Abstract
Incidence and Malignancy Rates Classified by The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) – An 8-year Tertiary Center experience in Thailand.
Background
Fine-needle aspiration (FNA) of the thyroid is considered the best diagnostic tool for preoperative evaluation of thyroid nodules. The introduction of The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) in 2010 provided the opportunity to establish worldwide standard for reporting and terminology guidelines for diagnostic categories. It is recommended that every institution evaluates the risk of malignancy (ROM) in each category for quality improvement process.
Aim
To assess the effectiveness of TBSRTC method at our institution using cyto-histological correlation.
Method
A retrospective 8-year (2010–2017) audit of thyroid FNA done by thyroid specialists at Theptarin hospital. The FNA results were classified according to TBSRTC. Histopathology reports for operated cases were used to correlate cytology and final histopathology.
Results
A total of 2735 thyroid FNA from 2115 patients (mean age 45.7 ± 13.1 years, female 89.8%) were examined. The rates of non-diagnostic, benign, atypia of undetermined significance (AUS), follicular neoplasm, suspected for malignancy, and malignant cases were 21.1%, 66.6%, 4.7%, 2.4%, 1.8%, and 3.3% respectively. There were 188 patients (9%) who underwent surgical resection with available histopathology. Malignancy rates in operated thyroid nodules were 20.0%, 4.2%, 9.4%, 23.5%, 57.1%, and 90.3% for categories 1 to 6, respectively. The sensitivity, specificity, negative predictive value, and positive predictive value were 96.6%, 88.5%, 95.8%, and 90.3, respectively.
Conclusions
Preoperative diagnosis of thyroid nodules using TBSRTC in our hospital was comparable with other studies. The uniform diagnostic criteria of the Bethesda System help avoid misinterpretation while sharing local experience with international benchmarks.
Keywords: Fine-needle aspiration (FNA), The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC), Risk of malignancy (ROM), Thailand
Introduction
Thyroid nodules are common, and sometimes incidental detection could be detected on imaging studies that include the neck [1]. Although most nodules are benign, the risk of possible thyroid carcinoma is still a great clinical concern. Previous studies found a 5% malignancy rate associated with clinically apparent thyroid nodules [2], [3]. Fine needle aspiration (FNA) of the thyroid plays a vital role for the evaluation of thyroid nodules since the 1970s to distinguish between benign and malignant disease [4]. FNA has been established as a cost-effectiveness diagnostic tool to select the appropriated patients for thyroid surgery [5].
The ultimate goal of FNA is to obtain cytologic material sufficiently to render a diagnosis of benign or malignant confidently. Until 2010, the reporting and interpretation of the cytology of thyroid aspirates had caused considerable confusion over the years in comparing results from different settings. The introduction of The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) in 2010 provided the opportunity to establish worldwide standard reporting and terminology guidelines diagnostic category. Bethesda classification system divides cytologic findings into six categories associated with escalating risk of malignancy (ROM) [6]. The categories include non-diagnostic (Bethesda I), benign (Bethesda II), atypia or follicular lesion of undetermined significance (Bethesda III), follicular neoplasm or suspicious for follicular neoplasm (Bethesda IV), suspicious for malignancy (Bethesda V) and malignant (Bethesda VI). The percentage of non-diagnostic specimens in thyroid FNA has been estimated to be between 1 and 20% and the observed risk of malignancy in these nodules was higher than that in patients with the cytological outcome classified as benign lesion [7].
Implementation of the Bethesda System improves the method to classify the risk of thyroid cancer and serves as a platform for performance benchmarking. It is recommended that every institution evaluates the ROM in each category for quality improvement process. The Bethesda system recommends that repeat biopsy should be done no earlier than 3 months after the initial biopsy to avoid false-positive results from inflammatory cellular changes [6]. However, delaying a repeat FNA can put stress on the patient and may delay surgical intervention in patients who deserve thyroid removal. Therefore, optimal timing for a repeat FNA poses a dilemma in the management of patients with non-diagnostic FNA results [8].
The reliability and accuracy of any reporting system is built on experience, not only with cytologic interpretations but years of follow-up of cytologic specimens and their correlations with the histologic diagnoses whenever available [9]. Therefore, the objectives of this study are to assess the effectiveness of TBSRTC method by cyto-histological correlation at our institution in Bangkok and also to determine if the recommended 3-month waiting period is necessary to accurately obtain a diagnostic result in previously non-diagnostic FNA results.
Materials and methods
Study settings
A retrospective 8-year (2010–2017) audit of thyroid FNA done by thyroid specialists at Theptarin Hospital, a specialized endocrine center in Bangkok, was performed. The majority of patients undergoing FNA with or without an ultrasound-guide using 27G or 25G needles with 4–6 passes per nodule. A minority of patients (less than 20%) had undergone a modified technique non-aspiration fine needle capillary sampling (FNCS). The obtained sample material was smeared and fixed in alcohol, and then stained with hematoxylin and eosin. The final cytopathologic finding was reported by using the TBSRTC criteria which state that one sample is adequate if it contains a minimum of 6 groups of well observed follicular cells, with at least 10 cells per group. All smears were evaluated and interpreted by experienced cytopathologists. Histopathology reports for operated cases were used to correlate cytology and final histopathology. In case of repeated preoperative FNA, only the latest cytological diagnosis was considered.
Patients with initial non-diagnostic FNA results who underwent repeat biopsy of the same thyroid nodule were also identified. The interval between initial and repeated FNAs of the same lesion was determined and results were compared. This retrospective study was approved by the Institutional Review Board of Theptarin Hospital (Certificate of Approval No. 05/2017).
Statistical analysis
Data was presented as mean (±standard deviation, SD) or median (interquartile range, IQR) for continuous data, and categorical variables were presented as proportions. Comparisons between two groups were done using an unpaired Student’s t-test for continuous data and Chi-square test for categorical data. Final histopathology was compared with initial FNA results to provide a ROM for each Bethesda category. The accuracy of FNA test to diagnose benign (Bethesda II) and malignant (Bethesda VI) lesions was expressed in four dimensions (sensitivity, specificity, positive predictive value and negative predictive value). Patients with initial non-diagnostic FNA results who underwent repeat biopsy of the same thyroid nodule were divided into an early repeated FNA (<3 months) and a late repeated FNA (≥3 months). The final FNA results of both groups were compared with Chi-square test in each Bethesda category. All statistical analyses were conducted using the Statistical Package for the Social Sciences (version 22.0; SPSS, Chicago, IL, USA). P-value ≤0.05 was considered statistically significant.
Results
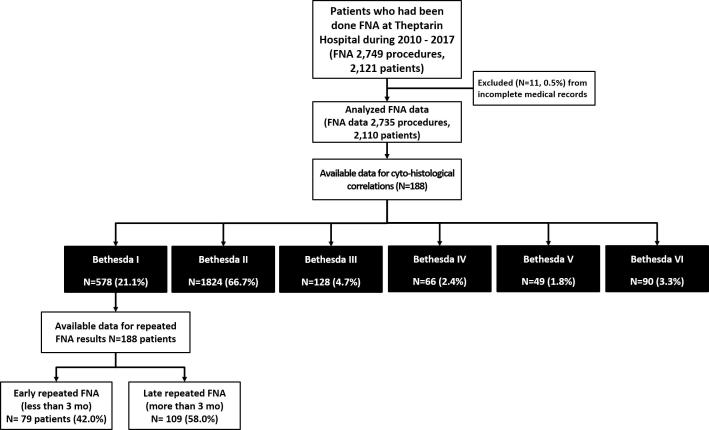
As shown in Fig. 1. 2749 FNA procedures were done at Theptarin Hospital from 2010 to 2017. A total of 2735 thyroid FNA from 2110 patients (mean age 45.7 ± 13.1 years, female 89.8%, mean size of nodules 3.2 ± 1.3 cm) were examined. Ultrasound-guided FNA was performed in only 15.3% of FNA procedures. The rate of non-diagnostic, benign, atypia of undetermined significance (AUS), follicular neoplasm, suspected for malignancy, and malignant cases was 21.1%, 66.7%, 4.7%, 2.4%, 1.8%, and 3.3% respectively. No statistical significance was found in the rate of non-diagnostic results between non-ultrasound-guided FNA versus ultrasound-guided FNA (21.3% vs. 20.3%, p-value 0.534). A total of 188 patients (9%) underwent surgical resection with available histopathology. Malignancy rate in operated thyroid nodules were 20.0%, 4.2%, 9.4%, 23.5%, 57.1%, and 90.3% for categories I to VI, respectively. Of the operated patients, 2 (1.0%) had a false-negative diagnosis (both cases had benign FNA results but turned out to be follicular carcinoma at the time of operation) and 6 (3.1%) a false-positive diagnosis. The details of cyto-histological correlations of FNA thyroid according to TBSRTC system are shown in Table 1. The diagnostic accuracy of FNA to diagnose benign (Bethesda II) and malignant (Bethesda VI) lesions revealed the sensitivity, specificity, negative predictive value, and positive predictive value and their 95% CIs were 0.97 (0.92–1.01), 0.89 (0.80–0.97), 0.96 (0.90–1.02), and 0.90 (0.83–0.98) respectively.
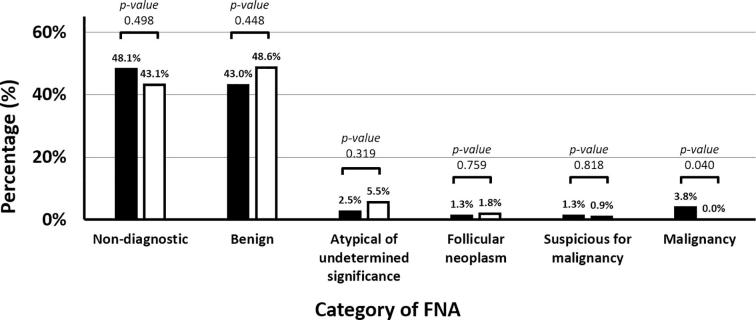
Fig. 2.
Results of early repeated FNA (black bar) compared with late repeated FNA (white bar).
In 188 patients with initial non-diagnostic results, repeated FNA were performed at the median time interval of 5 months (IQR 0–15 months). An early repeated FNA (<3 month) were done in 79 patients (42.0%). No increase in the percentage of non-diagnostic FNA results was observed in these patients when compared with late repeated FNA (≥3 months) (48.1% vs. 43.1%, p-value 0.329) but early repeated FNA revealed malignancy results in 3 cases (3.8%). Results of early repeated FNA compared with late repeated FNA according to TBSRTC system are displayed in Fig. 2. Of the operated patients (13 cases), the risk of malignancy from initial non-diagnostic FNA was found at 53.8%.
Discussions
In this retrospective study, we found that preoperative diagnosis of thyroid nodules using TBSRTC in our hospital was comparable with other studies in both previous local and international studies (summarized in Table 2). However, we noted relatively low rates of risk of malignancy in AUS category (ROM less than 10%). Another key finding from this study is that diagnostic yield of repeat FNA biopsies was not related to the time period between initial and subsequent FNA which were consistent with other studies [8], [10]. Therefore, patients with suspicious features of thyroid nodules should receive a repeat FNA as soon as needed, rather than waiting 3 months in order to rule out the presence of malignancy. In some patients with the repeated FNA revealed non-diagnostic results again, very carefully monitoring or diagnostic thyroidectomy should be offered from the high prevalence of malignancy [11].
The uniform reporting system for thyroid FNA is important to select which patients should be referred for surgery. In the past, pattern recognition was used as a method to categorize thyroid nodules into “typically benign” and “typically malignant” [12]. However, reproducible malignancy risk stratification especially intermediate categories and non-diagnostic FNA definition remained uncertain and variable between institutes until the development of TBSRTC. This system gained international acceptance and yielded dependable and reproducible risk of malignancy in each cytologic category [13], [14]. The ROM in the AUS category has been a subject of intensive research in the past decade. However, the diagnostic category of AUS is based on cytomorphologic interpretation and is relatively subjective. Therefore, TBSRTC was updated in 2018 to refine the category of atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) by sub-classifying this category regarding the presence or absence of nuclear atypia and also introduction of a new thyroid pathological diagnosis of “non-invasive follicular tumor with papillary-like nuclear features (NIFTP)” [15]. As a result of these revised items, ROM of AUS/FLUS (including NIFTP) increased from 5–15% to 10–30% but remain relatively unchanged (6–18%) if excluding NIFTP as a diagnosis of benign thyroid condition [16]. However, to date, the majority of published studies reference the original Bethesda categories. Therefore, we still relied on the original version of TBSRTC to benchmark ROM in our study.
The technique of FNA may be suction assisted with syringe or non-aspiration capillary technique or fine-needle capillary sampling (FNCS) [17]. In our experience, FNCS is practiced by only a few thyroidologists in our center and non-diagnostic rate has been slightly lower when compared with traditional suction assisted FNA technique (non-diagnostic rate 18.8% versus 22.3%). Theoretically, FNCS could avoid negative pressure from aspirations and causing less tissue damage and bleeding [18]. However, the familiarity in each technique might be more important factor than particular technique. Other influential factors include sampling error, the nature of the sampling tissues (solid or cystic lesions), adequacy criteria from reporting system, discrete decisions of pathologists, etc. In our study, the rate of non-diagnostic FNA was up to one-fifth of FNA samplings which was higher than the recommendation from experts in TBSRTC committees (non-diagnostic samples ideally should be limited to no more than 10% of thyroid FNAs) [6]. The problems of non-diagnostic FNAs are worldwide problems and ROM of this category is quite high at 16.8% from a recent meta-analysis [7]. In our study, the ROM of non-diagnostic samples was also high at 21.1%. The usual management of this category is to repeat ultrasound-guided FNA in 3–12 months depends on suspicious features from ultrasound findings [14]. However, our study was consistent with other studies that repeated FNA before 3 months did not affect outcomes of repeated FNA [8], [10], [19], [20]. Therefore, early repeated FNA should be offered to patients with worrisome features of thyroid nodules to rule out malignancy. Moreover, ultrasound guidance is strongly recommended to target suspicious area inside thyroid nodules accurately if repeated FNAs are required.
It should be noted that FNA cannot distinguish between benign and malignant follicular and Hürthle cell lesions because the demonstration of capsular and/or vascular invasion is required to diagnose non-papillary thyroid tumors [18]. From our experience in the FNA categories of “follicular neoplasm” and “suspicious for malignancy”, the ROM from both categories was lower than recommended by the original TBSRTC guidelines [6]. The detailed histological diagnosis revealed that follicular adenoma accounted for 30% in these categories. The discrepancy of ROM in the category of suspicious for malignancy might come from intrinsic incompleteness of sampling that occurs in thyroid nodules, low surgical follow-up rates in our study, or skills of cytopathologists. False-positive diagnoses (Bethesda VI but no malignancy detected in surgically removed thyroids) in our study was quite high at 9.7% when compared with previous studies (reported incidence ranges from 0% to 7.7%) [7], [13]. Interpretative errors from degenerative changes, or inexperienced cytopathologists might be possible reasons. Therefore, adequate counseling for patients regarding the range of ROM in each FNA category should be excelled and continuing internal quality control in reporting thyroid FNA according to TBSRTC should be performed to establish each institute ROM from each FNA category [21], [22].
Several limitations of this study should be noted. First, the calculated ROM based on the number of cases with available histological follow-up was quite low in our study from preferably conservative management. However, this reflects the real-world clinical practice in the era of patient-centered care. We acknowledged our limitation regarding the very low surgical follow-up results of repeated FNA nodules and risk of malignancy of non-diagnostic FNA needed to be interpreted cautiously in the context of limited surgical rate. Moreover, the false positive from early FNA need to be further studied more with the larger sample sizes of definite histological diagnosis. Second, the original TBSRTC was still used in this retrospective study which might be difficult to compare data with the recent or near-future publications that use the updated TBSRTC 2017. However, whether or not the revised ROM will be applicable to Asian patients need to be further studied because of the lower prevalence of NIFTP in the Asian series when compared with Caucasian series [23], [24]. Third, the practice of ultrasound-guided FNA in our institute had not been widely performed which might affect the relatively high rate of ROM in the non-diagnostic FNA category. Efforts should be made to reduce the rate of non-diagnostic FNA as much as possible. Forth, molecular-based testing modalities are not available in Thailand yet [25]. Therefore, the role of adjunctive molecular studies testing of FNA specimens to influence ROM could not be studied in our series.
In conclusions, preoperative diagnosis of thyroid nodules using TBSRTC in our series was comparable with other studies. The uniform diagnostic criteria of the Bethesda System help yield dependable, reproducible malignancy risk stratification and also sharing local experience with international benchmarks. All centers which practice thyroid FNAs should periodically audit the correlation between cytology and final histopathology in order to improve accuracy of FNA and communication between specialists. Moreover, diagnostic yield of repeat FNA biopsies are not related to the time period between initial and subsequent FNA. The relatively high percentage of risk of malignancy in non-diagnostic FNA is an important diagnostic issue so patients with suspicious features of thyroid nodules should receive a repeat FNA as soon as needed.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
Acknowledgments
The authors wish to thank Dr. Tinapa Himathongkam for her English editing and the staffs of Theptarin Hospital for all their supports and helps. Parts of this manuscript had previously been presented as a poster at the 18th International Endocrinology Congress 2018, Cape Town, South Africa and also at the 34th annual meeting of the Royal College of Physicians of Thailand (RCPT) 2018, Chonburi, Thailand.
Funding
No source of funding was applied in this retrospective study.
Authors’ contributions
TY,BS, and CP performed the statistical analyses, interpreted the data and drafted the manuscript. NS, PS, KS, and LN contributed to interpretation of the data and revised the manuscript critically before submission. PS, KS, and HT made substantial contributions to the discussion of results. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Ethical approval and consent to participant
This retrospective study is approved by the Ethics board committee of Theptarin Hospital (No.05/2017). No inform consent to participant was required as a retrospective study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2018.12.004.
Appendix A
Fig. 1.
Flow diagram of study.
Table 1.
The details of cyto-histological correlations of FNA thyroid according to TBSRTC system.
| n | Benign | Follicular adenoma | Hurthle cell adenoma | Papillary thyroid CA | Follicular carcinoma | Medullary CA | Anaplastic CA | |
|---|---|---|---|---|---|---|---|---|
| Non-diagnostic | 15 | 8 (53.3%) | 4 (26.7%) | 0 (0.0%) | 3 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Benign | 48 | 38 (79.2%) | 8 (16.7%) | 0 (0.0%) | 0 (0.0%) | 2 (4.2%) | 0 (0.0%) | 0 (0.0%) |
| Atypical of undetermined significance | 30 | 15 (50.0%) | 11 (36.7%) | 1 (3.3%) | 3 (10.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Follicular Neoplasm | 15 | 4 (26.7%) | 4 (26.7%) | 3 (20.0%) | 3 (20.0%) | 1 (6.7%) | 0 (0.0%) | 0 (0.0%) |
| Suspicious for malignancy | 18 | 1 (5.6%) | 6 (33.3%) | 0 (0.0%) | 9 (50.0%) | 1 (5.6%) | 1 (5.6%) | 0 (0.0%) |
| Malignant | 62 | 4 (6.5%) | 1 (1.6%) | 0 (0.0%) | 55 (88.7%) | 0 (0.0%) | 1 (1.6%) | 1 (1.6%) |
| Total | 188 | 70 (37.2%) | 34 (18.1%) | 4 (2.1%) | 73 (38.8%) | 4 (2.1%) | 2 (1.1%) | 1 (0.5%) |
Table 2.
Risk of Malignancy (ROM) per each category of the Bethesda system compared with other series.
| TBSRTC category on FNA | ROM in our study (N = 188) | Limlunjakorn et al. (2017)1 (N = 457) | Cibas et al. (2009)2 (Estimated) | Bongiovanni et al. (2012)3 (N = 6362) | Sheffield et al. 20144 (N = 8044) | Krauss et al. 20165 (N = 8214) |
|---|---|---|---|---|---|---|
| Non-diagnostic | 20% | 19% | 1–4% | 16.8% | 18.7% | 12% |
| Benign | 4% | 14% | 0–3% | 3.7% | 6.5% | 5% |
| Atypical of undetermined significance | 9% | 38% | 5–15% | 15.9% | 28.3% | 17% |
| Follicular Neoplasm | 24% | 21% | 15–30% | 26.1% | 33.1% | 25% |
| Suspicious for malignancy | 57% | 82% | 60–75% | 75.2% | 65% | 72% |
| Malignant | 90% | 94% | 97–99% | 98.6% | 98.6% | 98% |
Limlunjakorn P, et al. J Med Assoc Thai 2017;100:783–92.
Cibas ES, et al. Am J Clin Pathol 2009;132:658–65.
Bongiovanni M, et al. Acta Cytol 2012;56:333–9.
Sheffield BS, et al. Expert Rev Endocrinol Metab 2014;9:97–110.
Krauss EA, et al. Arch Pathol Lab Med 2016;140:1121–31.
Appendix B. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Burman K.D., Wartofsky L. Clinical practice. Thyroid Nodules. N Engl J Med. 2015;373:2347–2356. doi: 10.1056/NEJMcp1415786. [DOI] [PubMed] [Google Scholar]
- 2.Dean D.S., Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901–911. doi: 10.1016/j.beem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Davies L., Welch H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 4.Miller J.M., Hamburger J.I., Kini S.R. Diagnosis of thyroid nodules by fine needle aspiration and needle biopsy. JAMA. 1979;241:481–484. [PubMed] [Google Scholar]
- 5.Song J.S.A., Dmytriw A.A., Yu E., Forghani R., Rotstein L., Goldstein D. Investigation of thyroid nodules: a practical algorithm and review of guidelines. Head Neck. 2018;40:1861–1873. doi: 10.1002/hed.25160. [DOI] [PubMed] [Google Scholar]
- 6.Cibas E.S., Ali S.Z. NCI thyroid FNA state of the science conference. The bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 7.Krauss E.A., Mahon M., Fede J.M., Zhang L. Application of the Bethesda classification for thyroid fine-needle aspiration: institutional experience and meta-analysis. Arch Pathol Lab Med. 2016;140:1121–1131. doi: 10.5858/arpa.2015-0154-SA. [DOI] [PubMed] [Google Scholar]
- 8.Deniwar A., Hammad A.Y., Ali D.B., Alsaleh N., Lahlouh M., Sholl A.B. Optimal timing for a repeat fine-needle aspiration biopsy of thyroid nodule following an initial nondiagnostic fine-needle aspiration. Am J Surg. 2017;213:433–437. doi: 10.1016/j.amjsurg.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Migdal A.L., Sternberg S.B., Oshin A., Aronson M.D., Hennessey J.V. Building a quality management system for a thyroid nodule clinic. Thyroid. 2016;26:825–830. doi: 10.1089/thy.2015.0674. [DOI] [PubMed] [Google Scholar]
- 10.Lubitz C.C., Nagarkatti S.S., Faquin W.C., Samir A.E., Hassan M.C., Barbesino G. Diagnostic yield of nondiagnostic thyroid nodules is not altered by timing of repeat biopsy. Thyroid. 2012;22:590–594. doi: 10.1089/thy.2011.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coorough N., Hudak K., Jaume J.C., Buehler D., Selvaggi S., Rivas J. Nondiagnostic fine-needle aspirations of the thyroid: is the risk of malignancy higher? J Surg Res. 2013;184:746–750. doi: 10.1016/j.jss.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1996 ;15:84–9. [DOI] [PubMed]
- 13.Bongiovanni M., Spitale A., Faquin W.C., Mazzucchelli L., Baloch Z.W. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56:333–339. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 14.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cibas E.S., Ali S.Z. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27:1341–1346. doi: 10.1089/thy.2017.0500. [DOI] [PubMed] [Google Scholar]
- 16.Li W., Sciallis A., Lew M., Pang J., Jing X. Implementing noninvasive follicular thyroid neoplasm with papillary-like nuclear features may potentially impact the risk of malignancy for thyroid nodules categorized as AUS/FLUS and FN/SFN. Diagn Cytopathol. 2018;46:148–153. doi: 10.1002/dc.23866. [DOI] [PubMed] [Google Scholar]
- 17.Kamal M.M., Arjune D.G., Kulkarni H.R. Comparative study of fine needle aspiration and fine needle capillary sampling of thyroid lesions. Acta Cytol. 2002;46:30–34. doi: 10.1159/000326712. [DOI] [PubMed] [Google Scholar]
- 18.Baloch Z.W., LiVolsi V.A. Fine-needle aspiration of thyroid nodules: past, present, and future. Endocr Pract. 2004;10:234–241. doi: 10.4158/EP.10.3.234. [DOI] [PubMed] [Google Scholar]
- 19.Singh R.S., Wang H.H. Timing of repeat thyroid fine-needle aspiration in the management of thyroid nodules. Acta Cytol. 2011;55:544–548. doi: 10.1159/000334214. [DOI] [PubMed] [Google Scholar]
- 20.Lee H.Y., Baek J.H., Yoo H., Kim J.K., Lim M.K., Chu Y.C. Repeat fine-needle aspiration biopsy within a short interval does not increase the atypical cytologic results for thyroid nodules with previously nondiagnostic results. Acta Cytol. 2014;58:330–334. doi: 10.1159/000363277. [DOI] [PubMed] [Google Scholar]
- 21.Singh Ospina N., Castaneda-Guarderas A., Ward R., Brito J.P., Maraka S., Zeballos Palacios C. Patients' knowledge about the outcomes of thyroid biopsy: a patient survey. Endocrine. 2018 doi: 10.1007/s12020-018-1639-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Bychkov A., Kakudo K., Hong S.W. Current thyroid FNA practices in Asia – a missing voice. J Pathol Transl Med. 2017;51:517–520. doi: 10.4132/jptm.2017.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikiforov Y.E., Seethala R.R., Tallini G., Baloch Z.W., Basolo F., Thompson L.D. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bychkov A., Jung C.K., Liu Z., Kakudo K. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol. 2018;29:276–288. doi: 10.1007/s12022-018-9519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keelawat S., Rangdaeng S., Koonmee S., Jitpasutham T., Bychkov A. Current status of thyroid fine-needle aspiration practice in Thailand. Pathol Transl Med. 2017;51:565–570. doi: 10.4132/jptm.2017.08.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.