Comments on the JAR are then collected from the PRAC, CAT, and CHMP to formalize the day 180 JAR for transmission to the applicant. If all issues from the day 120 LoQ are considered solved, a draft opinion may be issued; otherwise, the applicant will be required to address a list of outstanding issues (LoOI) during another clock-stop. Subsequently, the evaluation phase restarts on day 181 with the submission of responses to the LoOI or with an oral explanation (OE), if requested. Then, the CAT (co)-rapporteurs prepare an updated JAR, which also includes RMP considerations and which is commented on by the CAT, CHMP, PRAC, and EMA. This leads to the adoption of a CAT draft opinion on day 204, which is followed by the adoption of the CHMP AR and CHMP opinion on day 210. Following issuance of a positive opinion by the CHMP, an EC decision will hypothetically be announced on day 277. (B) When a request for accelerated assessment is granted by the CAT, the initial MAA evaluation phase is reduced to 120 days and includes assessment steps similar to the standard procedure for ATMPs. The first assessment phase will generate ARs resulting in the presentation of the LoQ on day 90. A shorter, 30-day clock-stop is then implemented. At the end of this period, the second assessment phase leads to the circulation of the CAT JAR on day 106 and of the LoOI or CHMP positive opinion as appropriate on day 120. A second clock-stop is not expected after this stage, and the CHMP requests the submission of the written responses without timeline interruptions, ideally on day 121. A final 30-day evaluation leads then to the CAT draft opinion and ultimately to the CHMP opinion on day 150. The CHMP can decide to switch to the standard timetable at any time of the review process. Following issuance of the CHMP opinion, an EC decision will hypothetically be announced on day 217 in the case of accelerated assessment. Full details of the evaluation of ATMP MAAs are provided in the EMA publication, Procedural Advice on the Evaluation of Advanced Therapy Medicinal Products in Accordance with Article 8 of Regulation (EC) No. 1394/2007 (January 25, 2018).
The procedure starts on day 0 with the electronic submission of the MAA package, and, upon successful validation to confirm that all requisite modules are included and complete (note that validation generally takes a few weeks), day 1 is declared to indicate that the procedure is underway. Between day 1 and day 120, the rapporteur and co-rapporteur lead the review of the dossier by their teams. At day 80, the rapporteur and co-rapporteur independently submit an initial assessment report (AR) to the CHMP and the CAT, which is also sent to the applicant. The day 80 ARs comprise a discussion on how the quality, safety, and efficacy of the product have been evaluated; a provisional recommendation on whether or not the product may be authorised (based on a positive or negative benefit-risk balance); and a draft list of questions on outstanding issues to be solved for a positive opinion to be granted.
Upon receipt of the day 80 ARs, together with the PRAC rapporteur’s RMP AR, which follows on day 94, the CHMP agrees on the provisional recommendation and the outstanding issues to be solved, and it prepares the consolidated list of questions (LoQ) to be provided to the applicant, together with the rapporteur and co-rapporteur assessments, on day 120. A clock-stop is then implemented to give the applicant a period of time to respond to the outstanding issues by preparing answers to the LoQ. The provisional recommendation on whether the product under review may be authorised depends on the type of questions asked at day 120, and these will be classified as either major objections or other concerns. If major objections on quality, safety, or efficacy are raised, the provisional recommendation will be that the medicine cannot be authorised unless the major objections can be resolved within the time frame of the MAA procedure. Other concerns, in the absence of major objections, will allow a provisional recommendation on authorisation to be supported, but they still require resolution within the time frame of the procedure.
The typical time frame for answering day 120 questions is 90 days, but this may be extended, typically by another 90 days, with appropriate justification. Submission of answers to the LoQ triggers the procedure to restart on day 121. Between days 121 and 180, the answers are reviewed by the rapporteur and co-rapporteur teams, and their suitability for addressing the major objections and/or other concerns is evaluated. At day 150, the rapporteur and co-rapporteur provide a joint AR (the day 150 JAR) to the CHMP for endorsement, which is also provided to the applicant prior to the CHMP-endorsed list of outstanding issues (LoOI) being communicated on day 180. Unless all outstanding issues (i.e., major objections and other concerns raised at day 120) are considered solved on day 180, at which point a CHMP opinion can be given, another clock-stop is then implemented for the applicant to respond to the outstanding issues, typically within 30 days prior to the CHMP opinion being given on day 210.
In the event that a CHMP positive opinion is issued on day 180 or day 210, it is sent to the EC for issuance of an MA within 67 days. If a negative opinion is issued, this can be appealed by the applicant, obligating the CAT to review the validity of the initial opinion using only the data submitted in the original MAA, i.e., the applicant cannot submit new data for review (uniQure’s Glybera was authorised following review of an initial negative opinion19). Regardless of the type of opinion issued, the EMA publishes the outcomes of all MAA reviews in a number of ways on its website as part of its commitment to transparency. All opinions are published as press releases and in the CAT and CHMP monthly reports. In addition, for those medicines that receive an MA from the EC following endorsement of the CHMP positive opinion, a European public AR (EPAR) is published on the EMA website. An EPAR is a multi-part publication that includes a summary of how the positive opinion was reached, a detailed report on the assessment process that includes non-commercially sensitive information taken from the (co-)rapporteur ARs, details of post-authorisation procedures completed (e.g., variations), and product-specific information such as the summary of product characteristics (SmPC; a legal document that provides information for healthcare professionals on how to use the medicine and that is updated during the product life cycle; it is developed from the equivalent clinical-stage investigator’s brochure document included in a CTA submission), package leaflet (the package insert provided with a medicine to inform patients on how to use it), and product label(s).
For MAAs that are refused, a refusal EPAR, including a question and answer document and an AR, is published. EPARs therefore represent a highly valuable source of information for developers of a prospective new medicine that are published based on the EMA’s commitment to transparency.20 MAHs must ensure that the product is compliant with the terms of its MA. Pursuant to Regulation (EC) No. 1049/2001, the EMA also commits to transparency beyond this minimal legal requirement, and it is possible for anyone to submit a request for further, non-commercially sensitive information not published on the EMA website (and not protected by any of the other exemptions from disclosure set out in the above regulation).
The Hospital Exemption Scheme for ATMPs
The early years of cell and gene therapy saw some products being developed in university hospital environments as experimental medicines for the benefit of patients with no other treatment options and without commercial gain for the university or hospital. Although a global industry around cell and gene therapies is now developing, in which biotechnology and pharmaceutical companies are key players, the non-commercial supply of such therapies to individual patients may still take place in certain circumstances.
Provision is made under the ATMP regulation for ATMPs not intended for commercial development to be supplied to patients without requiring an MA to be granted pursuant to Directive 2001/83/EC. This is made possible by the hospital exemption scheme, which exempts ATMPs from the centralised procedure if they are for use in a hospital within an individual member state on a non-routine basis, under the exclusive responsibility of a medical practitioner, and they comply with an individual medical prescription for a custom-made product for a named patient. The manufacturing of such products needs to be authorised by the NCA. The intention not to commercialize an ATMP, therefore, underlines the difference between supply under the hospital exemption scheme versus supply under compassionate use, and clinical data generated under the hospital exemption scheme cannot typically be used to support an MAA, because, unlike clinical trials, the scheme is not governed by the principles of GCP.
In the current world of commercial cell and gene therapies, the hospital exemption scheme lives an uncomfortable existence because the potential continued use of non-commercial therapies under the scheme can threaten the profitability of MAHs who have made significant financial investment into commercial product development. Furthermore, the hospital exemption scheme has been implemented in different ways in different member states with respect to the definition of use of a product on a non-routine basis. The true value of the scheme may be interpreted as providing treatment options where none exist, for example, when a patient presents for urgent treatment but is unable to join a clinical trial or when compassionate use is not an option.
From a medicinal product quality perspective, ATMPs used under the hospital exemption scheme should, according to the ATMP regulation, be of equivalent quality to ATMPs developed for commercialization. Again, quality requirements are implemented differently across member states, but in Italy, for example, product quality equivalent to that required for a phase II trial is expected. For complex ATMPs, e.g., gene-modified cells, the investment in CMC development4, 21 needed to demonstrate process robustness and product consistency is highly significant, and it should not be underestimated for hospital exemption use.
The Type of MA Applied for Depends on the Target Patient Population and the Need for the Medicine
Depending on the extent of clinical data obtained during development, an MA via the centralised procedure may be granted in three ways: standard MA, conditional MA, and MA under exceptional circumstances. In all cases, the MAA is reviewed according to the centralised procedure, and eligibility for the appropriate route to MA for any particular ATMP is determined by dialogue with the EMA during the development phase.
Standard MA
A standard MA is awarded when specific obligations (see below) to further demonstrate the quality, safety, and efficacy—or the benefit-risk balance—of the medicinal product under evaluation are not required in addition to the data presented in the MAA to support the granting of the MA. In other words, a standard MA is awarded on the basis of a positive benefit-risk balance being supported by comprehensive clinical data at the time of the MAA. In accordance with article 14 (1–3) of Regulation (EC) No. 726/2004, a standard MA is initially valid for 5 years from the date of the EC decision, after which it may be renewed on application. Once renewed, the MA is valid for an unlimited period, unless the EMA decides, on justified grounds relating to pharmacovigilance (e.g., exposure of an insufficient number of patients to the medicinal product concerned), to mandate one additional 5-year renewal.
A standard MA would typically be applied for when clinical data are not limiting, for example, because it is possible to perform clinical trials in sufficiently large numbers of patients and provide a statistically significant demonstration of safety and efficacy based on a therapeutically relevant endpoint, or when extensive clinical experience has been gained in the target patient population, including those for which an orphan medicinal product is being developed for a rare disease. A standard MA can still be subject to certain post-authorisation committments, usually in relation to safety (as discussed later).
Conditional MA
A conditional MA (CMA) may be applied for when an unmet medical need supports the availability of a medicine to patients prior to the comprehensive clinical data, normally required for an MA to be granted, being available. As such, medicines eligible for CMA typically include those aimed at treating, preventing, or diagnosing seriously debilitating or life-threatening diseases, and it may be possible to submit the CMA application upon completion of phase II studies to expedite the medicine’s availability (Figure 4). The CMA route is considered when comprehensive clinical data may not readily be obtained, e.g., when developing for a rare disease that by definition has a small target patient population, but when it is likely that the applicant will be in a position to provide comprehensive clinical data. Indeed, in most circumstances, orphan designation will qualify a medicinal product for the CMA route if significant benefit can be demonstrated over existing treatments or if unmet medical need is established because no suitable treatments already exist. However, the CMA route is not obligatory for orphan medicinal products if the applicant can justify that the clinical data available support a standard MA (of the orphan ATMPs that have been granted an MA to date [Tables 1 and 3], Strimvelis, Alofisel, Yescarta, and Kymriah received standard MAs while Holoclar and Zalmoxis received CMAs).
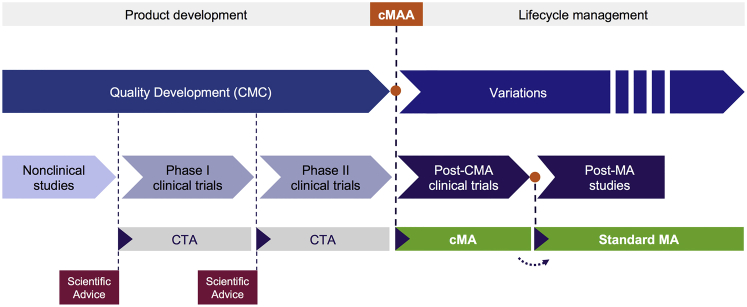
Figure 4.
Acceleration of Clinical Development via the CMA Procedure
The CMA procedure enables orphan medicinal products and medicinal products addressing unmet medical needs to be supplied earlier to the market based on pre-pivotal trial data, if the data support an initial positive benefit-risk balance and qualifying criteria are met. The scenario shown in the figure envisions a CMA on the basis of phase II data, potentially based on a surrogate endpoint rather than a clinical endpoint, although other scenarios are possible depending on the product and its particular development pathway (as agreed with the EMA during scientific advice or protocol assistance). Following conditional approval, further studies must be performed to confirm the positive benefit-risk balance via clinical endpoints if a surrogate endpoint was used for the CMA or in increased patient numbers if only limited patients were enrolled in the pre-CMA trials. The CMA is reviewed annually until sufficient data are available to support conversion to a standard MA. See also Figures 1 and 2.
Table 3.
ATMPs to Have Received an EU MA between 2009 and 2018
| ATMP (MAH) | Class | Active Substance | Drug Product | Pharmaceutical Form | Full Therapeutic Indication | Regulatory Status |
|---|---|---|---|---|---|---|
| Yescarta (Kite Pharma EU) | GTMP (autologous) | axicabtagene ciloleucel:a autologous T cells transduced with retroviral vector encoding an anti-CD19 CD28/CD3-zeta chimeric antigen receptor (CAR) with a target dose of 2 × 106 anti-CD19 CAR-positive viable T cells/kg. | Yescarta 0.4–2 × 108 cells dispersion for infusion | dispersion for infusion (cryopreserved) | indicated for the treatment of adult patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) and primary mediastinal large B cell lymphoma (PMBCL), after two or more lines of systemic therapy | standard MA orphan medicinal product PIP deferral priority medicine currently authorised |
| Kymriah (Novartis Europharm) | GTMP (autologous) | tisagenlecleucel:a autologous human T cells genetically modified ex vivo using a lentiviral vector encoding an anti-CD19 chimeric antigen receptor (CAR). | Kymriah 1.2 × 106–6 × 108 cells dispersion for infusion | dispersion for infusion (cryopreserved) | pediatric and young adult patients up to 25 years of age with B cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse; adult patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) after two or more lines of systemic therapy | standard MA orphan medicinal product PIP deferral priority medicine currently authorised |
| Alofisel (TiGenix NV/Takeda Pharmaceutical) | TEP (autologous) | darvadstrocel:a expanded human allogeneic mesenchymal adult stem cells extracted from adipose tissue (expanded adipose stem cells (eASC)) | Alofisel 5 million cells/mL suspension for injection | suspension for injection (fresh) | indicated for the treatment of complex perianal fistulas in adult patients with non-active/mildly active luminal Crohn’s disease, when fistulas have shown an inadequate response to at least one conventional or biologic therapy; Alofisel should be used after conditioning of fistula | standard MA PIP deferral currently authorised |
| Spherox (CO.DON) | TEP (autologous) | 10–70 spheroids/cm2 spheroids of human autologous matrix-associated chondrocytes | Spherox 10–70 spheroids/cm2 implantation suspension | implantation suspension (fresh) | repair of symptomatic articular cartilage defects of the femoral condyle and the patella of the knee (International Cartilage Repair Society [ICRS] grade III or IV) with defect sizes up to 10 cm2 in adults | standard MA PIP deferral currently authorised |
| Zalmoxis (MolMed) | SCTMP (allogeneic) | allogenic T cells genetically modified with a retroviral vector encoding for a truncated form of the human low affinity nerve growth factor receptor (ΔLNGFR) and the herpes simplex I virus thymidine kinase (HSK-TK Mut2) | Zalmoxis 5–20 × 106 cells/mL dispersion for infusion | dispersion for Infusion (cryopreserved) | indicated as adjunctive treatment in haploidentical hematopoietic stem cell transplantation (HSCT) of adult patients with high-risk hematological malignancies | CMA orphan medicinal product PIP deferral currently authorised |
| Strimvelis (Orchard Therapeutics) | GTMP (autologous) | autologous CD34+ enriched cell fraction that contains CD34+ cells transduced with retroviral vector that encodes for the human ADA cDNA | one or more ethylene vinyl acetate (EVA) bags which contain an autologous CD34+ enriched cell fraction that contains CD34+ cells transduced with retroviral vector that encodes for the human ADA cDNA sequence. The concentration is 1–10 million CD34+ cells/mL | dispersion for infusion (fresh) | indicated for the treatment of patients with severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID), for whom no suitable human leukocyte antigen (HLA)-matched related stem cell donor is available | standard MA orphan medicinal product PIP completed at the time of MAA currently authorised |
| Imlygic (Amgen) | GTMP (recombinant HSV-1 vector) | talimgene laherparepveca | Imlygic 106 plaque-forming units/mL solution for injection: 1 mL deliverable volume at 1 million plaque-forming units/mL Imlygic 108 plaque-forming units/mL solution for injection: 1 mL deliverable volume at 100 million plaque-forming units/mL | solution for injection (cryopreserved) | indicated for the treatment of adults with unresectable melanoma that is regionally or distantly metastatic (stages IIIB, IIIC, and IVM1a) with no bone, brain, lung, or other visceral disease | standard MA PIP deferral currently authorised |
| Holoclar (Chiesi Farmaceutici) | TEP (autologous) | ex vivo expanded autologous human corneal epithelial cells containing stem cells | 79,000–316,000 cells/cm2 living tissue equivalent | living tissue equivalent (fresh) | treatment of adult patients with moderate to severe limbal stem cell deficiency (defined by the presence of superficial corneal neovascularization in at least two corneal quadrants, with central corneal involvement, and severely impaired visual acuity), unilateral or bilateral, due to physical or chemical ocular burns; a minimum of 1–2 mm2 of undamaged limbus is required for biopsy | CMA orphan medicinal product PIP deferral currently authorised |
| Provenge (Dendreon Pharmaceuticals) | SCTMP (autologous) | sipuleucel-T:a autologous peripheral-blood mononuclear cells activated with prostatic acid phosphatase-granulocyte macrophage-colony stimulating factor | 50 million CD54+ cells/250 mL dispersion for infusion | dispersion for infusion (fresh) | indicated for treatment of asymptomatic or minimally symptomatic metastatic (non-visceral) castrate-resistant prostate cancer in male adults in whom chemotherapy is not yet clinically indicated | standard MA PIP waiver no longer authorised (withdrawn) |
| Maci (Vericel) | combined ATMP (autologous) | matrix-applied characterized autologous cultured chondrocytes | 0.5–1 million cells/cm2 implantation matrix | implantation matrix (fresh) | indicated for the repair of symptomatic, full-thickness cartilage defects of the knee (grades III and IV of the Modified Outerbridge Scale) of 3–20 cm2 in skeletally mature adult patients | standard MA PIP deferral authorisation currently suspended |
| Glybera (uniQure) | GTMP (recombinant AAV vector) | alipogene tiparvoveca | Glybera 3 × 1012 genome copies/mL solution for injection | solution for injection (cryopreserved) | indicated for adult patients diagnosed with familial lipoprotein lipase deficiency (LPLD) and severe or multiple pancreatitis attacks despite dietary fat restrictions; the diagnosis of LPLD has to be confirmed by genetic testing; the indication is restricted to patients with detectable levels of LPL protein | MA under exceptional circumstances orphan medicinal product PIP deferral no longer authorised (withdrawn) |
| Chondrocelect (TiGenix) | TEP (autologous) | characterized viable autologous cartilage cells expanded ex vivo expressing specific marker proteins | 4 million autologous human cartilage cells in 0.4 mL cell suspension, corresponding to a concentration of 10,000 cells/μL | implantation suspension (fresh) | repair of single symptomatic cartilage defects of the femoral condyle of the knee (International Cartilage Repair Society [ICRS] grade III or IV) in adults; concomitant asymptomatic cartilage lesions (ICRS grade I or II) might be present | standard MA PIP completed at the time of MAA no longer authorised (withdrawn) |
The information presented for each ATMP is taken from their EPARs.
Active substance descriptions may also include international non-proprietary names (INNs). INNs are globally recognized generic names used to identify the active ingredient in a medicine. Note: as discussed in text, Spark Therapeutic’s Luxturna was the 13th ATMP to receive an MA (in late 2018). However, at the time of writing, the Luxturna EPAR was not published on the EMA website and thus further details on this product are not included in the table.
In all cases under which a CMA is granted, the benefit-risk balance of the product must be considered positive pending confirmation from the comprehensive clinical data, which the applicant is expected to provide within a certain time frame post-authorisation. The subsequent provision of the pending comprehensive clinical data, for example, to corroborate the initial (potentially phase II) data presented for a therapy addressing an unmet medical need, would be a specific obligation required of an MAH to which a CMA is granted. Indeed, as the terminology suggests, a CMA stipulates certain conditions, i.e., specific obligations, that must be fulfilled post-authorisation if the MA is to be maintained by ultimate conversion to a standard MA. Other specific obligations that may apply include additional clinical trials to confirm safety and efficacy in larger patient numbers for the designated orphan medicinal products. A CMA is granted under article 14(7) of Regulation (EC) No. 726/2004, is initially valid for 1 year, and may be renewed annually.
For an urgent or unmet medical need, a CMA may be granted when initial efficacy, with a positive benefit-risk balance, is demonstrated through a surrogate clinical endpoint, such as a biomarker, rather than a direct therapeutic measure. Confirmation of efficacy through a direct endpoint, rather than the surrogate endpoint, may constitute either the post-authorisation specific obligation or another requisite post-authorisation measure (see below). Further insight into all medicinal products authorised in the EEA via a CMA is contained in a EMA report on 10 years of experience with CMAs, published in 2017.22 With regard to ATMPs, information is provided on Holoclar, but not on Zalmoxis, because the latter product was not authorised at the time of the report compilation.
MA under Exceptional Circumstances
MA under exceptional circumstances (ECMA) applies only in those extreme situations where a disease is so rare or a clinical endpoint is so difficult to measure—for either scientific or ethical reasons—that the comprehensive safety and efficacy data required for a standard MA are never expected to be obtained. Unlike a CMA, an ECMA is therefore unlikely to ever be converted to a standard MA, and indeed the expectation is that it would not be. Consequently, an ECMA is granted subject to the applicant agreeing to specific obligations to monitor the ongoing safety of the product and to notify the competent authorities of any incident relating to its use and actions to be taken. The accumulated clinical data are reviewed in an annual re-assessment procedure to continuously evaluate the benefit-risk balance and monitor the completion, and ongoing relevance, of the specific obligations required of the MAH. ECMAs are granted under article 14(8) of Regulation (EC) No. 726/2004 and valid for 5 years, and continuation of the MA shall be linked to the annual re-assessment.
Typically, medicinal products licensed via an ECMA would be for rare or ultra-rare diseases, and they would likely have an orphan medicinal product designation. However, while designated orphan medicinal products may automatically qualify for licensing under a CMA, orphan medicinal products are eligible for approval under exceptional circumstances only if the criteria considered for the approval under exceptional circumstances (i.e., the improbability that comprehensive clinical data will be provided) are fulfilled. Certainly, it is difficult to envisage that products, which can only be provided to patients under the exceptional circumstance route, could reach the market without the incentives provided by orphan medicinal product designation.
As with all medicinal products, the benefit-risk balance must be positive for an ECMA to be granted, even though this is likely to be based on limited data from small patient numbers. Medicinal product quality must nonetheless be equivalent to that required for a standard or CMA. The fact that only a limited number of patients may benefit from a product marketed via the ECMA route can cause potential problems for return on investment into development for the MAH, particularly if reimbursement is difficult to obtain in some member states. Indeed, uniQure’s Glybera, an orphan medicinal product and the first non-cell-based GTMP to be licensed globally, was authorised in 2012 under exceptional circumstances based on the extremely low prevalence of lipoprotein lipase deficiency (LPLD), a rare autosomal recessive inherited condition with a calculated prevalence in the EU of 0.02 in 10,000; but, reimbursement issues led to MA withdrawal in 2017.
Recommendations, Post-Authorisation Measures, Registries, and Risk Management Plans: Ongoing Monitoring of ATMPs following Authorisation
As discussed above, specific obligations are assigned on a medicinal product-specific basis during the MAA review process, and they are conditions on which either a CMA or ECMA is granted. During the evaluation of an MAA via any of the standard, conditional, or exceptional circumstance routes, the CHMP may request that the applicant should provide additional data post-authorisation when it is necessary from a public health perspective to complement the available data with additional data on the safety, and sometimes the efficacy or quality, of an authorised product. Such requests are imposed as either recommendations or post-authorisation measures (PAMs). Recommendations usually concern the optimization of certain quality aspects of the product or considerations for extending the patient population. Such recommendations are not binding conditions of the MA, but they should be seen as important considerations with regard to the potential future use of a medicinal product by the MAH. Fulfillment of recommendations is usually evaluated through a variation procedure, through which changes to the registered details of the MA are amended. PAMs represent commitments by the MAH to generate further data to enable the assessment of the safety or efficacy of medicinal products in the post-approval setting.
Following authorisation of a medicinal product, all MAHs are also mandated to continue monitoring the safety and efficacy (benefit-risk balance) of their products on an ongoing basis. The mechanism for the ongoing reporting of the benefit-risk balance is the periodic safety update report (PSUR), and this will include the reporting of outcomes from studies performed as post-authorisation specific obligations and/or PAMs. The ongoing benefit-risk balance assessment may require that the PAMs include post-authorisation safety studies (PASSs) and/or post-authorisation efficacy studies (PAESs), if imposed. A PASS is defined as “any study relating to an authorised medicinal product conducted with the aim of identifying, characterising or quantifying a safety hazard, confirming the safety profile of the medicinal product, or of measuring the effectiveness of risk management measures,” while a PAES is defined as a study “considered important for complementing available efficacy data in the light of well-reasoned scientific uncertainties on aspects of the evidence of benefits that is to be, or can only be, addressed post-authorisation.”
Patient registries are another way in which the safety of medicines is monitored on an ongoing basis. Patient registries are organized systems that use observational methods to collect uniform data on a population defined by a particular disease, condition, or exposure and that is followed over time. The EMA launched an initiative to make better use of existing registries, and facilitate the establishment of high-quality new registries, in September 2015. This Initiative for Patient Registries aims to develop a more systematic and standardized approach to the role of registries in the benefit-risk evaluation of medicines authorised in the European community. In particular, the initiative addresses collaboration among physician associations, patient associations, academic institutions, national agencies responsible for overseeing healthcare services, and potential users of registry data such as medicine regulators and pharmaceutical companies. As discussed later, registries are being used for the long-term safety monitoring of a number of the ATMPs currently authorised in the EU.
Although medicinal products are authorised on the basis of a positive benefit-risk balance in the specified indication, risks (e.g., adverse events) of varying severity and likelihood of occurrence will be evident. Not all risks will have been identified at the time of MAA, and some will only be discovered and characterized post-authorisation. Since July 2012, all new MAAs have been required to also include an RMP, in which the risk management system considered necessary to identify, characterize, and minimize a medicinal product’s risks post-authorisation is documented. In this respect, the main principle of risk management is to ensure that the benefits of a particular medicinal product exceed its risks by the greatest achievable margin.
The key elements of the RMP are the following: (1) the safety specification, which describes the current safety profile and identifies important potential risks to be managed or studied further; (2) the pharmacovigilance plan, which describes activities to characterize and quantify clinically relevant risks and to identify new adverse reactions; and (3) the risk minimization plan, which describes the planning and implementation of risk minimization measures and how their effectiveness is evaluated. As such, the RMP is a dynamic document that changes (to both add and remove safety measures, as necessary) as knowledge regarding a medicinal product’s safety profile increases over time. RMPs for ATMPs should additionally focus on specific risks associated with these products, including risks to living donors and of germline transformation and transmission of vectors.
Accelerating Clinical Development and the Advent of the PRIME Scheme
Conditional Approval, Adaptive Licensing, and Accelerated Assessment of MAAs
As discussed above, a standard MA would typically be applied for when comprehensive clinical data can be provided at the time of MAA. By contrast, a CMA (or conditional approval) provides a mechanism by which an innovative medicine addressing an unmet medical need can be made available for market supply as early as a positive benefit-risk balance indicated by sufficient clinical data is demonstrated. Renewal on an annual basis then ensures that the benefit-risk balance is monitored while further clinical trials are performed, as a commitment by the MAH, to confirm safety and efficacy, such that data are obtained to enable the CMA to be converted to a standard MA later. CMA is, therefore, a strategic way of providing therapies to patients who may have no or limited treatment options in a timely manner.
Conditional approval was introduced in 2006 through Regulation (EC) No. 507/2006. EMA’s experience with the procedure has shown that, while intended to focus on unmet needs of small patient populations, many CMAAs have been the result of late requests by applicants during EMA evaluations after it became apparent that either a standard MA would not be granted or a broader therapeutic indication not supported. Furthermore, the datasets used to approve CMAs may differ from those normally required by HTA bodies in their assessments, a situation that could delay or prevent medicine reimbursement by national healthcare systems. In recent years, the EMA has investigated certain ways in which the CMA procedure could be better implemented for its intended use, and in 2014 the adaptive pathways pilot was launched for this purpose.
Adaptive pathways is a conceptual approach to medicine development for addressing high unmet medical needs where it is difficult to collect data via traditional routes. In this respect, medicines considered suitable for adaptive pathways are envisaged to require an iterative development plan, with an initial (conditional) approval either in a restricted patient population, followed by expansion to wider patient populations, or based on a clinically relevant surrogate endpoint, with later confirmation of the benefit-risk balance from broader clinical experience. This broader clinical experience should also involve the gathering of evidence through real-world use to supplement clinical trial data (e.g., from patient registries). Finally, early involvement of patients and HTA bodies in discussions on a medicine’s development plan are also considered important. The adaptive pathways scheme ran as a pilot between 2014 and 2016,23 and it enrolled 18 developmental products, two of which were ATMPs (bluebird bio’s LentiGlobin BB305, an autologous ex vivo lentiviral vector-transduced CD34+ cell therapy for beta-thalassemia major that is currently under MAA review, and Pluristem Therapeutics’ PLX-PAD, a placenta-derived, mesenchymal stromal cell product for critical limb ischemia). The scheme as originally conceived is no longer active, but the learnings from it are being applied to adaptive licensing, particularly in the context of an adapted scientific advice procedure known as parallel consultation, which involves the EMA, EUnetHTA, HTA bodies, patient representatives, and healthcare professionals.
In addition to conditional approval, another way in which innovative medicines can be supplied to the market earlier than allowed by the standard MA procedure is via the accelerated assessment procedure. Accelerated assessment is a procedural tool that is applied to qualifying medicines to reduce the centralised procedure review period from 210 to 150 days in total, i.e., the EC decision on whether to grant an MA is reached more quickly (Figure 3B). Medicinal products are eligible for accelerated assessment if they are of major public health interest, in particular from the viewpoint of therapeutic innovation.
The concepts of unmet medical need and therapeutic innovation are key to the implementation of conditional approval or accelerated assessment to provide earlier market access to medicines. In the EU, unmet medical need means a condition for which there exists no satisfactory method of diagnosis, prevention, or treatment authorised in the European community, or, if such a method exists, a new medicinal product will provide a major therapeutic advantage. For medicines with the potential to fulfill these criteria, the EMA gives high value to the acceleration of their clinical development programs to ensure that patients can benefit from new treatments at the earliest opportunity.
Until recently, accelerated assessment was not possible in the context of a CMA. Furthermore, accelerated assessment itself does not accelerate a clinical development program. In 2016, the EMA published new guidance documents24, 25 to address certain shortcomings or inefficiencies identified in the implementation of conditional approval and accelerated assessment following an extensive review of experience gained with them. The main ways in which accelerated assessment was addressed include the following: (1) more detailed guidance on how to justify fulfillment of major public health interest, which is the basis of a request for an accelerated assessment; (2) optimization of the assessment timetable by better balancing evaluation phases to reach a CHMP opinion within the 150 days after the start of an MAA procedure; (3) emphasis on the importance of early dialogue with the EMA so that accelerated assessment can be planned well ahead of the submission; and (4) enabling accelerated assessment in the context of a CMA.
For conditional approval, the revised guideline emphasizes the importance to medicine developers of planning a CMA prospectively and engaging in early dialogue with the EMA and other stakeholders, for example, through parallel consultation. In addition, the revisions include the following: (1) clarification on the fulfillment of unmet medical needs, i.e., medicines providing major improvements in patient care over existing therapies can be eligible in certain cases; (2) clarification of how a positive benefit-risk balance is to be substantiated where there are less complete data, with further guidance on the level of evidence that must be provided at the time of authorisation and the data that can be provided after authorisation; (3) encouragement of early scientific advice and prospective planning of a CMA to expedite assessment; and (4) updated guidance on the extent and type of data required to be included in annual renewal submissions.
Moreover, this review of conditional approval and accelerated assessment not only resulted in the publication of revised scientific guidelines on these procedures but also led to the introduction of the PRIME scheme.
The PRIME Scheme: Expedited Development of Priority Medicines
The PRIME scheme was launched in 2016, coincident with the revised guidance documents on CMA and accelerated assessment being published. While the adaptive pathways concept, or parallel consultation as it now is, focuses on medicines with non-standard development pathways, the PRIME scheme focuses on expediting and optimizing the development of priority medicines in the EU. Priority medicines are defined as those medicines that may offer a major therapeutic advantage over existing treatments or may be of benefit to patients with no other treatment options, i.e., priority medicines address an unmet medical need.
The basis of the PRIME scheme is to enhance interactions and enable early dialogue between developers of promising medicines and the EMA, thus optimizing development plans and expediting the evaluation of MAAs such that novel effective treatments can be made available to patients as early as possible. As such, the PRIME scheme uses relevant tools and procedures already available in the regulatory framework, particularly scientific advice (for early and enhanced dialogue) and accelerated assessment (for expedited MAA review). Early dialogue, which begins with a kick-off meeting soon after a medicine is granted eligibility to the scheme, is aimed at optimizing clinical trial designs through prospectively planned scientific advice at key milestones, such that data suitable for MAA are generated quickly and efficiently. The kick-off meeting is led by a rapporteur appointed from the CAT, and it also involves a multidisciplinary team of experts that provide input into the overall development plan and regulatory strategy. The appointment of a rapporteur early in development as opposed to at the time of MAA is a key part of the PRIME support mechanism provided by the EMA, and the rapporteur provides continuous support (enhanced interactions) prior to MAA. Scientific advice is also expected to involve additional stakeholders, such as HTA bodies, as and when appropriate to expedite market access, while eligibility to accelerated assessment will be confirmed at the earliest opportunity to allow the procedure to be efficiently managed. Priority medicines are additionally eligible for CMA, if supported by the development strategy, and they can also be evaluated by the CHMP for compassionate use while clinical trials are being conducted.
The aims of the PRIME scheme mean that eligibility should be granted as early as possible, with phase II clinical evidence being the typical entry point for all products enrolled to date.26 Since coming into effect 2 years ago, there are already more ATMPs (17 currently, 20 cumulatively; Table 2) in the PRIME scheme than there are currently authorised ATMPs (eight; Tables 1 and 3). Of all the medicinal products currently in the scheme, over 40% are ATMPs, and this is indicative not only of the recent growth of the industry but also of the importance of ATMPs in addressing unmet medical needs. Indeed, of the total PRIME scheme applications received in the 2 years of the scheme, 25% were related to ATMPs26 (and more are currently under evaluation). Details and outcomes of PRIME scheme applications are updated monthly in the CHMP monthly meeting reports published by the EMA.
Table 2.
ATMPs in the PRIME Scheme
| ATMP | Therapeutic Indication | Start Date | Company |
|---|---|---|---|
| NY-ESO-1c259Ta | metastatic synovial sarcoma | July 21, 2016 | Adaptimmune Therapeutics |
| DNX-2401b | recurrent glioblastoma | July 21, 2016 | DNAtrix |
| LentiGlobin BB305a,c,d | transfusion-dependent beta-thalassemia | September 15, 2016 | bluebird bio |
| ATA129a | EBV-associated post-transplant lymphoproliferative disorder | October 13, 2016 | Atara Biotherapeutics |
| JCAR017a | relapsed and refractory DLBCL | December 15, 2016 | Juno Therapeutics |
| AVXS-101a,e | pediatric spinal muscular atrophy type 1 | January 26, 2017 | Avexis |
| BMN 270a | hemophilia A | January 26, 2017 | BioMarin Pharmaceutical |
| PF-06838435/SPK-9001a | hemophilia B | February 23, 2017 | Spark Therapeutics |
| AMT-061a | severe hemophilia B | April 21, 2017 | uniQure |
| Vocimagene miretrorepveca | high-grade glioma | July 20, 2017 | Tocagen |
| xbb2121 | relapsed and refractory multiple myeloma | November 9, 2017 | bluebird bio |
| AAV2/8-hCARp.hCNGB3 | achromatopsia associated with defects in CNGB3 | February 22, 2018 | MeiraGTx Holdings |
| AT132c | X-linked myotubular myopathy | May 31, 2018 | Audentes Therapeutics |
| NLA101b | HSCT | May 31, 2018 | Voisin Consulting |
| Autologous T cells transduced with retroviral vector encoding an anti-CD19 CD28/CD3-zeta chimeric antigen receptor (KTE-X19) | relapsed or refractory mantle cell lymphoma | May 31, 2018 | Kite, a Gilead Company |
| Lenti-Da | cerebral adrenoleukodystrophy | July 26, 2018 | bluebird bio |
| OTL-300 | transfusion-dependent beta-thalassemia | September 20, 2018 | Orchard Therapeutics |
There are 17 ATMPs in the PRIME scheme as of the fourth quarter 2018. These are all GTMPs and were accepted on the basis of non-clinical plus clinical exploratory data, except for AAV2/8-hCARp.hCNGB3, which was accepted on the basis of non-clinical plus first time-in-human tolerability data. AMT-061 was accepted based on clinical data obtained with its predecessor product, AMT-060. One other ATMP not included in the table has also been granted eligibility to the PRIME scheme, but it was withdrawn by the applicant. Both Yescarta and Kymriah, two CAR T cell products that now hold an MA, entered the PRIME scheme during clinical development. DLBCL, diffuse large B cell lymphoma; HSCT, hematopoietic stem cell transplantation.
These products also have FDA breakthrough therapy designation.
These products also have FDA fast track designation.
These products also have FDA RMAT designation.
This product is currently under MAA evaluation.
This product also has Japan PMDA sakigake designation.
CMC Considerations for Accelerated Development
Accelerated clinical development as enabled by conditional approval, adaptive licensing, and the PRIME scheme aims to expedite the generation of clinical data supporting a positive benefit-risk balance of a medicinal product. Nonetheless, regardless of whether or not clinical development is expedited, an MA for any medicinal product will only be granted if, in addition to a positive benefit-risk balance, the quality (or CMC) development is sufficiently advanced to demonstrate that commercial supply of a medicinal product can be ensured. The CMC requirements for market supply of ATMPs are significant,4, 27 and expedited clinical development does not reduce the CMC data needed in the MAA to demonstrate that the manufacturing process is robust, reproducible, validated, and controlled to enable ongoing supply of a characterized ATMP released using validated analytical methods, at a scale that can meet the commercial demands of patient treatment. CMC development activities must, therefore, be planned, managed, and executed to keep pace with clinical development (Figure 2). In this respect, early development of a commercially viable process is recommended to avoid the delays that process changes during development inevitably bring.21, 27
MAA Experience with ATMPs from 2009 to 2018
Since the ATMP regulation came into force, 13 ATMPs have received an MA from the EC (Tables 1 and 3). These include seven ATMPs with orphan medicinal product status, two ATMPs with a CMA (both of which are orphan medicinal products), one ATMP (also an orphan medicinal product) with an MA under exceptional circumstances, and 10 ATMPs granted a standard MA (of which six are orphan medicinal products) (Table 3). PIPs were completed for three of these ATMPs at the time of MAA, waived for one ATMP, and deferred for eight ATMPs (Table 3). Of these 12 ATMPs, four are no longer marketed in the EU. Vericel’s Maci is currently suspended following the closure of its EU manufacturing site, whereas the MAs for TiGenix’s Chondrocelect, uniQure’s Glybera, and Dendreon Pharmaceuticals’ Provenge were withdrawn by the MAHs for commercial or reimbursement issues. Maci and Provenge are, however, currently marketed in the United States,28, 29, 30 as are Amgen’s Imlygic,31 Kite’s Yescarta,32 and Novartis’s Kymriah, other ATMPs with an EU MA. Yescarta and Kymriah have now progressed through the PRIME scheme to MA, and they represent the first chimeric antigen receptor (CAR) T cell products to be marketed in Europe, in addition to being the first human medicinal products of any type to be brought to market through PRIME (one more ATMP, bluebird bio’s LentiGlobin BB305, is currently under MAA evaluation). Both Kymriah and Yescarta, together with Strimvelis, were initially submitted for accelerated assessment, but the MAA reviews reverted to the standard timetable during the procedure. Both Kymriah and Yescarta are also orphan medicinal products in more than one indication. Review of the EPARs for the currently authorised ATMPs provides insights into their development programs and basis for approval.
Yescarta is an autologous T cell therapy in which a patient’s own immune cells are engineered to express a CAR directed against CD19-expressing malignancies. In the EU, Yescarta is indicated for the treatment of adult patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) and primary mediastinal large B cell lymphoma (PMBCL) after two or more lines of systemic therapy. DLBCL and PMBCL are aggressive subtypes of non-Hodgkin lymphoma (NHL). PRIME scheme support was given to Yescarta based on preliminary clinical evidence from the following: (1) NCI study 09-C-0082, a phase I open-label study of anti-CD19 CAR T cells in patients with advanced B cell malignancies; and (2) initial outcomes for patients with refractory DLBCL treated in the phase 1 portion of the pivotal single-arm multicenter phase I/II clinical study (ZUMA-1). The MA was based on clinical efficacy from the phase II portion of ZUMA-1, which enrolled adults with refractory DLBCL, PMBCL, DLBCL arising from follicular lymphoma, and other rarer forms of aggressive NHL, including high-grade B cell lymphomas. Additionally, to provide context for interpretation of the response rates observed in ZUMA-1, a retrospective, global, patient-level, pooled analysis (SCHOLAR-1) of historical outcome data for patients with refractory aggressive NHL treated with previously available therapies was conducted. Post-authorisation requirements included the setup of an educational program for patients and healthcare professionals, the qualification of hospitals and associated centers to dispense the therapy, the submission of PSURs at specific intervals, and the execution of a non-interventional, registry-based PASS. The PASS is intended to further characterize identified risks and evaluate other potential risks. At the time of MAA submission, PIP measures were deferred.
Kymriah is indicated for the treatment of the following: (1) B cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse in children and young adults (≤25 years of age); and (2) relapsed or refractory DLBCL after two or more lines of systemic therapy in adult patients. Similar to Yescarta, Kymriah is an autologous CAR T cell ATMP directed against CD19. Eligibility for PRIME was granted according to the presentation of data on initial clinical efficacy (phase II, single-arm, multicenter CCTL019B2202 trial). The EMA’s support regarding the development program and regulatory strategy included recommendations to address comparability between manufacturing sites and processes, advice on developing a risk minimization plan and generating a registry for long-term safety data collection, and advice on the PIP. At the time of MAA, evidence of safety and efficacy was presented based on the outcomes of one phase I/IIA trial (CTL019-B2101J IA 2017) and three phase II multicenter trials (CCTL019-B2202 IA 2017,33 CCTL019-B2205J IA, and CCTL019-C2201). All three studies were single arm and open label. Interestingly, Kite’s SCHOLAR-1 study was referred to as an external control for the Kymriah studies. Kymriah was required to commit to the same post-authorisation measures as Yescarta, with the additional requirement of a PAES study of pediatric patients under 3 years of age with ALL until 2023 and for patients with relapsed or refractory DLBCL until mid-2022.
The two ATMPs granted a CMA are Holoclar in 2015 and Zalmoxis in 2016, both of which are orphan medicinal products. As discussed earlier, Holoclar was authorised on the basis of retrospective clinical trials designed from previously conducted named-patient treatments.16 In the main safety and efficacy study, treatment success was recurrent in a sufficient percentage of the patients, and long-term data from the 10-year follow-up indicated treatment persistence; therefore, the benefit-risk balance for Holoclar was considered favorable. However, because the studies presented were retrospective, Holoclar received a CMA with the specific obligation to complete a PASS multinational clinical trial by the end of 2020 for the collection of additional efficacy and safety data. The long-term safety profile will also need to be supported by a registry for the collection of data from routine clinical practice. The MAH committed to acquire additional safety data in pediatric patients as part of the approved PIP, which was not completed because some measures were deferred.
The orphan medicinal product designation of Zalmoxis was confirmed at the time of MAA on an estimated incidence of around 0.21 in 10,000 individuals. The MAA included data from a multicenter, international, single-arm, phase I/II study34 with the primary objective of immune reconstitution after treatment, while a randomized controlled phase III study with disease- or progression-free survival as the primary endpoint was ongoing. Because only limited clinical data were available from the studies (45 patients in total), data from a surrogate control group from the European Blood and Marrow Transplant (EBMT) Society databases were compiled and compared to the study outcomes. As post-authorisation obligations for pharmacovigilance, a clinical study report with data on efficacy is required for the phase III study by 2021, and an additional clinical study report on long-term safety and efficacy outcomes from a non-interventional PASS trial is required by 2022. The MAH is also conducting two interventional PIP studies to assess safety in pediatric patients, to be completed by 2022.
Two other ATMPs with orphan medicinal product designations, Alofisel and Strimvelis, have each received a standard MA despite the rare disease status of their therapeutic indications. Alofisel is the first allogeneic SCTMP to be authorised, and it was granted orphan medicinal product designation on an estimated disease incidence of no more than 3.47 in 10,000 individuals. The clinical evidence provided at the time of the initial MAA included data from a phase III, randomized, double-blind, parallel group, placebo-controlled, multicenter study (Cx601-0302, ADMIRE-CD35, 36) to assess the safety and efficacy of the product administered in a full single-dose to adult Crohn’s disease patients, with complex perianal fistula(s) with inadequate response to at least one conventional or biologic therapy. The primary endpoint was complete remission. Data from a second single-arm, open-label supportive study (Cx601-0101) were presented to assess safety and efficacy in patients receiving an initial injection of 20 million cells only, and an additional 40 million cells in case of incomplete closure at week 12, with a primary endpoint of incidence of treatment-related adverse events. Updates at week 52 for the pivotal study (Cx601-0302) were provided as part of the response to the day 120 LoQ, enabling a positive benefit-risk balance to be concluded. Nonetheless, post-authorisation measures were stipulated, including a requirement to generate data on the safety and efficacy of repeated administrations in a subset of the target population. To fulfill this obligation, results from an ongoing phase III study approved by the FDA (Cx601-0303) are expected in the form of a PASS clinical study report by 2022. Alofisel is being evaluated for pediatric use, with the conclusion of the PIP planned for 2025.
Strimvelis was the first autologous ex vivo stem cell gene therapy to be authorised when it received a standard MA in 2016. Strimvelis is indicated for the treatment of pediatric patients with severe combined immunodeficiency resulting from adenosine deaminase deficiency (ADA-SCID), an ultra-rare disease affecting children with an estimated prevalence 0.04 people in 10,000. The MAA presented the outcomes of one pivotal open-label, non-randomized, single-arm phase I/II clinical study (AD1116511), which used a historical reference as comparator,37 supported by two open-label pilot trials (AD1117056 pilot 1 and AD1117054 pilot 2), results from three patients treated under a compassionate use program (AD1117064 CUP), results from a pivotal long-term follow-up study (AD1115611 LTFU), and supporting data from a named-patient program (200893 NPP). Strimvelis, therefore, presents an interesting case in which compassionate use and named-patient treatment data were accepted in support of data from clinical studies, likely because of the ultra-rare status of ADA-SCID. The primary endpoint was defined as 3-year survival for the pivotal phase I/II trial and as survival for the pivotal long-term follow-up trial. The benefit-risk balance was considered positive based on the strong evidence of efficacy for Strimvelis in ADA-SCID patients, who otherwise do not survive beyond 1 to 2 years. Nonetheless, certain unfavorable effects were recognized, including the onset of autoimmunity in 66% of the treated patients. In addition, concerns were raised related to the genetic modification of the CD34+ cells contained in the product. The Strimvelis manufacturing process uses a retroviral vector, which presents concerns for potential mutagenesis and oncogenesis, although no malignancies have yet been detected in the treated patients. The MAH was required to complete post-authorisation measures, including a non-interventional PASS to investigate the long-term safety and efficacy of Strimvelis, with patients being expected to enroll in a dedicated registry for the collection of data related to immunogenicity, insertional mutagenesis, oncogenesis, and hepatic toxicity. Notably, because of the prevalence of ADA-SCID, a final clinical study report is not expected to be submitted until 2037, with 15-year follow-up data from 50 patients.
The two other currently authorised ATMPs with a standard MA are Imlygic and CO.DON’s Spherox. Imlygic is an in vivo GTMP indicated for unresectable metastatic melanoma. The multicenter, international clinical trial data presented for safety and efficacy evaluation in melanoma patients included an open-label, single-arm phase II study (002/03); a randomized, open-label, controlled phase III study (005/05); and extension studies for each of these studies (002/03-E and 005/05-E, respectively). Primary efficacy was confirmed by an independent endpoints assessment committee. Additional data were provided from one human pharmacokinetic study and four other efficacy and safety studies. However, the CAT and CHMP considered that it was not possible to conclude that an effect on overall survival following treatment with Imlygic was positive because of uncertainties in specific population subgroups, and, therefore, post-authorisation measures were required, including clinical study reports on the multicenter, phase II, single-arm trial performed in subjects with unresected, stage IIIB to IVM1c melanoma, which was ongoing at the time of evaluation. The MAH was also requested to perform additional post-authorisation clinical trials to generate further efficacy data and monitor the impact of Imlygic on disease progression, because specific validated biomarkers for the disease were unavailable. Other post-authorisation measures stipulated included the submission of an updated RMP as a result of risk management system modifications and whenever a benefit-risk balance milestone is achieved, the submission of clinical study reports for trials aimed at assessing the correlation of treatment with surgery versus surgery only, and the submission of preliminary efficacy outcomes from studies evaluating IMLYGIC in combination with pembrolizumab. Imlygic is also currently being evaluated for use in children (2–18 years of age) in a PIP expected to be completed in 2027.
Spherox is a recently approved TEP to be used for the treatment of articular cartilage defects of the femoral condyle and the knee patella (ICRS grade III or IV), with defect sizes of up to 10 cm2 in adults. The MAA clinical data evaluation was based on two prospective, randomized, open-label, multicenter pivotal clinical trials. The phase II study (cod 16 HS 1438) aimed to define the dose in adults affected by large defects in the knee (4–10 cm2), while the phase III study (cod 16 HS 13) is being conducted in adults presenting smaller defects (1–4 cm2), with both studies using the KOOS (Knee Injury and Osteoarthritis Outcome Score) system to obtain assessment of the primary efficacy endpoints. Further data provided in support of the MAA included the outcomes from retrospective clinical trials, investigator-initiated studies, and investigational studies in pediatric patients (cod 16 HS 16 and cod 16 HS 17 paed). Both CAT and CHMP concluded that the overall benefit-risk balance was positive, although post-authorisation measures were required, including submission of the conclusive clinical data from the phase III study (ongoing at the time of application) and a PAES trial report based on the same study, with collection of 6-month follow-up data, by March 2021. Additional post-authorisation obligations included re-validation of the potency assay correlated to the efficacy outcome of the phase III trial.
Cell and Gene Therapies in the United States and Japan
The term ATMP is an EU-specific term for cell- and gene-based therapies developed for commercial use. Regulatory frameworks for cell- and gene-based therapies also exist in other global jurisdictions,39 and non-EU countries where these therapies are in active development include the United States, Canada, Australia, Japan, and Korea. In the ICH regions of the United States and Japan, regulatory frameworks around cell and gene therapies have been further elaborated in recent years.
United States
In the United States, cell and gene therapies are recognized and regulated by the U.S. Food and Drug Administration (FDA) as a particular subset of biologic medicinal products known as cellular and gene therapy products, subjecting them to the biologics license application (BLA) procedure for commercialization (a BLA is the equivalent of an EU MAA) under Section 351 of the Public Health Service Act. Such cellular and gene therapy products are, therefore, regulated in a similar way to ATMPs, while human cells, tissues, or cellular or tissue-based products (HCT/Ps) are products containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient. HCT/Ps are considered to be minimally manipulated products intended for homologous use only, and they are regulated under section 361 of the Public Health Service (PHS). With the introduction of the FDA’s 21st Century Cures Act, enacted on December 13, 2016, some cellular and gene therapy products may now also be granted a regenerative medicine advanced therapy (RMAT) designation. According to the 21st Century Cures Act, a drug is eligible for RMAT designation if the following apply: (1) the drug is a regenerative medicine therapy, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, except for those regulated solely under section 361 of the Public Health Service Act and part 1271 of Title 21, Code of Federal Regulations; (2) the drug is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and (3) preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such a disease or condition.
The benefit of RMAT designation is that it qualifies the investigational drug for FDA support equivalent to that given to other drugs with fast track and breakthrough therapy designations (introduced through the FDA Modernization Act of 1997 and the FDA Safety and Innovation Act of 2012, respectively). These latter designations, breakthrough therapy in particular, are similar to the EMA’s PRIME designation, and, together with priority review designation and accelerated approval, they form part of the FDA’s expedited programs for serious conditions40 that can be applied to drugs targeting an unmet medical need. In the United States, drugs targeting an unmet medical need are defined as therapies against severe or life-threatening diseases with no current therapy option. If therapies are available, the medicinal product must demonstrate some advantage over available therapy to be eligible for any of the expedited programs described below. This is, therefore, similar to the requirement for orphan medicinal products in the EU to demonstrate an advantage (significant benefit) over available therapies to qualify for conditional approval.
Fast track designation (FTD) can be requested at any time during development, but it is particularly applicable to therapies showing the potential based on pre-clinical or early clinical data to address an unmet medical need for a serious condition (as defined in FDA guidance). FTD provides the opportunity for more frequent meetings with the FDA to discuss the development plan and eligibility for priority review (a procedure similar to the EMA’s accelerated assessment that shortens the BLA period to a maximum of 6, rather than 10, months). FTD also allows access to rolling review, a procedure in which BLA dossier sections may be submitted separately for review upon completion, rather than waiting to submit the dossier in its entirety for contemporaneous evaluation. However, all dossier sections must still be made available for review within the BLA evaluation period, and the specific details of the rolling review must be agreed upon with the FDA up front.
To qualify for breakthrough therapy designation (BTD), a drug should treat a serious condition, and preliminary clinical evidence should indicate that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies. BTD provides all the benefits of FTD plus intensive FDA guidance on the development program beginning as early as phase I, with a commitment to providing this support from FDA senior advisors. Several cellular and gene therapy products have been granted BTD (Table 4).
Table 4.
Cell and Gene Therapies in FDA-Expedited Development Programs
| Product | Therapeutic Indication | Designation Type and Date | Company |
|---|---|---|---|
| Humacyl | vascular access for hemodialysis | FTD July 2014 RMAT March 2017 | Humacyte |
| ATA129a | EBV-associated post-transplant lymphoproliferative disorder | BTD February 2015 | Atara Biotherapeutics |
| LentiGlobin BB305a | severe sickle cell disease | BTD February 2015 RMAT October 2017 | bluebird bio |
| NY-ESO-1c259Ta | metastatic synovial sarcoma | BTD February 2016 | Adaptimmune Therapeutics |
| AVXS-101a | pediatric spinal muscular atrophy type 1 | BTD July 2016 | Avexis |
| PF-06838435/SPK-9001a | hemophilia B | BTD July 2016 | Spark Therapeutics |
| JCAR017a | relapsed and refractory DLBCL | BTD December 2016 RMAT October 2017 | Juno Therapeutics |
| AMT-061a | severe hemophilia B | BTD January 2017 | uniQure |
| RVT-802 | Di George syndrome | RMAT April 2017 | Enzyvant |
| Ixmyelocel-T | dilated cardiomyopathy | RMAT May 2017 | Vericel |
| jCell | retinitis pigmentosa | RMAT May 2017 | jCyte |
| Stratagraft | thermal burns | RMAT July 2017 | Mallinckrodt Pharmaceuticals |
| ATIR101 | leukemia | RMAT September 2017 | Kiadis Pharma |
| AST-OPC1 | spinal cord injury | RMAT October 2017 | Asterias Biotherapeutics |
| BMN 270a | hemophilia A | BTD October 2017 | BioMarin Pharmaceutical |
| MultiStem | ischemic stroke | RMAT October 2017 | Athersys |
| Vocimagene miretrorepveca | high-grade glioma | BTD October 2017 | Tocagen |
| CEVA101 | traumatic brain injury | RMAT November 2017 | Cellvation (Fortress Biotech) |
| MPC-150-IM | heart failure | RMAT December 2017 | Mesoblast |
| Ixmyelocel-T | dilated cardiomyopathy | RMAT May 2017 | Vericel |
| EB-101 | recessive dystrophic epidermolysis bullosa | RMAT January 2018 | Abeona Therapeutics |
| CAP-1002 | Duchenne muscular dystrophy | RMAT February 2018 | Capricor Therapeutics |
| AmnioFix injectable | osteoarthritis of the knee | RMAT March 2018 | MiMedx Group |
| ABO-102 | Sanfilippo syndrome type A | RMAT April 2018 | Abeona Therapeutics |
| Lenti-Da | cerebral adrenoleukodystrophy | BTD May 2018 | bluebird bio |
| AT132a | X-linked myotubular myopathy | FTD September 2017 RMAT August 2018 | Audentes Therapeutics |
| NLA101a | HSCT | FTD August 2018 | Nohla Therapeutics |
| FCX-013 | scleroderma | FTD September 2018 | Fibrocell Science |
| ADVM-022 | wet age-related macular degeneration | FTD September 2018 | Adverum Biotechnologies |
As of the fourth quarter 2018, there are five cell and gene therapies with FDA fast track designation (FTD), ten with breakthrough therapy designation (BTD), and 18 with RMAT designation. Of note, LentiGlobin BB305 and JCAR017 were granted BTD first and obtained RMAT designation at a later stage. BTD and RMAT designations are similar in principle to the EMA’s PRIME scheme, and all products with BTD are also in the PRIME scheme. Differences between BTD and RMAT designations are explained in the text. DLBCL, diffuse large B cell lymphoma; EBV, Epstein-Barr virus; HSCT, hematopoietic stem cell transplantation.
These products are also in the EMA PRIME scheme.
RMAT designation differs from BTD primarily in that the qualifying criteria are different. BTD is potentially applicable to any drug intended to treat a serious condition, while RMAT designation is for regenerative medicine therapies (cellular and gene therapy products) intended to treat, modify, reverse, or cure a serious condition. Furthermore, while preliminary clinical evidence for BTD should indicate that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies, preliminary clinical evidence for RMATs should indicate that the drug has the potential to address unmet medical needs for a serious disease or condition (i.e., potentially in the absence of available therapies). RMAT designation gives access to all features of the BTD program, as well as to early interaction with the FDA for discussions on potential surrogate or intermediate endpoint inclusion in the trial design. It also provides support for potential accelerated approval and how post-approval requirements may be satisfied. Accelerated approval (not to be confused with the EMA’s accelerated assessment procedure) is similar in principle to the EMA’s CMA in that it is a procedure to allow drugs for serious conditions that fill an unmet medical need to be approved based on an intermediate or surrogate endpoint, with studies to confirm clinical benefit being completed post-approval. Draft guidance on how current guidance on the FDA’s expedited programs for serious conditions applies to RMATs was published in 2017,41 and cellular and gene therapy products with a current RMAT designation are shown in Table 4.
Priority review is highly valued as a way of bringing new medicines to market quickly in the United States, because it enables revenues to be generated earlier than if the standard review timetable was followed. As a way of incentivizing the development of medicines for rare pediatric diseases, the FDA runs a voucher system through which medicine developers who successfully obtain a rare pediatric disease designation (RPDD) for their product may qualify for a voucher that can be redeemed for priority review of a subsequent marketing application for a different product. This is one way in which the FDA aims to offset the low return on investment associated with developing drugs for rare diseases, i.e., orphan medicinal products. Other FDA incentives for the development of orphan medicinal products include tax credits worth up to 50% of the development costs, a 7-year period of market exclusivity, and the waiving of marketing application review fees and annual FDA product fees. The value of priority review vouchers is shown in the fact that they are often traded between companies (legally) for hundreds of millions of dollars.
One cellular and gene therapy product to obtain an RPDD is Spark Therapeutics’s Luxturna, an adeno-associated virus-based in vivo gene therapy that received a BLA in December 2017. Together with Kymriah (Novartis)42 and Yescarta (Kite, a Gilead Company), Luxturna is one of three gene therapies to receive a BLA in late 2017, with Kymriah being the first gene therapy to be approved by the FDA. The approvals of these three products are widely considered to represent the coming of age of the gene therapy industry, but this should not detract from the earlier EU authorisation of gene therapies, including Glybera and Strimvelis. A number of cell-based products are also authorised in the United States, including products such as Maci, Imlygic, and Provenge, that have also received an EU MA (Table 1).
Japan
In Japan, medicinal products manufactured from human cells, genes, or tissues have been regulated under the Pharmaceuticals and Medical Devices Act (PMD Act) since November 2014, which was introduced to replace the previously established Pharmaceutical Affairs Law (PAL) and create a new regulatory pathway for such products. The PMD Act is enforced via a number of ministerial ordinances, notifications, and administrative notices (regulations). Under the PMD Act, medicinal products comprising human cells, genes, or tissues that will be marketed are now regulated generically as regenerative medical products (RMPs) by the Pharmaceuticals and Medical Devices Agency (PMDA), the agency responsible for evaluating medicinal products for use in clinical trials or for MA. RMPs are defined as processed human cells or gene therapy products that are intended to be used for the reconstruction, repair, or formation of structures or functions of the human body or for the treatment or prevention of human diseases.
The PMD Act introduced a new pathway for the approval of RMPs that is distinct from the traditional pathway for small molecule drugs. Under the PMD Act, an RMP can obtain expedited CMA on the basis of safety and predicted probable efficacy demonstrated in early stage clinical trials. CMA of an RMP under the PMD Act is time limited and lasts for a maximum of 7 years, during which time the applicant is required to perform the later-stage trials that will be required for subsequent full MA. If these trials are not performed or if the data from them are considered inadequate to support full MA, the product must be withdrawn from the market at the end of the 7-year conditional authorisation period at the latest.
To date, four RMPs have received an MA in Japan (Table 1). Jacc (an autologous cultured cartilage product) and Jace (an autologous cultured epidermis product) were initially authorised under the PAL prior to 2015, and Jace was re-authorised under the PMD Act as an RMP in 2016. Temcell, an allogeneic mesenchymal stem cell product for the treatment of acute GvHD, was the first RMP to be fully authorised under the PMD Act in September 2015. Temcell is essentially the same as Mesoblasts’s Prochymal, which was the first stem cell therapy to be granted an MA anywhere in the world when it was approved for the same indication by Health Canada in 2012. HeartSheet (an autologous skeletal myoblast preparation using cell sheet technology) was granted a 5-year conditional approval, also in September 2015, on the basis of data from a clinical study with no control arm, that used a surrogate endpoint, and included only a small patient number—conditions that precluded a full approval. The conditions applied for full approval of HeartSheet within 5 years include demonstration of efficacy in 60 patients and superiority to current existing treatment in 120 patients. HeartSheet remains the only RMP to have received conditional approval in Japan.
The PMDA’s priority review system for innovative therapies targeting an unmet medical need is the sakigake designation, which was introduced in 2015. It is similar in principle to the EMA’s PRIME scheme and the FDA’s BTD in that its aim is to facilitate the rapid authorisation of new medicines; however, none of these schemes should be considered equivalent in how they are implemented by the respective regulatory agencies. In Japanese, “sakigake” means “pioneer.” To qualify for the sakigake designation, a medicinal product must be for a disease or condition with an urgent need of innovative therapy that is initially developed and submitted for authorisation in Japan and has high efficacy in early stage (phase I/II) clinical trials. To be considered an innovative therapy with an urgent need, the product should possess a new and different mechanism of action to currently authorised products and treat either a serious life-threatening disease or a chronic disease that leads to the deterioration of patient quality of life and for which there is currently no viable treatment.
The benefits of sakigake designation include the following: (1) prioritized consultation (scientific advice) with PMDA, with the meeting taking place 1 month, rather than 2 months, after submission of the briefing documents; (2) scope for extensive consultation prior to submission of the marketing application, including a requirement to enter into consultation early in development once the designation is granted during phase I/II; (3) accelerated review of the marketing application, targeting review within 6 months rather than 12 months and enabling submission of phase III study data after submission of the marketing application; (4) assignment of a PMDA concierge to facilitate an efficient development program and marketing application process; and (5) implementation of specific post-authorisation safety measures, including extended follow-up (over 10 years) and global information dissemination.
Sakigake designations are published annually by PMDA on their website, and sakigake designations have been published for two RMPs (AVXS-101 and NY-ESO-1 siTCRTM) in 2018. Of these, AVXS-101 (in development by Avexis) is a gene therapy product for the treatment of spinal muscular atrophy type 1 that has also received PRIME designation in the EU and BTD in the United States (Tables 2 and 4). NY-ESO-1 siTCRTM (in development by Takara Bio and Otsuka Pharmaceutical) is a gene-modified cell therapy product for the treatment of synovial sarcoma. NY-ESO-1-based products are also in accelerated development schemes in the EU and United States (Tables 2 and 4).
Conclusions and Future Perspectives
A decade has now passed since the ATMP regulation came into force, requiring developers of cell- and gene-based therapies meeting the definitions of ATMPs to obtain an MA, according to the centralised procedure, to place their products on the EU pharmaceutical market. So far, 13 ATMPs have received an EU MA, and this rate of new product authorisation is widely considered to be low compared to the authorisation rates of other types of medicinal products. However, the number of authorised ATMPs is expected to increase further in 2019, with three other ATMPs are currently under MAA review (Table 1). This increased growth is being driven particularly by the coming of age of gene therapy development, with many such products now in clinical trials in addition to those already on the international markets, and also by the application of enhanced regulatory agency support to the development of ATMPs addressing unmet medical needs. In the EU, the EMA’s PRIME scheme is the main mechanism by which such support is provided, and it is interesting to note that over one-third of the medicines in the PRIME scheme are ATMPs and that all of these ATMPs are gene therapies. Regulatory agency support schemes also operate in the United States (breakthrough designation and RMAT designation) and Japan (sakigake designation), and these schemes are actively contributing to the progression of cell and gene therapies in these territories too.
The PRIME scheme is a clear example of how the European regulatory landscape is being evolved by the EMA to facilitate the timely provision of innovative medicines to the broader patient populations (i.e., not just to those patients enrolled in clinical trials). Indeed, rather than being a static framework, the EU regulatory framework is constantly evolving for the benefit of patients and medicine developers. For cell and gene therapies, the EU ATMP regulation was the first move in this respect. This was elaborated further by the risk-based approach to development and description of the technical requirements expected of ATMPs being developed for commercialization laid down in Directive 2009/120/EC. Lessons from the experience with ATMP development since the introduction of the ATMP regulation are also being applied to the evolution of regulatory guidance and procedures. The CAT publishes a work plan annually on the activities it is addressing, with current notable examples being revision of the Guideline on Safety and Efficacy Follow-up and Risk Management of Advanced Therapy Medicinal Products and the development of specific guidance on the requirements for ATMPs in clinical trials. In late 2017, Good Manufacturing Practice (GMP) guidelines specific to ATMPs were published to adapt the GMP framework to novel aspects of these products, such as decentralised manufacture, final product reconstitution at the treatment center, and release of out-of-specification (autologous) products. The GMP for ATMPs guidelines are published as Part IV of EudraLex Volume 4 on Good Manufacturing Practice, and they are considered as stand-alone guidelines that should be used independently of, and not in conjunction with, GMP guidelines for other types of medicinal products. As discussed above, specific GCP guidelines for ATMPs are now also under revision.
One particular development that will help facilitate clinical trials with investigational medicinal products that contain or consist of genetically modified organisms (GMOs) in particular will be the harmonization of GMO authorisations across the European community. GMOs are subject to both clinical trial and GMO legislation. When applying for a clinical trial, the CTA documentation required is common to all EU member states, but GMOs are typically approved for use separately from the CTA submission, and additional country-specific information is required to be submitted to an agency other than the national competent authority. Recent outputs from the Joint EC-DG Health and Food Safety and European Medicines Agency Action Plan on ATMPs regarding harmonization of clinical trial requirements for GMOs include a question-and-answer document, a good practice guideline, and a common application form adopted by a number of member states (Table S3), plus a repository of national regulatory requirements on the EC website to describe the different current requirements among member states.
The number of ATMPs in the PRIME scheme in its first 2 years is a very encouraging sign that points to a significantly increased rate of ATMP authorisations in the next few years. Similarly, in the United States, there are currently 28 cellular and gene therapy products with BTD and/or RMAT designation, which grants these products FDA support similar to that granted by the EMA to ATMPs in the PRIME scheme. Further cellular and gene therapy product authorisations can, therefore, be anticipated soon in the United States. The USA regulatory framework around cellular and gene therapy products has evolved significantly in recent years, for example, with the introduction of RMAT designation and publication of new guidance documents. Very recently, the FDA published six new draft guidance documents on both the CMC and clinical development of cellular and gene therapy products (particularly gene therapies). The initial focus of the clinical guidance is hemophilia, a condition for which there are a number of products in late-stage clinical trials (Table 4). Some of these products are also under development in the EU (Table 2), and wide global reach of new cell and gene therapies can, therefore, be anticipated. The EU authorisations of Yescarta, Kymriah, and Luxturna in 2018, all of which were licensed in the United States in 2017 as the first gene therapies to receive FDA approval, is an important step toward this. Another recently announced FDA initiative, called Initial Targeted Engagement for Regulatory Advice on CBER products (INTERACT), will foster pre-clinical trial dialogue between the FDA and medicine developers to focus on CMC and clinical development issues early.
In summary, after a slow start, the cell and gene therapy industry is poised to deliver a number of promising new medicines in the EU and global markets, supported by tailored and evolving regulatory schemes focused on their bespoke and expedited development.
Author Contributions
Conceptualization, A.L.; Investigation, G.D. and A.L.; Data Curation, G.D.; Writing – Original Draft, Review & Editing, G.D. and A.L.; Visualization, G.D. and A.L.; Supervision, A.L.; Project Administration, A.L.
Conflicts of Interest
G.D. is an employee of VivaBioCell S.p.A., a company fully acquired by NantCell, Inc., part of NantWorks LLC. A.L. is an employee of Kite, a Gilead Company, the MAH of Yescarta. A.L. is a former employee of Chiesi Farmaceutici S.p.A., the MAH of Holoclar, and GlaxoSmithKline, the original MAH of Strimvelis.
Acknowledgments
G.D. acknowledges support from the Interreg V–A Italia–Slovenija 2014–2020 (ARTE 1473178135). The authors give special thanks to Hilary Jones (EU Regulatory Law, Gilead Sciences) for expert review of the pre-submission manuscript.
Footnotes
Supplemental Information includes four tables and can be found with this article online at https://doi.org/10.1016/j.omtm.2019.01.010.
Supplemental Information
The EU regulatory framework for ATMPs includes a number of Directives and Regulations. Many are applicable to all human medicinal products while some are ATMP-specific.
References
- 1.Hanna E., Rémuzat C., Auquier P., Toumi M. Advanced therapy medicinal products: current and future perspectives. J. Mark. Access Health Policy. 2016;4:1–10. doi: 10.3402/jmahp.v4.31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boráň T., Menezes-Ferreira M., Reischl I., Celis P., Ferry N., Gänsbacher B., Krafft H., Lipucci di Paola M., Sladowski D., Salmikangas P. Clinical Development and Commercialization of Advanced Therapy Medicinal Products in the European Union: How Are the Product Pipeline and Regulatory Framework Evolving? Hum. Gene Ther. Clin. Dev. 2017;28:126–135. doi: 10.1089/humc.2016.193. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho M., Sepodes B., Martins A.P. Regulatory and Scientific Advancements in Gene Therapy: State-of-the-Art of Clinical Applications and of the Supporting European Regulatory Framework. Front. Med. (Lausanne) 2017;4:182. doi: 10.3389/fmed.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detela G., Lodge A. Manufacturing process development of ATMPs within a regulatory framework for EU clinical trial & marketing authorisation applications. Cell Gene Ther. Insights. 2016;2:425–452. [Google Scholar]
- 5.Monaco L., Faccio L. Patient-driven search for rare disease therapies: the Fondazione Telethon success story and the strategy leading to Strimvelis. EMBO Mol. Med. 2017;9:289–292. doi: 10.15252/emmm.201607293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Commission ENTR/6283/00 Rev 4. Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another, 27.03.2014. 2014. https://ec.europa.eu/health/sites/health/files/files/orphanmp/2014-03_guideline_rev4_final.pdf
- 7.European Commission 2016/C 424/03. Commission notice on the application of articles 3, 5 and 7 of regulation (EC) no 141/2000 on orphan medicinal products. 2016. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:JOC_2016_424_R_0003&from=EN
- 8.European Medicines Agency EMA/19529/2018. Annual report on the use of the special contribution for orphan medicinal products. Year 2017. 2018. https://www.ema.europa.eu/documents/report/annual-report-use-special-contribution-orphan-medicinal-products-2017_en.pdf
- 9.Tomasi P.A., Egger G.F., Pallidis C., Saint-Raymond A. Enabling Development of Paediatric Medicines in Europe: 10 Years of the EU Paediatric Regulation. Paediatr. Drugs. 2017;19:505–513. doi: 10.1007/s40272-017-0261-1. [DOI] [PubMed] [Google Scholar]
- 10.European Commission. European Medicines Agency EMA/542202/2018. European Medicines Agency and European Commission (DG Health and Food Safety) action plan on paediatrics. 2018. https://www.ema.europa.eu/documents/report/european-medicines-agency-european-commission-dg-health-food-safety-action-plan-paediatrics_en.pdf
- 11.European Commission EudraLex - EU legislation. 2015. https://ec.europa.eu/health/documents/eudralex/
- 12.European Commission. European Medicines Agency European Commission-DG Health and Food Safety and European Medicines Agency action plan on ATMPs. 2018. https://www.ema.europa.eu/documents/other/european-commission-dg-health-food-safety-european-medicines-agency-action-plan-advanced-therapy_en-0.pdf
- 13.European Commission Targeted stakeholder consultation on the draft guidelines on good clinical practice for advanced therapy medicinal products. Consultation document. 2018. https://ec.europa.eu/health/sites/health/files/files/advtherapies/2018_gcp_atmp_en.pdf
- 14.Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 15.Rama P., Bonini S., Lambiase A., Golisano O., Paterna P., De Luca M., Pellegrini G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Milazzo G., De Luca M., Pellegrini G. Holoclar: first of its kind in more ways than one. Cell Gene Ther. Insights. 2016;2:183–197. [Google Scholar]
- 17.Pellegrini G., Ardigò D., Milazzo G., Iotti G., Guatelli P., Pelosi D., De Luca M. Navigating Market Authorisation: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl. Med. 2018;7:146–154. doi: 10.1002/sctm.17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Commission EudraLex volume 2. Pharmaceutical legislation on notice to applicants and regulatory guidelines for medicinal products for human use. Volume 2B. Notice to applicants: medicinal products for human use. Presentation and format of the dossier Common Technical Document (CTD) 2008. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/b/update_200805/ctd_05-2008_en.pdf
- 19.Watanabe N., Yano K., Tsuyuki K., Okano T., Yamato M. Re-examination of regulatory opinions in Europe: possible contribution for the approval of the first gene therapy product Glybera. Mol. Ther. Methods Clin. Dev. 2015;2:14066. doi: 10.1038/mtm.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papathanasiou P., Brassart L., Blake P., Hart A., Whitbread L., Pembrey R., Kieffer J. Transparency in drug regulation: public assessment reports in Europe and Australia. Drug Discov. Today. 2016;21:1806–1813. doi: 10.1016/j.drudis.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Salmikangas P., Menezes-Ferreira M., Reischl I., Tsiftsoglou A., Kyselovic J., Borg J.J., Ruiz S., Flory E., Trouvin J.H., Celis P. Manufacturing, characterization and control of cell-based medicinal products: challenging paradigms toward commercial use. Regen. Med. 2015;10:65–78. doi: 10.2217/rme.14.65. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency EMA/471951/2016. Conditional marketing authorisation. Report on ten years of experience at the European Medicines Agency. 2017. https://www.ema.europa.eu/documents/report/conditional-marketing-authorisation-report-ten-years-experience-european-medicines-agency_en.pdf
- 23.European Medicines Agency EMA/276376/2016. Final report on the adaptive pathways pilot. 2016. https://www.ema.europa.eu/documents/report/final-report-adaptive-pathways-pilot_en.pdf
- 24.European Medicines Agency EMA/CHMP/671361/2015 Rev. 1. Guideline on the scientific application and the practical arrangements necessary to implement the procedure for accelerated assessment pursuant to article 14 (9) of regulation (EC) no 726/2004. 2016. https://www.ema.europa.eu/documents/scientific-guideline/guideline-scientific-application-practical-arrangements-necessary-implement-procedure-accelerated/2004_en.pdf
- 25.European Medicines Agency EMA/CHMP/509951/2006, Rev. 1. Guideline on the scientific application and the practical arrangements necessary to implement commission regulation (EC) no 507/2006 on the conditional marketing authorisation for medicinal products for human use falling within the scope of regulation (EC) no 726/2004. 2016. https://www.ema.europa.eu/documents/scientific-guideline/guideline-scientific-application-practical-arrangements-necessary-implement-commission-regulation-ec/2006-conditional-marketing-authorisation-medicinal-products-human-use-falling_en.pdf
- 26.European Medicines Agency EMA/242980/2018. PRIME: a two-year overview. 2018. https://www.ema.europa.eu/documents/report/prime-two-year-overview_en.pdf
- 27.Flory E., Gasparini P., Jekerle V., Palomäki T., Celis P., Boráň T., McBlane J.W., Borg J.J., Kyselovic J., Lipnik-Stangelj M. Regulatory viewpoints on the development of advanced stem cell–based medicinal products in light of the first EU-approved stem cell product. Cell Gene Ther. Insights. 2015;1:109–127. [Google Scholar]
- 28.Saris D., Price A., Widuchowski W., Bertrand-Marchand M., Caron J., Drogset J.O., Emans P., Podskubka A., Tsuchida A., Kili S., SUMMIT study group Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Two-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2014;42:1384–1394. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 29.Brittberg M., Recker D., Ilgenfritz J., Saris D.B.F., SUMMIT Extension Study Group Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018;46:1343–1351. doi: 10.1177/0363546518756976. [DOI] [PubMed] [Google Scholar]
- 30.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 31.Collichio F., Burke L., Proctor A., Wallack D., Collichio A., Long P.K., Ollila D.W. Implementing a Program of Talimogene laherparepvec. Ann. Surg. Oncol. 2018;25:1828–1835. doi: 10.1245/s10434-018-6361-5. [DOI] [PubMed] [Google Scholar]
- 32.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciceri F., Bonini C., Stanghellini M.T., Bondanza A., Traversari C., Salomoni M., Turchetto L., Colombi S., Bernardi M., Peccatori J. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 35.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Kazemi-Shirazi L., ADMIRE CD Study Group Collaborators Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 36.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Kazemi-Shirazi L., ADMIRE CD Study Group Collaborators Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2018;154:1334–1342.e4. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Hassan A., Booth C., Brightwell A., Allwood Z., Veys P., Rao K., Hönig M., Friedrich W., Gennery A., Slatter M., Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation and European Society for Immunodeficiency Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120:3615–3624. doi: 10.1182/blood-2011-12-396879. quiz 3626. [DOI] [PubMed] [Google Scholar]
- 38.Becher C., Laute V., Fickert S., Zinser W., Niemeyer P., John T., Diehl P., Kolombe T., Siebold R., Fay J. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J. Orthop. Surg. Res. 2017;12:71. doi: 10.1186/s13018-017-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodge A., Detela G., Barry J., Ginty P., Mount N. Chapter 10: global regulatory perspective for MSCs. In: Viswanathan S., Hematti P., editors. Mesenchymal Stromal Cells: Translational Pathways to Clinical Adoption. Academic Press; 2017. pp. 243–287. [Google Scholar]
- 40.Food and Drug Administration Guidance for industry: expedited programs for serious conditions – drugs and biologics. 2014. https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf
- 41.Food and Drug Adminstration Expedited programs for regenerative medicine therapies for serious conditions: draft guidance for industry. 2017. https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm585414.pdf
- 42.Bach P.B., Giralt S.A., Saltz L.B. FDA approval of tisagenlecleucel: Promise and complexities of a $475 000 cancer drug. JAMA. 2017;318:1861–1862. doi: 10.1001/jama.2017.15218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The EU regulatory framework for ATMPs includes a number of Directives and Regulations. Many are applicable to all human medicinal products while some are ATMP-specific.