Abstract
Purpose
Numerous studies on thromboembolic prevention for non-valvular atrial fibrillation (NVAF) have shown either equal or better efficacy and safety of non-vitamin K oral anticoagulants (NOACs) compared to warfarin, even for patients aged ≥75 years. Data on elderly patients, in particular, octogenarians, are lacking. Paradoxically, this population is the one with the highest risk of bleeding and stroke with a worse prognosis. This study aims to describe safety and effectiveness of NOACs in an elderly comorbid population.
Patients and methods
REGIstry of patients on Non-vitamin K oral Anticoagulants (REGINA) is a prospective observational study enrolling consecutive NVAF patients started on NOACs and followed up to 1 year (at 1, 6, 12 months). The primary endpoint was the incidence rate of major bleeding (MB) and clinically relevant non-major bleeding (CRNMB). The secondary endpoints were the incidence of 1) stroke or systemic embolism, 2) hospitalization, 3) death, and 4) drug-related adverse events.
Results
We enrolled 227 patients aged 81.6±6.1 years (range 67–95 years; ≥80 years in 59.4%). The median CHA2DS2-VASc was 5 (IQR 4–5) and HAS-BLED was 4 (IQR 3–5). The estimated glomerular filtration rate was 59.27±24.12 mL/min. During follow-up, only 10 MB and 23 CRNMB occurred, with a total incidence of 4.4% (95% CI: 1.7%–7.17%) and 5.7% (95% CI: 2.68%–8.72%), respectively. There were 2 cerebral ischemic events, with a total incidence of 0.88% (95% CI: 0.84%–0.92%), 23 NOAC-related hospitalizations, no NOAC-related deaths, and 4 minor drug-related adverse effects.
Conclusion
In a population of aged and clinically complex patients, mainly octogenarians, NOACs were safe and effective.
Keywords: NVAF, anticoagulation, elderly, octogenarians, cardiac rehabilitation, NOAC
Introduction
Non-vitamin K oral anticoagulant (NOAC) therapy has been increasingly used for stroke prevention in non-valvular atrial fibrillation (NVAF)1 due to its proven effectiveness and safety. Real-world data have confirmed Phase III trial results,2 even showing further advantages in the older population,3,4 which shows the highest risk of bleeding, as well as stroke with a worse prognosis.5 In elderly patients, NOACs seem to be more effective than and as safe as vitamin K antagonists (VKA).6 Nevertheless, real-world prospective studies in this population, including octogenarians, are still scarce. On the contrary, these patients are more and more frequent in clinical practice. The decision-making process to initiate them to a chronic anticoagulant therapy can be difficult due to the risk/benefit ratio.
We, therefore, aimed to analyze data from the local REGIstry of patients on Non-vitamin k oral Anticoagulants (REGINA) to assess the efficacy and safety of NOACs in an elderly and comorbid population, such as the one cardiologists increasingly follow in the cardiac rehabilitation setting.
Patients and methods
REGINA is a single-center, prospective, observational study that was conducted at the IRCCS Istituti Clinici Scientifici Maugeri, Milano, Italy. The Local Ethics Committee (Comitato Etico ICS Maugeri SpA SB, Pavia) approved the study (1053CE). It adhered to the Declaration of Helsinki, and each patient signed a written informed consent form.
NVAF patients on NOAC treatment followed at our inpatient or outpatient cardiac rehabilitation clinic were consecutively enrolled from April 14, 2015 to July 31, 2016. All patients were followed up to 1 year. Follow-up (FU) visits were scheduled at 1, 6, and 12 months from the first NOAC dose. Patients with severe cognitive impairment (Mini-Mental State Examination score <20), active cancer, and/or any-end stage disease and those who had undergone cardiac surgery in the previous 12 months were excluded. NOAC choice was based on the knowledge and expertise of each prescriber to ensure the collection of real-life data. Note that edoxaban was not available in Italy at the time of enrollment. Updated international and national guidelines’ recommendations and appropriate local diagnostic–therapeutic instructions were available on our institution’s Intranet. Periodic meetings for the discussion of clinical cases were scheduled throughout the study period.
The primary endpoint of the study was represented by the incidence rate of major bleeding (MB) and/or clinically relevant non-major bleeding (CRNMB), defined according to International Society on Thrombosis and Haemostatis (ISTH) criteria.7,8
The secondary endpoints included the incidence of 1) stroke or systemic embolism, 2) hospitalization, 3) death, and 4) any drug-related adverse event.
Data collection
A specific electronic case report form (eCRF; Promeditec, Milan, Italy) was developed for this study. On enrollment, eCRF mandatory fields included age, gender, weight, height, calculated body mass index (BMI), heart rhythm (sinus rhythm or atrial fibrillation), concurrent therapies, Cumulative Illness Rating Scale (CIRS)9 to quantify the presence of comorbidities (CIRSc) and their severity (CIRSs), CHA2DS2-VASc,10 and HAS-BLED11 for all patients. Each participant underwent a complete physical examination. Baseline blood tests were also obtained, with hemoglobin (HGB), platelet count (PLT), serum creatinine, ALT, and AST being mandatory. The estimated glomerular filtration rate (eGFR) was calculated through the Cockcroft–Gault formula.12 In the eCRF, medical history details were also recorded.
At each FU visit, a detailed medical history, including specific, multiple questions regarding treatment adherence and adverse effects, and a repeated physical examination were obtained. The results of repeated blood tests, occurrence of death, hospitalization, undercurrent disease, and any relevant information were recorded in the eCRF.
Statistical analyses
Baseline characteristics are presented as percentages for categorical variables and medians (IQR) or mean ± SD for continuous variables. The chi-squared test was used for univariate comparisons of categorical variables between groups. The unpaired t-test (or Wilcoxon rank sum test when appropriate) was used for continuous variables. The incidence rate of outcomes per 100 patient-years of FU and corresponding 95% CIs are presented. All available FU data were used in the calculation of these rates. We chose to apply on-treatment analysis to describe the safety and efficacy of NOAC treatment in this cohort of real-life patients. Therefore, we calculated the incidence rates on the population available at each time point. Kaplan–Meier curves were realized to describe event-free survival.
Results
We consecutively enrolled 227 patients (F/M 105/122), of whom 183 (80.6%) were inpatients. Only three patients dropped out at FU. Patients’ age was 81.6±6.1 years (range 67–95 years). A large proportion of patients (59.4%) were aged ≥80 years. BMI was 26±5 kg/m2, and eGFR was 59.27±24.12 mL/min. Patients with permanent NVAF numbered 125 (55%), those with paroxysmal NVAF were 81 (35.7%), and those with persistent NVAF numbered 21 (9.3%). Median CHA2DS2-VASc was 5 (IQR, 4–5) and median HAS-BLED was 4 (IQR, 3–5), thus grading this population at high risk for both stroke and bleeding. Furthermore, CIRSc was 4.99±1.74 and CIRSs was 1.94±0.29, demonstrating that the enrolled population was also characterized by a very high clinical complexity. The prevalence of the main comorbidities is detailed in Table 1, where the proportion of patients with a CIRSc >3 is also reported.
Table 1.
Patients’ clinical complexity
| Comorbidities | n (%) |
|---|---|
|
| |
| Hypertension | 156 (68.7) |
| CHF | 99 (43.6) |
| Diabetes | 57 (25.1) |
| CAD | 65 (28.7) |
| Previous MI | 47 (20.7) |
| PAD | 63 (27.7) |
| Previous stroke or TIA | 42 (18.5) |
| CIRSc >3 | 180 (79.3) |
Note: Values are expressed as n, total number, and (%), percentage.
Abbreviations: CAD, coronary artery disease; CHF, chronic heart failure; CIRSc, Cumulative Illness Rating Scale complexity; MI, myocardial infarction; PAD, peripheral artery disease; TIA, transient ischemic attack.
Naïve patients to any type of anticoagulation numbered 96 (42.3%), while the remaining ones were switched from VKA. The switch to an NOAC was proposed to all eligible patients without contraindications, such as eGFR <30 mL/min, mechanical valve prostheses, and surgical valve disease. None of these patients refused the therapy change.
One-hundred and twenty-four patients (54.6%) were treated with apixaban, 45 (19.8%) with dabigatran, and 58 (25.6%) with rivaroxaban. Table 2 shows the clinical details of each subgroup, that is, according to the prescribed NOAC (drug and dose).
Table 2.
Patients’ clinical details according to the prescribed NOAC therapy
| Apixaban 2.5 mg BID (n=86) | Apixaban 5 mg BID (n=35) | Dabigatran 110 mg BID (n=31) | Dabigatran 150 mg BID (n=14) | Rivaroxaban 15 mg QD (n=18) | Rivaroxaban 20 mg QD (n=40) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age, years | 84.13±5.54 | 75.42±7.31 | 81.77±5.97 | 72.86±5.88 | 81.83±7.82 | 76.62±10.39 |
| Weight, kg | 69.28±15.39 | 76.11±13.7 | 67.65±12.15 | 76.97±17.97 | 64.86±9.56 | 73.99±13.98 |
| eGFR, mL/min | 46.62±15.75 | 72.11±24.67 | 54.55±20.55 | 87.58±28.82 | 54.02±21.73 | 73.48±22.72 |
Note: Values are expressed as n, total number, and mean ± SD.
Abbreviations: BID, bis in die; eGFR, estimated glomerular filtration rate; NOAC, non-vitamin K oral anticoagulant; QD, quaque die.
Overall, blood tests remained stable throughout the FU compared to baseline. We observed no changes in HGB (12.2±1.7, 12±1.6, 12.4±1.6, and 12.7±1.6 g/dL, respectively), PLT (230.8±85, 222.9±85.4, 227.3±72, and 224.3±86.4×109/L, respectively), ALT (21.3±13.1, 19.9±9.7, 21.3±15.1, and 19.2±5.8 units/L, respectively), AST (22.3±9.5, 20±7.9, 21.1±9.7, and 19.3±5.1 units/L, respectively), and creatinine levels (1.1±0.3, 1.06±0.4, 1.09±0.4, and 1.1±0.4 mg/dL, respectively). Finally, we observed a good self-reported adherence to NOACs treatment. A fully correct therapeutic scheme was followed by 92% of patients, while only 8% of patients reported having occasionally forgotten a tablet intake, never for more than two consecutive pills.
Primary endpoint
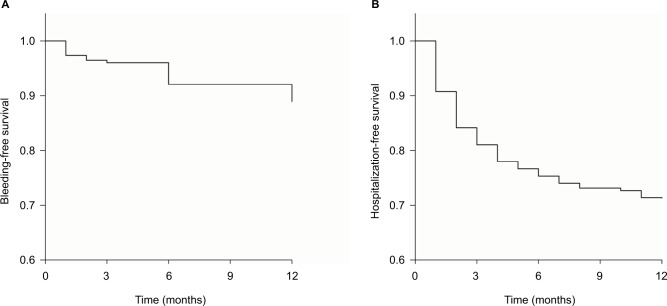
Upon completion of FU, MB occurred in only 10 patients, for a total incidence of 4.4% (95% CI: 1.73%–7.07%), and CRNMB occurred in 13, for a total incidence of 5.7% (95% CI: 2.68%–8.72%). There was no fatal bleeding. Out of ten MB cases, only one was intracranial and the patient underwent successful surgery, five were gastrointestinal, and four were urogenital. Forty percent of total bleeding occurred in the first 3 months of therapy and the remaining events were evenly spaced during the remaining FU period, as shown in the Kaplan–Meier plot of Figure 1A. The incidence of MB and CRNMB according to NOAC subgroups is presented in Table 3.
Figure 1.
Event-free survival: bleeding-free survival plot (A); hospitalization-free survival plot (B).
Table 3.
Occurrence of MB and CRNMB according to the prescribed NOAC therapy
| NOAC | MB (n=10) | CRNMB (n=13) |
|---|---|---|
|
| ||
| Apixaban 2.5 mg BID | 3 (3.49) | 4 (4.65) |
| Apixaban 5 mg BID | 2 (5.71) | 3 (8.57) |
| Dabigatran 110 mg BID | 1 (3.23) | 2 (6.45) |
| Dabigatran 150 mg BID | 3 (21.43) | 1(7.14) |
| Rivaroxaban 15 mg QD | 0 (0) | 3 (16.67) |
| Rivaroxaban 20 mg QD | 1 (2.5) | 0 (0) |
Notes: Values are expressed as n, total number, and (%), percentage.
Abbreviations: BID, bis in die; CRNMB, clinically relevant non-major bleeding; MB, major bleeding; NOAC, non-vitamin K oral anticoagulant; QD, quaque die.
Neither CHA2DS2-VASc nor HAS-BLED was correlated with the occurrence of MB or CRNMB.
Secondary endpoints
We observed the following: 1) two embolic events, namely, a transient ischemic attack (TIA) and a minor stroke, with a total incidence of 0.88% (95% CI: – 0.33% to 2.09%), that occurred at the ninth and fourth months of FU, respectively; 2) 69 hospitalizations (rate of 30.3% per year), of which 23 (33%) were NOAC related, while the other causes for the hospital admission were either congestive heart failure (45%) or infections (22%); all hospitalizations involved patients who had been enrolled in the study during their first hospital stay, truly representing hospital readmissions that mainly occurred in the first 3 months of FU (64% of the events), as shown in the Kaplan–Meier plot of Figure 1B; 3) 20 deaths (total death rate of 8.8% per year), of which 8 were because of cardiac causes (heart failure or sudden death) and 12 were due to pneumonia, other infections, or cancer; there were no NOAC-related deaths; and 4) rare drug-related adverse events, that is, only 4 patients, all on dabigatran, complained of dyspepsia, and a switch to a different NOAC was necessary in 3 of them.
Reduced dose of NOAC
A post hoc analysis of the data revealed that reduced dose (RD) of NOAC was used in 135 patients. Among these patients, RD was appropriately selected based on the Phase III trial criteria13–15 in 89 (66%) patients, while in the remaining patients, RD was prescribed based on clinical judgment. More specifically, 40% of patients on apixaban 2.5 mg bis in die (BID) met one criterion only, mainly, the age criterion, 32% of patients on dabigatran 110 mg BID and 50% of those on rivaroxaban 15 mg quaque die had either borderline eGFR or advanced age, in addition to high CIRS. Compared to the remaining population, that is, patients on full dose, those treated with an RD of apixaban were older and had lower body weight, eGFR, and HGB levels at enrollment (Table 4). Regarding the endpoints, in these RD groups, MB occurred in three patients with a total incidence of 3.49% (95% CI: 1.1%–5.87%) and CRNMB occurred in four patients with a total incidence of 4.65% (95% CI: 1.91%–7.38%), while embolic events occurred in two patients with a total incidence of 1.48% (95% CI: – 0.09% to 3.05%). These two embolic events consisted of a minor stroke in a patient with fulfilled RD criteria and a TIA in another patient with borderline criteria, that is, age of 82 years and creatinine equal to 1.5 mg/dL.
Table 4.
Clinical details of patients on a reduced dose of NOAC compared to those on full dose
| Apixaban 2.5 mg BID | Full dose | P-value | |
|
| |||
| Age, years | 84.13±5.54 | 77.74±8.52 | <0.001 |
| Weight, kg | 69.28±15.39 | 72±13.91 | 0.13 |
| eGFR, mL/min | 46.62±15.75 | 67.05±25.25 | <0.001 |
| CIRSc | 5.21±1.73 | 4.83±1.75 | 0.202 |
| CIRSs | 2±0.31 | 1.91±0.29 | 0.109 |
| HGB, g/dL | 11.8±1.68 | 12.59±1.72 | <0.001 |
|
| |||
| Dabigatran 110 mg BID | Full dose | P-value | |
|
| |||
| Age, years | 81.77±5.97 | 80±8.41 | 0.263 |
| Weight, kg | 67.65±12.15 | 71.62±14.84 | 0.263 |
| eGFR, mL/min | 54.55±20.55 | 60.06±24.64 | 0.348 |
| CIRSc | 5.5±2.04 | 4.93±1.7 | 0.158 |
| CIRSs | 2.01±0.31 | 1.94±0.3 | 0.234 |
| HGB, g/dL | 11.73±0.45 | 11.68±1.5 | 0.672 |
|
| |||
| Rivaroxaban 15 mg QD | Full dose | P-value | |
|
| |||
| Age, years | 81.83±7.82 | 80.11±8.16 | 0.367 |
| Weight, kg | 64.86±9.56 | 71.65±14.8 | 0.062 |
| eGFR, mL/min | 54.02±21.73 | 59.75±24.33 | 0.433 |
| CIRSc | 5.29±1.68 | 4.97±1.75 | 0.463 |
| CIRSs | 1.96±0.32 | 1.95±0.3 | 0.757 |
| HGB, g/dL | 12.32±1.6 | 12.24±1.77 | 0.7 |
Notes: Values are expressed as mean ± SD. P<0.05 was considered as significant.
Abbreviations: BID, bis in die; c, complexity; CIRS, Cumulative Illness Rating Scale; eGFR, estimated glomerular filtration rate; HGB, hemoglobin; NOAC, non-vitamin K oral anticoagulant; QD, quaque die; s, severity.
Discussion
REGINA is a single-center, prospective, observational study that enrolled an elderly population, treated with an NOAC for NVAF, mainly over 80 years old, and characterized by a high number of comorbidities and a high clinical complexity, as well as a high risk for stroke and bleeding. During a 1-year FU, there were rare embolic and few bleeding complications. Our data strengthen the concept that NOACs are safe and effective in the elderly, even in highly complex octogenarians.
Phase III trials clearly demonstrated NOACs’ effectiveness and safety in NVAF patients,1,2 although aged, frail, comorbid patients were represented to a lesser extent.16 In the last years, several real-world studies have confirmed the effectiveness and safety of NOACs in the elderly, although most of them were retrospective and/or based on health insurance databases.17–22 In the present study, in a cohort of 227 patients, with an average age of 81 years and about 60% of cases aged over 80 years, who had been prospectively observed up to 1 year, there were no fatal bleedings and MB occurred in only 4.4% (95% CI: 1.73%–7.07%), of which only 1 case was intracranial and life threatening. These data are in line with the results of a recent large retrospective study, which showed that compared to VKA, NOACs were associated with a lower rate of MB events.22
In addition to old age, we must underline that the population of the present study displayed very high-risk features. Indeed, all measured scores depicted a profile of high clinical complexity. Given these premises, in agreement with the literature,23 nearly one-third of the patients were rehospitalized during the observational period, mainly for cardiovascular causes. In keeping with several studies,18–22 only one-third of the hospitalizations were related to bleeding events. Furthermore, no deaths were related to the use of NOACs, although the overall death rate was 8.8% per year due to cardiac causes, infections, or cancer.
It is worth discussing that a high percentage (59%) of our population was treated with an NOAC at RD. As prespecified in the “Patients and methods” section, in order to collect real-world data, the selection of the specific NOAC and its dosage was based on the prescribers’ evaluation. In 34% of these patients, the RD was chosen based on clinical judgment rather than Phase III trials’ criteria14–16 and international consensus recommendations on NOAC use.24 Previous studies25,26 have reported the use of a lower-than-recommended dose of NOACs in clinical practice. Probably, the individual assessment of patients’ risks for bleeding often plays a major role in guiding the choice of drug and dosage, in particular, in the elderly. In fact, patients treated with RD were even more fragile than the remaining patients were, as they had lower body weight, worse eGFR, and higher CIRSc and CIRSs. Not surprisingly, the only two cardioembolic events occurred in this group of patients. It is known that NVAF patients with advanced age or low body weight or renal dysfunction have a higher risk of stroke and MB,27 irrespective of the chosen dosage or NOAC. It has to be emphasized that inappropriate dose reduction has been associated with a higher risk for embolism,28,29 while in other studies, it did not seem to be the case.26,30 Thus, transposing the recommendations of the randomized Phase III trials to the more complex real world remains a challenge for each clinician. Accordingly, the updated European Heart Rhythm Association (EHRA) practical guide on NOAC management,24 that is based on expert opinions, suggests dose reduction considering a number of clinical variables, not even mentioned in Phase III trials. While adequate dose prescription is certainly mandatory, such appropriateness remains to be further elucidated. Given the escalating complexity, we face in the daily clinical practice of geriatric patients, there is a growing need for a modern approach that integrates information from large trials and single patient’s evaluation.31
Lastly, we observed a good self-reported adherence to NOACs (eg, 92% compliance to the prescribed therapy) and a stability of blood tests throughout the FU period. Both these findings might depend on the peculiar organization that characterizes a prospective registry, in which both careful patient management and the periodic phone calls reduce the nonadherence and allow correction of possible undercurrent factors. Indeed, this might also have contributed to improve NOACs’ efficacy and safety. However, this is a mere speculation. Since adherence was considered high and events were low, no relation could be found between them.
A limitation of our study is the lack of a control group on VKA. According to the current guidelines, NOACs are often preferred; thus, it is extremely difficult to have comparable prospective data. Indeed, recent real-life studies that compared NOACs to VKAs were retrospective using the propensity score matching analysis.18,22 They showed that MB and overall bleeding episodes are less frequent in patients treated with NOACs rather than with VKA. We used the results of the aforementioned studies as a landmark to draw our conclusions.
Conclusion
Thus, despite the limited number of patients enrolled in this study, there is evidence that in a population of comorbid, clinically complex elderly NVAF patients, including octogenarians, NOACs were safe and effective. These results further encourage the use of NOACs in a population that is followed by the cardiologist and that is at very high risk of disabling events. There are also some clues that careful FU of elderly patients on NOAC therapy could contribute to improve their prognosis.
Acknowledgments
We thank Daniela Loi for her valuable help in scheduling follow-up visits for these elderly patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 2.Lamberts M, Staerk L, Olesen JB, et al. Major bleeding complications and persistence with oral anticoagulation in non-valvular atrial fibrillation: contemporary findings in real-life Danish patients. J Am Heart Assoc. 2017;(62):e004517. doi: 10.1161/JAHA.116.004517. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and meta-analysis. Circulation. 2015;132(3):194–204. doi: 10.1161/CIRCULATIONAHA.114.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Guo SD, Deng H, et al. Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-regression analysis. Age Ageing. 2018;47(1):9–17. doi: 10.1093/ageing/afx103. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim IS, Kim HJ, Kim TH, et al. Non-vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: a systematic review and meta-analysis. J Cardiol. 2018;72(2):105–112. doi: 10.1016/j.jjcc.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Schulman S, Angerås U, Bergqvist D, et al. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–204. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in nonsurgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 9.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 10.Lip GYH, Halperin JL. Improving stroke risk stratification in atrial fibrillation. Am J Med. 2010;123(6):484–488. doi: 10.1016/j.amjmed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committees and Investigators Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 16.Barco S, Cheung YW, Eikelboom JW, Coppens M. New oral anticoagulants in elderly patients. Best Pract Res Clin Haematol. 2013;26(2):215–224. doi: 10.1016/j.beha.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Coleman CI, Antz M, Bowrin K, et al. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT-US study. Curr Med Res Opin. 2016;32(12):2047–2053. doi: 10.1080/03007995.2016.1237937. [DOI] [PubMed] [Google Scholar]
- 18.Lip GY, Keshishian A, Kamble S, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost. 2016;116(5):975–986. doi: 10.1160/TH16-05-0403. [DOI] [PubMed] [Google Scholar]
- 19.Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staerk L, Fosbøl EL, Lip GYH, et al. Ischaemic and haemorrhagic stroke associated with non-vitamin K antagonist oral anticoagulants and warfarin use in patients with atrial fibrillation: a nationwide cohort study. Eur Heart J. 2017;38(12):907–915. doi: 10.1093/eurheartj/ehw496. [DOI] [PubMed] [Google Scholar]
- 21.Forslund T, Wettermark B, Andersen M, Hjemdahi P. Stroke and bleeding with non-vitamin K antagonist oral anticoagulant or warfarin treatment in patients with non-valvular atrial fibrillation: a population-based cohort study. Europace. 2018;20(3):420–428. doi: 10.1093/europace/euw416. [DOI] [PubMed] [Google Scholar]
- 22.Amin A, Keshishian A, Trocio J, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33(9):1595–1604. doi: 10.1080/03007995.2017.1345729. [DOI] [PubMed] [Google Scholar]
- 23.Krumholz HM. Post-hospital syndrome – an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidbuchel H, Verhamme P, Alings M, et al. ESC Scientific Document Group. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38(27):2137–2149. doi: 10.1093/eurheartj/ehw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giustozzi M, Vedovati MC, Verdecchia P, et al. Vitamin K and non-vitamin K antagonist oral anticoagulants for non-valvular atrial fibrillation in real-life. Eur J Intern Med. 2016;33:42–46. doi: 10.1016/j.ejim.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Barra ME, Fanikos J, Connors JM, et al. Evaluation of dose-reduced direct oral anticoagulant therapy. Am J Med. 2016;129(11):1198–1204. doi: 10.1016/j.amjmed.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JH, Andersson U, Lopes RD, et al. Apixaban for Reduction of Stroke and Other Thromboembolic Complications in Atrial Fibrillation (ARISTOTLE) Investigators Apixaban 5 mg twice daily and clinical outcomes in patients with atrial fibrillation and advanced age, low body weight, or high creatinine: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1(6):673–681. doi: 10.1001/jamacardio.2016.1829. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg BA, Shrader P, Pieper K, et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) II Investigators Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II) J Am Heart Assoc. 2018;7:e007633. doi: 10.1161/JAHA.117.007633. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69(23):2779–2790. doi: 10.1016/j.jacc.2017.03.600. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Keshishian A, Hamilton M, et al. Apixaban 5 and 2.5 mg twice-daily versus warfarin for stroke prevention in nonvalvular atrial fibrillation patients: comparative effectiveness and safety evaluated using a propensity-score-matched approach. PLoS One. 2018;13(1):e0191722. doi: 10.1371/journal.pone.0191722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628. doi: 10.1136/bmj.323.7313.625. [DOI] [PMC free article] [PubMed] [Google Scholar]