Abstract
Background:
Postoperative cardiac arrest is uncommon but associated with a high mortality risk in general surgery patients and is often preceded by postoperative complications. The relationships between prior complications and mortality after cardiac arrest in general surgery patients have not been completely evaluated.
Methods:
A retrospective, observational cohort of general surgery inpatients with cardiac arrest occurring after postoperative day (POD) #0 (and up to POD #30) was obtained from the 2012–2013 American College of Surgeons National Surgical Quality Improvement Program. Prior complication was defined as at least one of the following occurring prior to the POD of cardiac arrest: 1) acute kidney injury, 2) acute respiratory failure, 3) deep vein thrombosis/pulmonary embolus, 4) myocardial infarction, 5) sepsis/septic shock, 6) stroke, and/or 7) transfusion. The associations between prior complications and mortality after cardiac arrest were assessed using Cox proportional hazards models that adjusted for preoperative risk factors.
Results:
Of 1,352 patients with postoperative cardiac arrest, 746 patients (55%) developed at least one complication prior to cardiac arrest. Overall 30-day mortality was 71% (958/1,352) and was similar among patients with and without a prior complication (71% [533/746] vs. 70% [425/606], P=0.60). Patients with prior complications did not have an increased risk of mortality, compared to patients without prior complications, in adjusted Cox models (hazard ratio [HR] 1.03, 95% confidence interval [CI] 0.90–1.18, P=0.70). In addition, no prior complication was associated with increased mortality risk in individual analyses.
Conclusions:
Among general surgery patients with cardiac arrest after POD #0, complications occurring prior to cardiac arrest are common but are not associated with increased mortality risk.
Introduction
In-hospital cardiac arrest is a serious complication associated with high mortality, affecting over 200,000 adults in the United States per year with fewer than 20% surviving to hospital discharge.1 Among patients with cardiac arrest, surgical patients have characteristics distinguishing them from medical patients.2 Studies of cardiac arrest in surgical patients have tended to be older, single center studies focusing on anesthetic management,3 but recently, there has been a broader understanding of the incidence and risk factors of cardiac arrest and mortality in the surgical population.2–5 Fortunately, the incidence of cardiac arrest in surgical patients is low, with estimates ranging from 7 per 10,000 noncardiac surgeries for intraoperative cardiac arrest5 to 6 per 10,000 anesthetics for arrests in the operating room or postanesthesia care unit (PACU).4 Unfortunately, the mortality associated with cardiac arrest is high, with reported rates of 63% for intraoperative cardiac arrests5 and 58% for operating room/PACU cardiac arrests.4
While patient risk factors are important determinants of cardiac arrest and mortality, the development of postoperative complications may be a critical factor affecting outcomes after cardiac arrest, and other postoperative complications often precede cardiac arrest in surgical patients.3 In exploratory analyses, we identified potential interaction effects between cardiac arrest and other complications, indicating that they were not independent in their effects on mortality,6 but the specific relationships between complications and cardiac arrest have not been fully investigated. The prevention of postoperative complications represents an area where outcomes may be improved for those with cardiac arrest.7
Patients developing cardiac arrest are an extremely sick group of patients and closer examination could reveal characteristics that differentiate among those in this group. We evaluated general surgery patients experiencing cardiac arrest after postoperative day (POD) #0, up to POD #30, using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), hypothesizing that major postoperative complications occurring prior to cardiac arrest would be significantly associated with mortality. Our findings will enable further study to identify specific aspects in which the care of patients with cardiac arrest might be improved.
Methods
Overview
This study was not subject to local Institutional Review Board (IRB) review as it did not require access to protected health information. This is a retrospective, observational cohort study of general surgery inpatients experiencing cardiac arrest within 30 days of surgery using data from the 2012–2013 ACS-NSQIP participant use files. The ACS-NSQIPa is a validated, prospectively collected national dataset aimed at improving surgical quality and outcomes,8 with data collected from 374 sites in 2012 and 435 sites in 2013. Detailed descriptions of case inclusion criteria, systematic sampling process, quality measures, and variable descriptions are available from ACS-NSQIP.b Patients are followed for 30 days after surgery, including post-discharge, by the surgical clinical reviewer at each participating site to monitor postoperative outcomes. This article adheres to the STROBE guidelines for observational studies.c
Patient selection
Of the 1,195,825 initial records in the dataset, 349,027 patients were classified as being Inpatient and General Surgery (Supplemental Figure 1). Of these, 1,730 patients experienced cardiac arrest, defined as:
“The absence of cardiac rhythm or presence of chaotic cardiac rhythm which results in a cardiac arrest requiring the initiation of CPR, which includes chest compressions. Patients are included who are in a pulseless VT or Vfib in which defibrillation is performed and PEA arrests requiring chest compressions. Patients with automatic implantable cardioverter defibrillator (AICD) that fire but the patient has no loss of consciousness should be excluded.”
As the dataset does not allow for the determination of prior complications if cardiac arrest occurred on POD #0 (discussed more in detail below), the main analysis cohort consisted of the 1,352 patients experiencing cardiac arrest after POD #0.
Demographic and operative variables
Procedures were classified using the Clinical Classifications Software for Services and Procedures (Agency for Healthcare Research and Quality, Rockville, MD) based on the primary Current Procedural Terminology (American Medical Association, Chicago, IL) code.
Body mass index (kg/m2) was calculated from height and weight data and categorized as: <25, ≥25 and <30, ≥30, or missing. The estimated glomerular filtration rate (ml/min/1.73m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula incorporating creatinine, sex, age, and race9 and categorized into groups corresponding to the stages of chronic kidney disease:10 <30, ≥30 and <60, ≥60 and <90, ≥90, or missing. Prior history of cancer was determined as described previously.11 Continuous variables were grouped into deciles and plotted against the log(hazard ratio) for mortality after cardiac arrest to visualize the relationships. Based on these analyses, age and operative time were entered as continuous variables and hematocrit was categorized as ≤29, >29 and ≤35, >35 and ≤41, >41, or missing. Other variables were collected directly from the dataset.
Exposures and outcomes
For the primary analysis, the exposure variable was the development of one or more of the following seven postoperative complications prior to the day of cardiac arrest, as defined by the dataset (Supplemental Table 1): 1) acute kidney injury (AKI), 2) acute respiratory failure, 3) bleeding transfusions, 4) deep vein thrombosis/pulmonary embolus (DVT/PE), 5) myocardial infarction, 6) sepsis/septic shock, and 7) stroke. These complications were chosen based on prior work demonstrating their associations with postoperative mortality in general surgery patients.6 For planned secondary analyses, each individual complication was analyzed separately.
The primary outcome variable was mortality within 30 days of the surgical procedure. The dataset identifies the POD in which complications, cardiac arrest, and death occur.
Statistical analysis
Differences in the proportions or means of preoperative characteristics and comorbidities between patients with and without prior complications were compared with the t-test, the χ2-test, or Fisher’s exact test as appropriate. Differences in survival patterns between those with and without prior complications were analyzed using the Kaplan-Meier estimator12 and the log-rank test.13 Cox proportional hazard modeling was used to estimate the relative hazard for mortality after cardiac arrest, adjusting for confounders, comparing those with and without prior complications. Adjusted models included procedure class as well as variables associated with mortality in a prior study of postoperative cardiac arrest (age, chronic obstructive pulmonary disease [COPD], sepsis, cancer, and preoperative renal failure).3 In addition, we used stepwise multivariable Cox modeling to identify additional significant covariates. In the stepwise analysis, the previously mentioned variables were forced into the model while the following variables were subject to the stepwise criteria: female, white, body mass index, emergency, diabetic, mechanical ventilation, dyspnea, functionally dependent, current smoker, congestive heart failure, hypertension, ascites, steroid use, bleeding disorder, preoperative transfusion, estimated glomerular filtration rate, hematocrit, and log (operative time). A significance level of 0.1 was required to enter the model and a level of 0.2 was required to remain in the model.
Statistical analyses were performed using SAS Software version 9.4 (SAS Institute, Cary, NC) and GraphPad Prism 6.07 (GraphPad Software, Inc, La Jolla, CA). In the primary analysis of the associations between prior complications and mortality, statistical significance was determined with a P-value <0.05. Analysis of individual complications, 7 in total, were subject to Bonferroni correction to adjust for multiple comparisons14 (P<0.05/7=0.007).
A formal analysis plan was not filed with the IRB as this was an exempt study. The major post hoc change to the analysis involved the definition of prior complication. Initially, a prior complication was defined as one that occurred before the day of cardiac arrest OR on the day of cardiac arrest. However, it became apparent that for complications occurring on the same day as cardiac arrest, we were unable to determine which event occurred first, so complications occurring on the same day as cardiac arrest were no longer classified as a prior complication. As a result of this change, patients with cardiac arrest on POD #0 were excluded as a complication could not occur prior to POD #0. We include post hoc analyses of mortality comparing patients with cardiac arrest on POD #0 vs. after POD#0.
Sample size
As this is a retrospective, observational study, our sample size is limited by the available data. We estimated the sample size required to detect a relative hazard of 1.25 with α=0.05 and power=0.90, assuming equal sample sizes in each group (prior complication vs. no prior complication). Based on prior literature, we assumed a cumulative mortality of 70% in the no prior complication group.3 Other assumptions include a baseline event rate of 0.24/day in the no prior complication group, a censoring rate of 0.10/day, and an average follow-up of 30 days.d, 15 Based on these parameters, the required sample size is 1,159, so the actual sample size in the study (N=1,352) is adequate to detect a clinically meaningful difference in mortality between those with and without complications prior to cardiac arrest.
Results
Baseline characteristics of patients developing cardiac arrest
Of the 1,352 patient with cardiac arrest after POD #0, there were 746 (55%) patients who developed at least one complication prior to cardiac arrest (Table 1). Compared to those without prior complications, those with prior complications were more likely to be ASA PS 4–5, mechanically ventilated, have an emergent procedure, COPD, ascites, steroid use, preoperative sepsis/septic shock, preoperative transfusions, and a lower preoperative hematocrit. There were no significant differences in factors such as age, sex, and cancer diagnoses.
Table 1.
Preoperative characteristics of general surgery patients with postoperative cardiac arrest by complications prior to the day of cardiac arrest, American College of Surgeons National Surgical Quality Improvement Program, 2012–13
| No Complication |
Complication | P-value | |||
|---|---|---|---|---|---|
| 606 | (45) | 746 | (55) | ||
| Age (years) | 67.8 | (15) | 67.1 | (14) | 0.40 |
| Female | 250 | (41) | 323 | (43) | 0.45 |
| White | 386 | (64) | 472 | (63) | 0.87 |
| ASA Physical Status | <0.0001 | ||||
| 1 | 2 | (0.3) | 3 | (0.4) | |
| 2 | 60 | (10) | 52 | (7) | |
| 3 | 324 | (53) | 303 | (41) | |
| 4 | 194 | (32) | 326 | (44) | |
| 5 | 26 | (4) | 61 | (8) | |
| Emergency | 218 | (36) | 351 | (47) | <0.0001 |
| Diabetic | 176 | (29) | 242 | (32) | 0.18 |
| Mechanical Ventilation | 47 | (8) | 107 | (14) | <0.001 |
| Dyspnea | 102 | (17) | 115 | (15) | 0.48 |
| Chronic Obstructive Pulmonary Disease | 83 | (14) | 134 | (18) | 0.03 |
| Current Smoker | 95 | (16) | 159 | (21) | 0.01 |
| Congestive Heart Failure | 44 | (7) | 54 | (7) | 0.99 |
| Hypertension | 438 | (72) | 519 | (70) | 0.28 |
| Acute Renal Failure/Dialysis | 74 | (12) | 113 | (15) | 0.12 |
| Sepsis/Septic Shock | 195 | (32) | 354 | (47) | <0.0001 |
| Wound Infection | 70 | (12) | 84 | (11) | 0.87 |
| Functionally Dependent | 93 | (16) | 133 | (18) | 0.27 |
| Ascites | 19 | (3) | 51 | (7) | 0.002 |
| Steroid Use | 57 | (9) | 96 | (13) | 0.046 |
| Cancer | 182 | (30) | 224 | (30) | 1.00 |
| Bleeding Disorder | 100 | (17) | 140 | (19) | 0.28 |
| Preoperative Transfusion | 38 | (6) | 107 | (14) | <0.0001 |
| Body Mass Index (kg/m2) | 0.10 | ||||
| BMI <25 | 186 | (31) | 258 | (35) | |
| BMI ≥25 and <30 | 150 | (25) | 205 | (27) | |
| BMI ≥30 | 243 | (40) | 257 | (34) | |
| BMI Missing | 27 | (4) | 26 | (3) | |
| Hematocrit (%) | <0.0001 | ||||
| HCT ≤29 | 83 | (14) | 170 | (23) | |
| HCT >29 and ≤35 | 181 | (30) | 231 | (31) | |
| HCT >35 and ≤41 | 184 | (30) | 210 | (28) | |
| HCT >41 | 144 | (24) | 126 | (17) | |
| Missing | 14 | (2) | 9 | (1) | |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 0.04 | ||||
| eGFR <30 | 117 | (19) | 174 | (23) | |
| eGFR ≥30 and <60 | 145 | (24) | 198 | (27) | |
| eGFR ≥60 and <90 | 175 | (29) | 218 | (29) | |
| eGFR ≥90 | 157 | (26) | 146 | (20) | |
| eGFR Missing | 12 | (2) | 10 | (1) | |
Continuous variables expressed as mean (SD). Categorical variables expressed as counts (%).ASA, American Society of Anesthesiologists
There were 49 separate Clinical Classifications Software for Services and Procedures categories represented among patients with cardiac arrest. The category with the greatest number of patients was colorectal resection (36%), followed by small bowel resection (7.6%), and other hernia repair (7.1%) (Supplemental Table 2).
Prior complications and mortality in patients with cardiac arrest
There was no difference in 30-day mortality risk between cardiac arrest patients with prior complications and those without prior complications (71% [533/746] vs. 70% [425/606], P=0.60 χ2-test) (Table 2). Kaplan-Meier plots of post-cardiac arrest survival also demonstrated no significant differences in survival patterns between those with and without prior complications (Figure 1A).
Table 2.
Postoperative complications occurring prior to cardiac arrest in general surgery patients and associated 30-day mortality rates, American College of Surgeons National Surgical Quality Improvement Program, 2012–2013
| Complication Prior to Cardiac Arrest (Total N=1,352) |
Mortality |
||||||
|---|---|---|---|---|---|---|---|
| Mortality In Those Without Complication |
Mortality In Those with Complication |
||||||
| Complication | N | (%) | N | % | N | % | P-value |
| One or More Complications | 746 | (55) | 425 | (70) | 533 | (71) | 0.60 |
| Transfusion | 445 | (33) | 639 | (70) | 319 | (72) | 0.64 |
| Acute Respiratory Failure | 344 | (25) | 730 | (72) | 228 | (66) | 0.03 |
| Sepsis/Septic Shock | 189 | (14) | 823 | (71) | 135 | (71) | 0.85 |
| Acute Kidney Injury | 154 | (11) | 842 | (70) | 116 | (75) | 0.20 |
| Myocardial Infarction | 54 | (4.0) | 914 | (70) | 44 | (81) | 0.08 |
| DVT/PE | 46 | (3.4) | 923 | (71) | 35 | (76) | 0.43 |
| Stroke | 11 | (0.8) | 951 | (71) | 7 | (64) | 0.74† |
DVT, deep vein thrombosis; PE, pulmonary embolus.
Groups compared using Fisher’s exact test. Bonferroni adjusted criteria for individual complications is P<0.007
Figure 1.
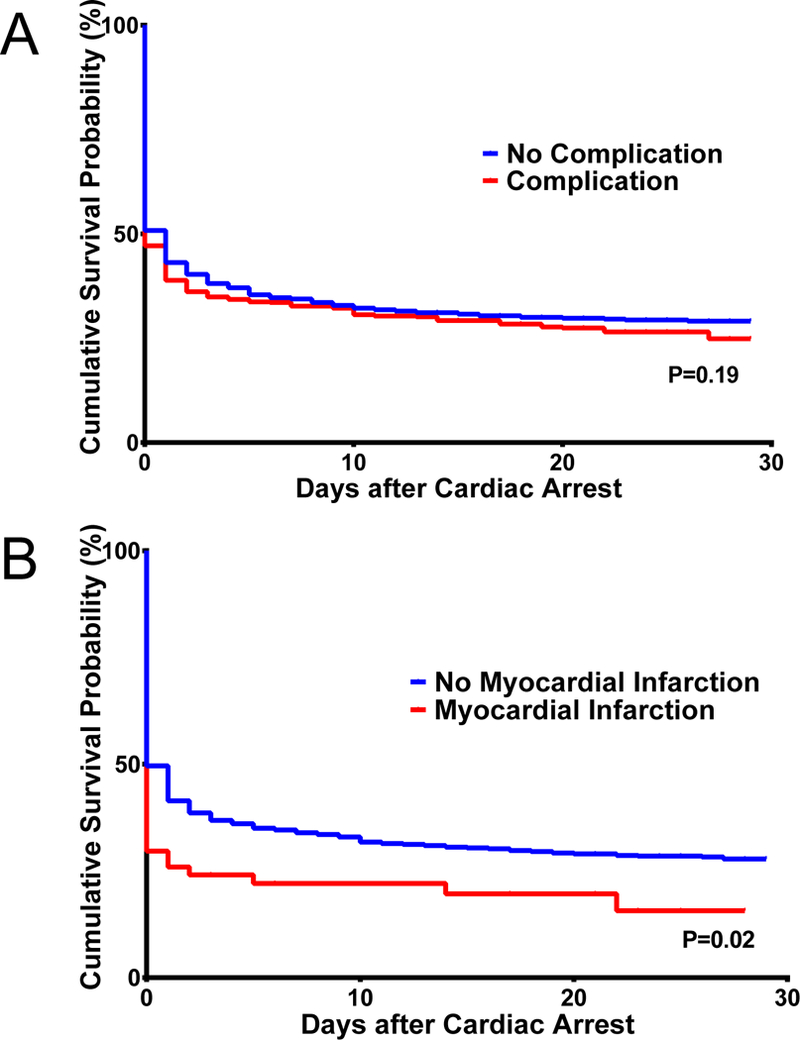
Among individual complications, transfusion (33% [445/1,352]) was the most common, followed by acute respiratory failure (25% [344/1,352]), and sepsis/septic shock (14% [189/1,352]) (Table 2). In contingency tables, mortality was lower among those with prior acute respiratory failure than in those without prior acute respiratory failure (66% [228/344] vs 72% [730/1,008], P=0.03 χ2-test). However, this result did not meet Bonferroni-adjusted criteria for statistical significance and no other individual complication was significantly associated with mortality. Kaplan-Meier plots demonstrated differences in post-cardiac arrest survival patterns between those with and without prior myocardial infarction (Figure 1B; P=0.02 log-rank test), but this did not meet Bonferroni-adjusted criteria for significance and no other individual prior complication was significantly associated with mortality.
Cox models for post-cardiac arrest mortality by prior complications
Compared to those without prior complications, those with a prior complication did not have significant associations in the hazard for mortality in unadjusted (HR 1.09, 95% confidence interval [CI] [0.96, 1.23], P=0.21) or adjusted models (HR 1.03, 95% CI [0.90, 1.18], P=0.70) (Table 3). Confounding variables were identified from prior studies3 (COPD, preoperative sepsis/septic shock, cancer, preoperative acute renal failure/dialysis, and operative procedure) and stepwise regression (preoperative ascites and estimated glomerular filtration rate).
Table 3.
Cox models for postoperative complications and 30-day mortality in general surgery patients with cardiac arrest, American College of Surgeons National Surgical Quality Improvement Program, 2012–2013.
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Postoperative Complication Occurring Prior to Cardiac Arrest | HR | CIb | P-Value | HR | CIb | P-Value |
| One or More Complications | 1.09 | [0.96, 1.23] | 0.21 | 1.03 | [0.90, 1.18] | 0.70 |
| Myocardial Infarction | 1.49 | [0.98, 2.26] | 0.01 | 1.40 | [0.92, 2.14] | 0.03 |
| Deep Vein Thrombosis/Pulmonary Embolus | 1.27 | [0.80, 2.02] | 0.17 | 1.32 | [0.82, 2.11] | 0.12 |
| Acute Kidney Injury | 1.15 | [0.88, 1.50] | 0.16 | 1.14 | [0.86, 1.51] | 0.21 |
| Transfusion | 1.07 | [0.89, 1.28] | 0.36 | 1.01 | [0.83, 1.22] | 0.91 |
| Sepsis/Septic Shock | 1.05 | [0.82, 1.35] | 0.57 | 1.05 | [0.81, 1.36] | 0.64 |
| Acute Respiratory Failure | 0.88 | [0.72, 1.08] | 0.10 | 0.88 | [0.71, 1.09] | 0.10 |
| Stroke | 0.77 | [0.28, 2.13] | 0.48 | 0.68 | [0.24, 1.90] | 0.31 |
HR, hazard ratio; CI, confidence interval. Note: Each row reflects separate Cox models for the given complication.
Adjusted for confounding variables: age, chronic obstructive pulmonary disease, preoperative sepsis/septic shock, cancer, preoperative acute renal failure/dialysis, preoperative ascites, estimated glomerular filtration rate, and operative procedure.
The CI for “One or More Complications” is a 95% CI. The CI for individual complications is a 99.3% CI. Note: Each row reflects separate Cox models for the given complication.
Among individual complications, compared to those without prior myocardial infarction, those with a prior myocardial infarction had an increased hazard for mortality in unadjusted (HR 1.49, 99.3% CI [0.98, 2.26]; P=0.01) and adjusted (HR 1.40, 99.3% CI [0.92, 2.14]; P=0.03) analyses, though neither result met the Bonferroni-adjusted criteria for significance. No other individual prior complication was significantly associated with mortality in unadjusted or adjusted Cox models.
Post hoc analyses: Postoperative day of cardiac arrest and survival after cardiac arrest
While our main analyses excluded patients with cardiac arrest on POD #0, we included these patients in post hoc analyses. Among all patients with cardiac arrest (N=1,730; Supplemental Figure 1), POD #0 was the POD with the greatest number of cardiac arrest events (Supplemental Figure 2), with 378/1,730 (22%) occurring on POD #0, including 28 patients with intraoperative cardiac arrest. The number of cardiac arrests declined in the subsequent days after the surgical procedure, with half of all cardiac arrest events occurring by POD #4. There were no significant differences in post-arrest survival among those experiencing cardiac arrest on POD #1, POD #2, or after POD #2 (Supplemental Figure 3A). When comparing post-cardiac arrest survival between those with cardiac arrest on POD #0 vs. after POD #0, there were significant differences in post-cardiac arrest survival patterns between these two groups (Supplemental Figure 3B).
Discussion
Using a retrospective, multicenter, high-quality surgical dataset, we found that in general surgery patients with cardiac arrest after POD #0, prior complications were identified in 55%, with blood transfusions and acute respiratory failure being the most common. Cardiac arrest was associated with very high mortality, with 71% of patients dying within 30 days of surgery. Overall, the development of a prior complication was not associated with an increased risk of mortality after cardiac arrest, and analyses of individual complications also did not demonstrate any prior complications that were associated with increased mortality risk. These findings suggest that knowledge of the complications that developed prior to postoperative cardiac arrest does not help to differentiate a patient’s risk of subsequent mortality.
A recent study by Kazaure et al.,16 also using the ACS-NSQIP, found that postoperative complications commonly preceded cardiac arrest in surgical patients, but as complications were evaluated as outcomes and not risk factors, conclusions about the specific contribution of complications to mortality risk could not be drawn, though there were thoughts that aggressive management and prevention of complications might reduce the incidence of postoperative cardiac arrest.7 While our study cannot address whether aggressive management does indeed reduce the incidence of cardiac arrest, it does suggest that prior complications do not affect mortality once cardiac arrest occurs. In addition, Kazaure et al. examined the entire ACS-NSQIP database, including all surgical specialties, and found baseline differences in the underlying patient populations. We chose to study only general surgery patients to reduce heterogeneity introduced by examining multiple surgical specialties, but note that heterogeneity also exists within general surgery patients with regards to surgical outcomes11 that necessitates accounting for the specific surgical procedure even within this category.
Much of the literature on perioperative cardiac arrest focuses on the intraoperative or immediate postoperative period up to 24 hours after the procedure,2,4,5,17 and the epidemiology of cardiac arrest events after this period is limited.3 Although it was not our original intent, by excluding patients with cardiac arrest on POD #0, our analysis focused on patients with cardiac arrest after POD #0 and factors affecting mortality in this group of patients have not been well studied. It may be thought that cardiac arrest during this time is not directly related to surgery or anesthesia and it remains to be determined if cardiac arrest and mortality are the inevitable result of the poor postoperative course of these patients or if interventions could have been performed to reduce the rate of complications and cardiac arrest events. The survival in this group is comparable to survival rates among all patients with in-hospital cardiac arrest,18 so they may be similar to those in the general medical population experiencing cardiac arrest.
Interestingly, we found that survival patterns after cardiac arrest were not affected by the POD on which cardiac arrest occurred in patients with cardiac arrest after POD #0. In contrast, post hoc analyses demonstrated better survival in patients with cardiac arrest on POD #0 compared to after POD #0, with both higher day of arrest survival (66% vs. 49%) and 30-day survival (47% vs 29%). In other studies, of cardiac arrests occurring within 24 hours of surgery, those that occurred in the operating room or in the PACU were associated with better survival compared to other locations2 and survival has improved over time in patients with cardiac arrest during general anesthesia,19 with less than a quarter of events attributable to anesthetic management.20 We cannot make definitive conclusions regarding cardiac arrests occurring on POD #0 as they were not the primary focus of the analysis, but based on these findings, it appears that there are differences between cardiac arrests occurring on POD #0 vs. after POD #0, and a more detailed understanding of the factors related to cardiac arrests occurring at different time points in the postoperative course is warranted.
We did not identify any individual complication that was associated with mortality when occurring prior to cardiac arrest, but myocardial infarction merits further discussion as the results hint at an association with mortality. Though it did not meet the statistical significance criteria when adjusted for multiple comparisons, the sample was not powered to evaluate myocardial infarction as an individual complication. Myocardial infarction occurs in 5% of patients undergoing noncardiac surgery, with most events diagnosed within 48 hours after the procedure.21 While speculative in nature at this time, we observed a temporal relationship between myocardial infarction and cardiac arrest affecting the risk of mortality as a sensitivity analysis found that myocardial infarctions occurring on the same day as or after the day of cardiac arrest were not associated with increased mortality risks (data not shown), but this finding will have to be validated in future studies.
It is likely that clinically significant perioperative myocardial ischemia is underdiagnosed as a recent study demonstrated that almost 60% of myocardial ischemic events did not meet the criteria for myocardial infarction but were nonetheless associated with increased mortality risk.22 Routine and early monitoring of postoperative troponin levels in high risk patients may be necessary to identify patients with myocardial ischemia that may not be symptomatic.22,23 We cannot identify patients with undiagnosed myocardial infarction or myocardial injury in our sample and it is unclear what impact these conditions would have had on our analyses. Thus, the associations between prior myocardial ischemia and mortality in cardiac arrest patients need to be evaluated in a larger study and the exposure should be expanded to include patients with myocardial injury as well as myocardial infarction.
Limitations of our study include its retrospective nature that precludes determination of causality. Though we identified complications that temporally preceded cardiac arrest, this does not necessarily mean that the complication itself caused any changes in mortality risk. The dataset does not contain some relevant clinical variables such as the cause of the cardiac arrest event, data on intraoperative management, and the location of the patient when the event occurred (PACU, intensive care unit, floor, etc.). More granular data, such as coding hours, instead of days, after surgery, would have been beneficial, especially in the first 24 hours after surgery. Other factors that have been shown to affect surgical outcomes, such as hospital variation,24 Do Not Resuscitate orders,25 rapid response teams,26 and the day of week of surgery (weekday vs. weekend)27 were not able to be addressed with this dataset.
Observational studies are subject to certain limitations.28 Misclassification bias may exist if the reporting of prior complications was inaccurate due to issues with follow-up or charting. For example, if the time period between the development of a complication and cardiac arrest was short, the complication might not have been recognized. Our a priori hypothesis was that prior complications would be associated with increased mortality risk after cardiac arrest, but the data did not support this finding. It is plausible to think that every patient with cardiac arrest had some complication even if it was not explicitly documented, and there may be important complications affecting cardiac arrest and mortality that were not collected in the dataset. There may be a lack of generalizability as the ACS-NSQIP has tended to include mainly large, academic hospitals so the results may not apply in smaller non-academic settings.29 Residual confounding is less of a concern as our study did not identify any significant associations between the exposures and outcome.
In conclusion, we found that among general surgery patients with cardiac arrest after POD #0, mortality was extremely high and many patients experienced major postoperative complications prior to cardiac arrest, but these complications did not appear to increase the risk of mortality. While further study is necessary to identify measures to reduce the risk of complications and mortality after cardiac arrest, the development of a major postoperative complication prior to cardiac arrest does not appear to be a distinguishing factor between those likely to have better or worse outcomes after cardiac arrest.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by a Mentored Research Award from the International Anesthesia Research Society, San Francisco, CA (MK) and Grant R49 CE002096 from the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA (GL). Its contents are solely the responsibility of the authors and do not necessarily reflect the official views of the funding agencies.
This work was presented in part at the American Society of Anesthesiologists’ Annual Meeting, October 2016, Chicago, IL.
Footnotes
Conflicts of Interest: None
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
https://www.facs.org/~/media/files/quality%20programs/nsqip/2013_acs_nsqip_puf_user_guide.ashx; Published November 2014.
http://www.strobe-statement.org/index.php?id=available-checklists; Accessed January 2, 2017.
Estimated with online sample size calculator: http://www.sample-size.net/sample-size-survival-analysis/; Accessed June 6, 2017.
Contributor Information
Minjae Kim, Department of Anesthesiology, Columbia University Medical Center, New York, NY, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, This author was involved in study design, conduct of study, data analysis, and manuscript preparation. Dr. Kim approved the final manuscript and attests to the integrity of the original data and analysis reported in this manuscript. Dr. Kim is the archival author. No conflict of interest exists..
Guohua Li, Department of Anesthesiology, Columbia University Medical Center, New York, NY, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, This author was involved in study design, data analysis, and manuscript preparation. Dr. Li approved the final manuscript and attests to the integrity of the original data and analysis reported in this manuscript. No conflict of interest exists..
References
- 1.Kolte D, Khera S, Aronow WS et al. Regional variation in the incidence and outcomes of in-hospital cardiac arrest in the United States. Circulation 2015;131:1415–25. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran SK, Mhyre J, Kheterpal S et al. Predictors of survival from perioperative cardiopulmonary arrests: a retrospective analysis of 2,524 events from the Get With The Guidelines-Resuscitation registry. Anesthesiology 2013;119:1322–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazaure HS, Roman SA, Rosenthal RA, Sosa JA. Cardiac arrest among surgical patients: an analysis of incidence, patient characteristics, and outcomes in ACS-NSQIP. JAMA Surg 2013;148:14–21. [DOI] [PubMed] [Google Scholar]
- 4.Nunnally ME, O’Connor MF, Kordylewski H, Westlake B, Dutton RP. The incidence and risk factors for perioperative cardiac arrest observed in the national anesthesia clinical outcomes registry. Anesth Analg 2015;120:364–70. [DOI] [PubMed] [Google Scholar]
- 5.Goswami S, Brady JE, Jordan DA, Li G. Intraoperative Cardiac Arrests in Adults Undergoing Noncardiac Surgery: Incidence, Risk Factors, and Survival Outcome. Anesthesiology 2012;117:1018–26. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Li G. 3: Interaction Effects of Multiple Complications on Postoperative Mortality in General Surgery [Abstract]. Crit Care Med 2015;43:1. [Google Scholar]
- 7.Zenilman ME. Cardiopulmonary resuscitation in surgical patients: it is all in the timing. JAMA Surg 2013;148:21–2. [DOI] [PubMed] [Google Scholar]
- 8.Fink AS, Campbell DA Jr., Mentzer RM Jr. et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility. Ann Surg 2002;236:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266. [PubMed] [Google Scholar]
- 11.Kim M, Brady JE, Li G. Variations in the risk of acute kidney injury across intraabdominal surgery procedures. Anesth Analg 2014;119:1121–32. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 13.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. J R Statist Soc A 1972;135:185–207. [Google Scholar]
- 14.Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt 2014;34:502–8. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 1983;39:499–503. [PubMed] [Google Scholar]
- 16.Kazaure HS, Roman SA, Sosa JA. Epidemiology and outcomes of in-hospital cardiopulmonary resuscitation in the United States, 2000–2009. Resuscitation 2013;84:1255–60. [DOI] [PubMed] [Google Scholar]
- 17.Braz LG, Modolo NS, do Nascimento P Jr. et al. Perioperative cardiac arrest: a study of 53,718 anaesthetics over 9 yr from a Brazilian teaching hospital. Br J Anaesth 2006;96:569–75. [DOI] [PubMed] [Google Scholar]
- 18.Chan PS, Krein SL, Tang F et al. Resuscitation Practices Associated With Survival After In-Hospital Cardiac Arrest: A Nationwide Survey. JAMA Cardiol 2016;1:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprung J, Warner ME, Contreras MG et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology 2003;99:259–69. [DOI] [PubMed] [Google Scholar]
- 20.Ellis SJ, Newland MC, Simonson JA et al. Anesthesia-related cardiac arrest. Anesthesiology 2014;120:829–38. [DOI] [PubMed] [Google Scholar]
- 21.Devereaux PJ, Xavier D, Pogue J et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011;154:523–8. [DOI] [PubMed] [Google Scholar]
- 22.Botto F, Alonso-Coello P, Chan MT et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120:564–78. [DOI] [PubMed] [Google Scholar]
- 23.van Waes JA, Nathoe HM, de Graaff JC et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013;127:2264–71. [DOI] [PubMed] [Google Scholar]
- 24.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009;361:1368–75. [DOI] [PubMed] [Google Scholar]
- 25.Morrison LJ, Neumar RW, Zimmerman JL et al. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation 2013;127:1538–63. [DOI] [PubMed] [Google Scholar]
- 26.Offner PJ, Heit J, Roberts R. Implementation of a rapid response team decreases cardiac arrest outside of the intensive care unit. J Trauma 2007;62:1223–7. [DOI] [PubMed] [Google Scholar]
- 27.Glance LG, Osler T, Li Y et al. Outcomes are Worse in US Patients Undergoing Surgery on Weekends Compared With Weekdays. Med Care 2016;54:608–15. [DOI] [PubMed] [Google Scholar]
- 28.Hill HA, Kleinbaum DG. Bias in Observational Studies. Encyclopedia of Biostatistics 2005;1. [Google Scholar]
- 29.Dimick JB, Osborne NH, Hall BL, Ko CY, Birkmeyer JD. Risk adjustment for comparing hospital quality with surgery: how many variables are needed? J Am Coll Surg 2010;210:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


