Highlights
-
•
Saccharomyces cerevisiae showed 76% Cu(II) removal efficiency.
-
•
The biosorption process was adjusted to the Langmuir isotherm.
-
•
The yeast presented maximum biosorption capacity of 4.73 mg g−1.
-
•
The analyzed variables influence the biosorption process.
Keywords: Biosorption, Saccharomyces cerevisiae, Potentially toxic metal
Abstract
With the industrial and population advances, the generation of effluents containing heavy metals has grown a lot. In this work, the commercial biomass of the yeast Saccharomyces cerevisiae Perlage® BB were carried out as Cu (II) ion biosorbent. The influence of some variables such as metal concentration, pH range, equilibrium time and biomass concentration were evaluated. The biosorption capacity was measured by adsorption isotherms, with the Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) models. The characterization of the biomass surface were investigated by Dispersive Energy X-Ray Fluorescence Spectrometry (EDX) and Atomic Force Microscopy (AFM). The results showed that the biomass presented good biosorption efficiency. The best fit of the data was obtained with the Langmuir model, detecting the maximum biosorption capacity of 4.73 mg g−1. By the methods used in the characterization of the biomass surface, it was possible to verify the presence of the Cu (II) ion in the yeast.
1. Introduction
Population and industrial advancement have led to increased generation of effluents and solid wastes which, if not disposed of in an environmentally sound manner, contaminate the soil, subsoil and groundwater by potentially toxic metals, such as the copper ion [1].
Copper is a chemical element that is found in nature in many different compounds and in molecular form, being found primarily in a variety of cells and tissues [2]. The oxidation state in which the copper ion is more toxic is the divalent state Cu (II). This is used in different industrial sectors, mainly in the manufacture of metal alloys and electrical equipment. The main forms of contamination of the environment by copper metal are mainly through mining, disposal of solid waste containing this metal species, worn plumbing pipes, the use of copper pans and electrical wiring materials [3,4].
Pollutions from potentially toxic metals cause serious environmental problems and human health because they are persistent and have adverse effects on the ecosystem and human health [5]. However, the lack of accurate information on copper levels in the environment makes it difficult to establish the impact on human health [6]. However, some studies have suggested that copper toxicity could induce changes in cellular activity, such as the regulation of lipid metabolism and resistance of tumor cells to chemotherapeutic drugs [2]. Therefore, the presence of inadequate amounts of copper in environments is detrimental to biological systems. Removal of excess metal from industrial effluents before they are released into the environment becomes essential for the maintenance of life [7,8].
Conventional techniques for the treatment of industrial effluents are based on physicochemical processes, the most used being electrophlotation and membrane filtration. However, these techniques have the disadvantage of high cost, making implementation difficult [9]. A technology that has demonstrated promise in the treatment of systems containing potentially toxic metals consists of biosorption, which can be done through numerous biomasses such as microorganisms, fruit skins and others [10].
Numerous studies have demonstrated that the adsorption processes involving biological adsorbents are quite promising and have low cost, good removal efficiency and environmentally correct advantages [11].
Biosorption is an adsorption process that involves the use of biomasses as adsorbents. They are called biosorbents, which have the advantage of not generating solid residues and do not produce toxic substances during the process [11].
In the biosorption process, one of the methods used does not depend on the metabolism, since the metal ions accumulate on the surface of the biosorbents materials. The efficient removal of potentially toxic metals is linked to the properties and composition of the biosorbents as well as some parameters such as pH, metal ion type, ion concentration, biosorbent concentration, solution volume, temperature And the time of contact [12].
In the literature, numerous biomasses are cited as promising for the biosorption of various metal species, such as microorganisms that have the ability to capture metal ions due to the diversity of functional groups present in their cell walls, such as carboxylic groups, hydroxyls, amines, phosphates, among others. The biosorption process may be related to multiple mechanisms, such as electrostatic interactions, complexation, ion exchange and micropecipitation [13].
The yeast Saccharomyces cerevisiae is widely used in various industries, such as breweries, wineries and bakeries [14]. Thus the biomass of this yeast is a biosorbent of low cost and good availability [13].
This study aims to assess the process biossortivo Cu (II) using a Saccharomyces cerevisiae commercially available for wine making purposes.
2. Materials and methods
2.1. Preparation of the biosorbent
The biosorption assays were performed with the yeast Saccharomyces cerevisiae originated from Perlage® BB. The strain of this yeast was isolated in Champagne territory having an excellent fermentation capacity coupled with low nutritional requirements, its alcoholic power and its cryophilic character. All biosorption assays were performed with the biomass rehydrated according to the ratio of 1, 0 g of biomass per 10 milliliters of distilled water at the maximum temperature of 28 °C, as recommended by the manufacturer.
2.2. Preparation of Cu (II)
Aqueous solutions of Cu (II) were prepared from copper sulfate (CuSO4) Vetec obtained from and distilled, deionized water for preparation of all solutions and reagents.
2.3. Biosorption assays
In these tests, the following variables were evaluated: metal concentration, pH, equilibrium time and biomass concentration. All tests were conducted in 500 ml Erlenmeyer flasks, under 200 rpm shakes at 30 °C and buffered for the pH range 5, except for the pH variation assay. The removal capacity and efficiency of the metal species under study were calculated according to Eqs. (1) and (2), respectively. Where Ci (mg L −1) is the initial and Ce concentration (mg L −1) concentration of Cu(II) ions in equilibrium in the liquid phase, m (g) the mass of the biosorbent, V (L) volume metal solution, while q (mg g −1) is the biosorption capacity and E (%) shows the biosorption efficiency.
| (1) |
| (2) |
The assays to vary Cu (II) concentration were performed with 2 g L−1 of biomass in 50 ml of metal solution at the concentrations of 25, 50, 75 and 100 mg L−1. The influence of the pH was assessed by mass of 2 g L −1 in 50 ml of metal solution at a concentration of 25 mg L −1 for 4 h contact with the pH ranges studied ranged from 3 to 6. The test for determining the biosorption equilibration time compared to Cu (II) was performed in the cellular concentration of 5 g L −1 and the presence of 10 mg L −1 of the metal solution, 6 and 24 h respectively. The study evaluated the effect of biosorbent concentrations was carried out with concentrations of 2, 8, 14 and 20 g L−1 of biomass in 50 ml of metal solution containing 25 mg L −1 of Cu(II) and 4 h contact information.
2.4. Study of adsorption isotherms
In this study, solutions were prepared with concentrations ranging from 5 to 50 mg L −1 of Cu (II) ions, buffered to pH 5 group, in biomass concentration, 5 g L-1 rehydrated by stirring at 200 RPM temperature 30 °C for 24 h contact. The supernatants were then sent for residual metal determination and analyzed according to the isotherms of Langmuir, Freundlich and Dubinin-Radushkevich.
2.5. Analytical determination of the metal
After the experiments were carried out the biomasses were separated by centrifugation for 5 min at 3000 rpm in Centrifuge Baby I 206 BL, FANEM. The supernatants were then referred for quantification of the residual metal by Atomic Absorption with Flame Spectrophotometer, Varian Spectra AA 55B at the Mineral Technology Center, CETEM.
2.6. Characterization of the biosorbent surface
For the characterization of the yeast Saccharomyces cerevisiae biomass were performed Fluorescence Spectrometry energy dispersive X - ray (EDX) and Atomic Force Microscopy (AFM). The analyzes were performed in 1,0 gs of biomass remaining in contact with metal 50 ml solution of 25 mg L−1 for 24 h, then centrifuged and washed. The analysis of a sample that remained in contact with Cu (II) ions and another Sample that had no contact with metal (white). The EDX analysis was performed on the X-ray Fluorescence Spectrophotometer by Dispersive Energy - EDX, model 720 - Shimadzu, Carried out at the Institute of Macromolecules of UFRJ, at the Laboratory of Catalysis for Polymerization (LCP). For AFM, the JPK Nanowizard Atomic Force Microscope from the METALMAT-UFRJ Surface Analysis Laboratory was used.
3. Results and discussion
3.1. Biosorption assays
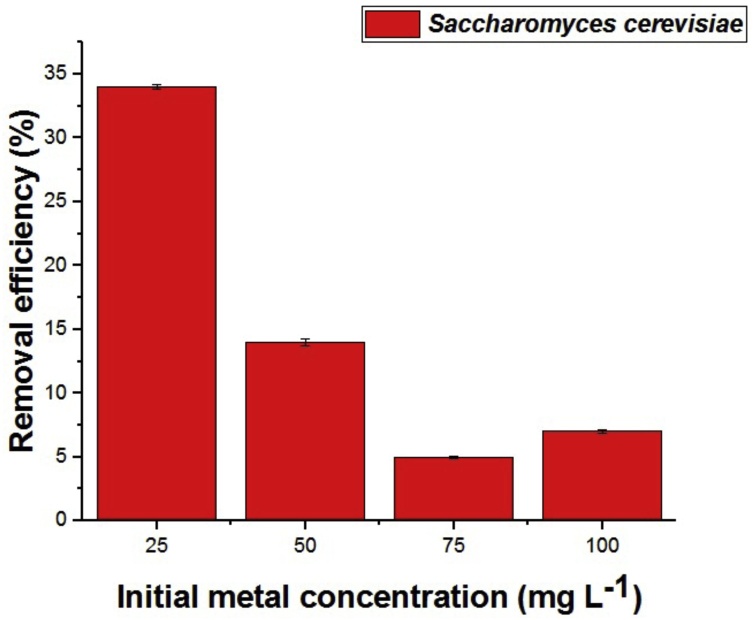
In Fig. 1 is shown biosorption efficiency, according to the amount of Cu (II) ions present in solution, relative to the biomass of Saccharomyces cerevisiae. The data indicate that biosorption, better efficiency (34%) was found at a concentration of 25 mg L −1, expressed as the metal concentration that increases there is a greater saturation of active sites biomass. Since this is confirmed by Amirnia, et al. [15] for biomass Saccharomyces cerevisiae biosorption in their studies of Cu (II) and Pb (II) using a continuous bioreactor system.
Fig. 1.
The effect of varying the initial concentrations of biomass for commercial yeast Saccharomyces cerevisiae Perlage® BB.
Farias [16] evaluated the fungus Penicillium corylophillum CCFIOC 4297 in potentially toxic metals biosorption. By changing the concentration of Ni (II) 10, 20 and 30 mg L−1 of metal removal efficiency found in the range of 5–58%.
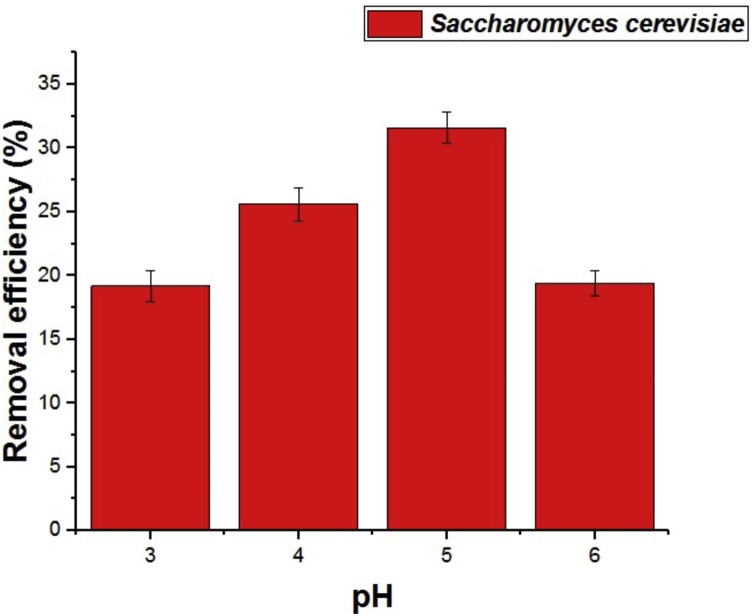
Fig. 2 shows the biosorption efficiency in relation to the pH study. The analysis of Fig. 2 reveals that the best biosorption efficiency was also found at pH 5 of approximately 32%, the drop in biosorption efficiency at pH 6 can probably be justified by the precipitation of the ion under study.
Fig. 2.
Effect of pH variation on biosorption of Cu(II) ions biomass using commercial yeast Saccharomyces cerevisiae Perlage® BB.
Sun, Yin and Yu [17] studied Al2 O3 nanoparticles in the adsorption of Cu (II) and Pb (II) and found that this adsorbent has such removal efficiency for both metal species at pH 5 ranges - 6.5, which are followed by a continuous and high removal efficiency of more than 90% up to pH 8.
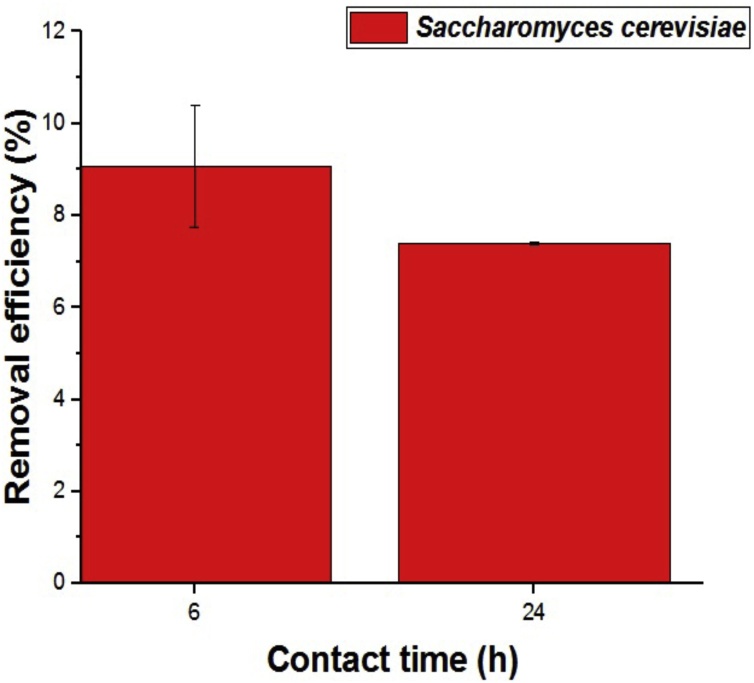
Fig. 3 shows the equilibrium study performed at the contact time band between the absorbent and the metal solution for 6 and 24 h. The results demonstrate that there was no significant variation in relation to the contact time from 6 to 24 h indicating that in 6 h the yeast biomass reaches the biosorption equilibrium.
Fig. 3.
The influence of the contact time on the biosorption of Cu(II) ions biomass using commercial yeast Saccharomyces cerevisiae Perlage® BB.
Ma et al. [18] evaluated the biosorption of Cd ions (II) and Pb(II) by Saccharomyces cerevisiae functioned by CaCO3 and showed that from 2 h contact time yeast biomass reached the biosorption balance of metallic species studied.
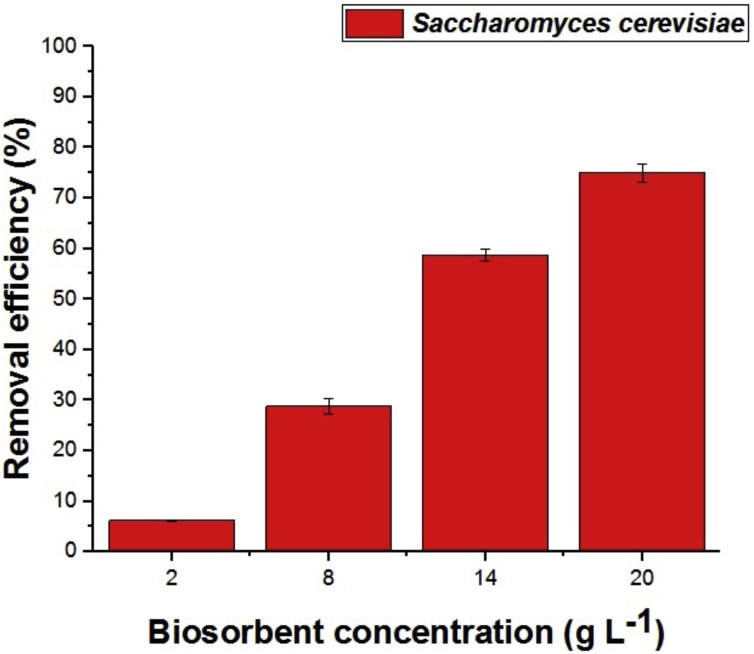
In Fig. 4 shows the results of the influence of the concentration of the biosorbent for biosorption efficiency of biomass commercial yeast Saccharomyces cerevisiae Perlage®BB. The data indicate that biosorption best efficiency was found at a concentration of 20 g L −1 and approximately 76%. Removal efficiency, increased with increasing biomass concentration, possibly due to the higher amount of uptake sites in the presence of more cells.
Fig. 4.
The influence of the concentration of the biosorbent biosorption of Cu(II) ions biomass using commercial yeast Saccharomyces cerevisiae Perlage® BB.
Buratto, Costa and Ferreira [19] studied the biomass Pleurotus ostreatus biosorption of Cu(II) and found 86% of maximum efficiency using 0.05 g biosorbent pH range 5 metal concentration of 10 mg L−1for 8 min of contact.
3.2. Adsorption isotherms
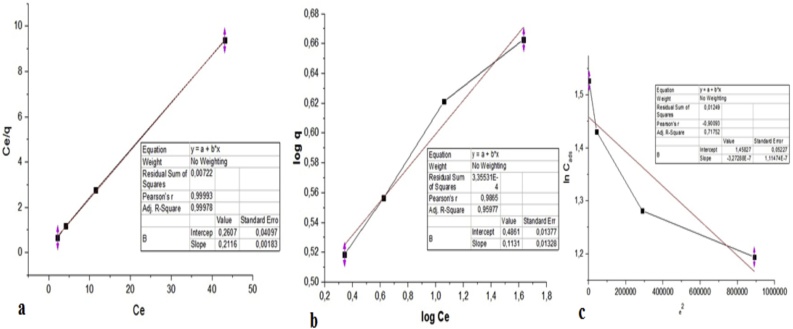
The biosorption balance Cu(II) relative to the biomass of Saccharomyces cerevisiae and the maximum biosorption capacity were modeled using Langmuir adsorption isotherms, Freundlich and Dubinin-Radushkevich (DR), Fig. 5. The data demonstrate that second correlation coefficient (R2), the Langmuir model (0.99) adjusted better to study biossortivo process, while models Freundlich (0.96) and Dubinin-Radushkevich (0.72) had coefficients lower.
Fig. 5.
Adsorption isotherm.A. Second Langmuir model; B. Freundlich and c. Dubinin-Radushkevich, (DR).
Other authors such as Amirnia, Ray and Margaritis [15] and Peng et al. [20] in his studies with Saccharomyces cerevisiae biomass also observed that the adsorption equilibrium isotherm is set to the model described by Langmuir.
In Table 1 are summarized the values found for the constant Langmuir-Freundlich and Dubinin Radushkevich. The value of RL for the Langmuir model was 0.2 indicating that for this model isotherm biossortivo the process is favorable. Clark [21] the fundamental parameter of the Langmuir model can be expressed by the constant (R) Which is related to the dimension separation factor or equilibrium parameter, where it provides for the adsorption system is favorable or unfavorable, values in the range of (0 <R L <1) indicate favorable adsorption.
Table 1.
Parameters of the Langmuir isotherm, Freundlich and Dubinin-Radushkevich for commercial biomass of the yeast Saccharomyces cerevisiae Perlage® BB.
| Langmuir | |
| Parameters | Cu (II) |
| RL | 0.2 |
| K | 4.73 |
| B | 0.81 |
| R2 | 0.99 |
| Freundlich | |
| Parameters | Cu (II) |
| KF | 3.1 |
| n | 0.12 |
| R2 | 0.96 |
| Dubinin-Radushkevich | |
| Parameters | Cu (II) |
| KDR | 0.38 |
| B | 3.27 × 10−7 |
| R2 | 0.72 |
| E | 1.24 |
Where to Langmuir isotherm: RL (Equilibrium parameter); K (maximum adsorption capacity corresponding to complete coverage of the monolayer mg g−1); b (equilibrium constant related to energy of adsorption mg g −1); for Freundlich K F (maximum adsorption capacity, mg g −1); n (energy heterogeneity of adsorption sites) and Dubinin-Radushkevich, KDR (constant related to adsorption capacity, mg g −1); B (constant energy adsorbate on the energy transfer to the adsorbent, kJ mol −1); and (adsorption energy, kJ mol −1).
The value of Freundlich constant KF related to the maximum adsorption capacity was 3.1 mg g−1 whereas for the Langmuir isotherm was 4.73 mg g-1.According to the value of KF found, the adsorptive process can be classified into small, medium, large and high. The value lodged during this study comes as small adsorptive be in the range from 0 to 24 mg g−1 [22].
According Cambuim [23] of the constant n Freundlich isotherm relates to the strength of adsorption values n constant in that range <1 indicates that the adsorption capacity is only reduced at high equilibrium concentrations. The n values found for Cu(II) lie in this range indicating that the adsorption is reduced.
Through the constant B of the Dubinin-Radushkevich isotherm model free energy is calculated in the adsorption process which can be characterized as physical or chemical adsorption. For Cu(II) ion is observed that this value was below 30 KJ Mol −1.According Paganini [24] and values in the range from 8 to 25 KJ Mol -1 are characterized as physical adsorption.
3.3. Characterization of the biosorbent surface
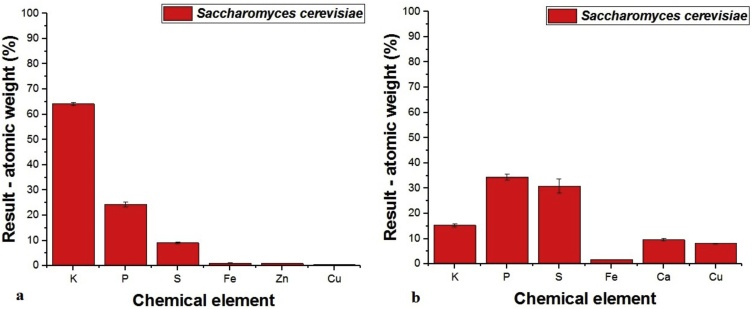
In Fig. 6 shows an elemental analysis of biomass of the yeast Saccharomyces cerevisiae Perlage ® BB through Dispersive Energy X-Ray Fluorescence Spectrometry (EDX). The results indicate that the element in greater quantity in the biomass that was not in contact with Cu(II) ions was potassium (64.1%) and only 0.57% copper was detected (Fig. 6a).
Fig. 6.
Analysis of biomass commercial yeast Saccharomyces cerevisiae Perlage ® BB EDX. A. No contact with Cu(II) ions; B. To contact with Cu(II) ions.
Limin, et al [25] evaluated the biomass of the yeast Saccharomyces cerevisiae provided by Harbin Brewing Group Co.Ltda., In the biosorption of Pb(II) ions. The EDX analysis detected that the biomass consists of the elements C, O, Na, Mg, P and K.
In Fig. 6b it is represented elemental analysis of biomass of the yeast Saccharomyces cerevisiae Perlage® BB in contact with Cu(II) by EDX. The results show that Cu (II) ions were sorbed by the biomass, since an increase in the amount of Cu(II) ions present in the biomass occurred, from 0.6% to 8.1%.
The EDX analysis shows that there was a decrease in the amount of K(I) in the biomass after contact with Cu(II) ions demonstrating that possibly ion exchange has occurred as observed by Limin, et al [25] who studied biomass provided by Saccharomyces cerevisiaeHarbin Brewing Group Co. Ltd., In the biosorption of Pb (II) ions.
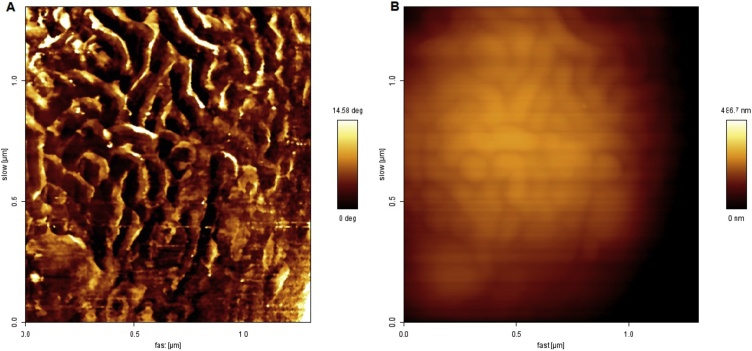
Analysis by Atomic Force Microscopy, (AFM) is a powerful tool for evaluation including biological surfaces. With (AFM) it is possible to obtain information about architecture and nanoscale structure [26].
In Fig. 7A and B is shown the atomic force micrograph of biomass commercial yeast Saccharomyces cerevisiae Perlage® BB in the absence of Cu(II) ions. Fig. 7A shows the phase contrast of the biomass surface, the analysis of this figure shows that little or no phase contrast exists on the surface of the biomass that remained without contact with Cu(II) ions. In Fig. 7B referring to the topography of the biomass surface, it is observed that the surface of the biomass appears in an apparently homogeneous form.
Fig. 7.
Atomic force micrograph of the surface of the biomass commercial yeast Saccharomyces cerevisiae Perlage ® BB in the absence of Cu(II) ions.(A) Phase contrast, image with a 1.5 μm dimension.(B) Topography, image with dimension of 1,5 μm.
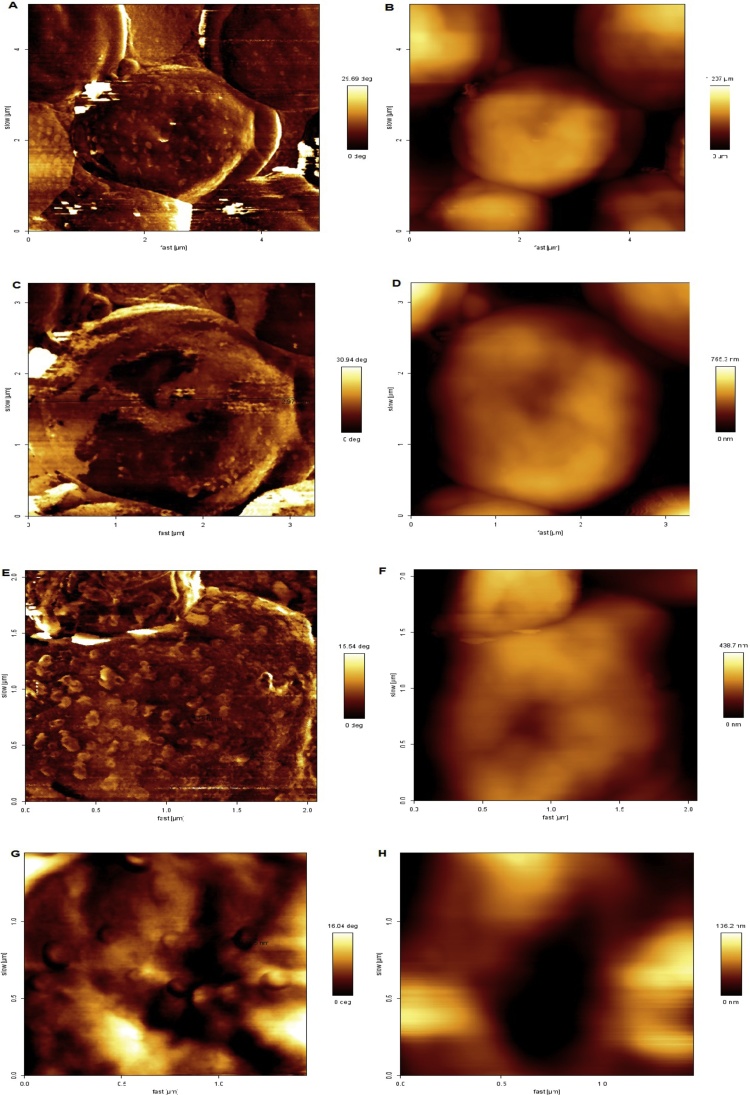
Fig. 8A to H shows the micrographs of the atomic force of the biomass in the presence of Cu(II) ions. The analysis of the images shows that in the presence of copper ions the biomass presented phase contrast, possibly indicating the presence of Cu(II) ions on the surface of the yeast biomass. The mean yeast size depicted in Fig. 8C was approximately 3 μm.
Fig. 8.
Atomic force micrograph of the surface of the biomass commercial yeast Saccharomyces cerevisiae Perlage ® BB in the presence of Cu(II) ions.(A) Phase contrast, 4 μm image size.(B) Topography, image with a dimension of 4 μm.(C) Phase contrast, an image with a 3 μm dimension.(D) Topography, image with a 3 μm dimension.(E) Phase contrast, image with dimension of 2 μm.(F) Topography, image with dimension of 2 μm.(G) Phase contrast, an image with a 1 μm dimension.(H) Topography, image with dimension of 1 μm.
As the micrographs receive cuts that reduce the size of the image (4 to 1 μm), there are spherical particles with an average diameter of 120 nm (Fig. 8G). The topography shown in Fig. 8H shows that the particles represented in Fig. 8G are made of a harder material, hence the existence of phase contrast. Therefore, the analysis of Fig. 8G and 8H possibly indicates that the particles displayed on the biomass surface are actually Cu(II) particles.
Chen, Wen and Wang [27] evaluated the surface of Saccharomyces cerevisiae before and after the biosorption ion Ag (I). Its results demonstrated that the surface of the biomass was modified after contact with Ag(I) ions showing some particles adhered to the surface with a diameter of 100 nm and ellipse format.
4. Conclusion
The results show that the commercial biomass Perlage® BB yeast Saccharomyces cerevisiae showed a good capacity and efficiency for the biosorption of potentially toxic metal Cu(II) and the variables that influence the biosorption process.
The adsorption isotherms for the study of Langmuir, Freundlich and Dubinin- Radushkevich (DR) showed that the model that set of Saccharomyces cerevisiaebiomass was Langmuir expresses a capacity of 4.73 mg g −1 biosorption. The characterization of the biomass surface by EDX and AFM showed that the Cu (II) ions were sorbed by the biomass.
Author declaration
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Acknowledgements
The present study was carried out with support of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – FinanceCode 001 and of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Contributor Information
Jéssica M. do Nascimento, Email: jessicanascimento@eq.ufrj.br.
Jorge Diniz de Oliveira, Email: jzinid@hotmail.com.
Andrea C.L. Rizzo, Email: arizzo@cetem.gov.br.
Selma G.F. Leite, Email: selma@eq.ufrj.br.
References
- 1.Akhigbe L., Ouki S., Saroj D. Disinfection and removal performance for Escherichia coli and heavy metals by silver-modified zeolite in a fixed bed column. Chem. Eng. J. 2016;295:92–98. [Google Scholar]
- 2.Gaetke L.M., Chow-Johnson H.S., Chow C.K. Copper: toxicological relevance and mechanisms. Arch. Toxicol. 2014;88:1929–1938. doi: 10.1007/s00204-014-1355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali R.M., Hamad H.A., Hussein M.M., Malash G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016;91:317–332. [Google Scholar]
- 4.AL-Mamun M., Chowdhury T., Biswas B., Absar N. Elsevier Inc.; 2018. Food Poisoning and Intoxication: A Global Leading Concern For Human Health. [Google Scholar]
- 5.Cai L.M., Wang Q.S., Luo J., Chen L.G., Zhu R.L., Wang S., Tang C.H. Heavy metal contamination and health risk assessment for children near a large Cu-smelter in central China. Sci. Total Environ. 2019;650:725–733. doi: 10.1016/j.scitotenv.2018.09.081. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J., Liang J., Hu Y., Zhang W., Liu H., You L., Zhang W., Gao M., Zhou J. Exposure risk of local residents to copper near the largest flash copper smelter in China. Sci. Total Environ. 2018;630:453–461. doi: 10.1016/j.scitotenv.2018.02.211. [DOI] [PubMed] [Google Scholar]
- 7.A.T. Fola, A.A. Idowu, A. Adetutu, Removal of Cu2+ from aqueous solution by adsorption onto quail eggshell Kinetic and isothermal studies, 5 (2016) 1–9.
- 8.Acharya J., Kumar U., Rafi P.M. Removal of heavy metal ions from wastewater by chemically modified agricultural waste material as potential adsorbent-a review. Int. J. Curr. Eng. Technol. 2018;8:526–530. [Google Scholar]
- 9.T. Cheng, C. Chen, C.H.U.I. Han, Y. Tian, Study on the Adsorption Properties of Ni (Ċ) by Linde Typr /. ee F (K) Zeolite, 07029 (2016).
- 10.Nascimento Jéssica Mdo, dos Santos Jonas Juliermerson S., de Oliveira Jorge D. Competitive biosorption of Cd(II), Pb(II) and Cr(III) using fungal biomass Pycnoporus sanguineus. J. Environ. Biotechnol. Res. 2017;6:123–127. [Google Scholar]
- 11.Costa F., Tavares T. Biosorption of nickel and cadmium in the presence of diethylketone by a Streptococcus equisimilis biofilm supported on vermiculite. Int. Biodeterior. Biodegrad. 2016;115:119–132. [Google Scholar]
- 12.Al-Homaidan A.A., Al-Houri H.J., Al-Hazzani A.A., Elgaaly G., Moubayed N.M.S. Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab. J. Chem. 2014;7:57–62. [Google Scholar]
- 13.Anagnostopoulos V.A., Vlachou A., Symeopoulos B.D. Immobilization of Saccharomyces cerevisiae on low-cost lignocellulosic substrate for the removal of Cd2+ from aquatic systems. J. Environ. Biotechnol. Res. 2015;1:23–29. [Google Scholar]
- 14.Hittinger C.T., Steele J.L., Ryder D.S. Diverse yeasts for diverse fermented beverages and foods. Curr. Opin. Biotechnol. 2018;49:199–206. doi: 10.1016/j.copbio.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Amirnia S., Ray M.B., Margaritis A. Heavy metals removal from aqueous solutions using Saccharomyces cerevisiae in a novel continuous bioreactor-biosorption system. Chem. Eng. J. 2015;264:863–872. [Google Scholar]
- 16.Farias Y.M.M. Universidade Federal do Rio de Janeiro; 2014. Biossorção De Metais Pesados Pelo Fungo Penicillium corylophilum.http://objdig.ufrj.br/61/dissert/815577.pdf [Google Scholar]
- 17.Sun W., Yin K., Yu X. Effect of natural aquatic colloids on Cu(II) and Pb(II) adsorption by Al2O3 nanoparticles. Chem. Eng. J. 2013;225:464–473. [Google Scholar]
- 18.Ma X., Cui W., Yang L., Yang Y., Chen H., Wang K. Efficient biosorption of lead(II) and cadmium(II) ions from aqueous solutions by functionalized cell with intracellular CaCO3mineral scaffolds. Bioresour. Technol. 2015;185:70–78. doi: 10.1016/j.biortech.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 19.Buratto A.P., Costa R.D., Ferreira Eda S. Application of fungal biomass of Pleurotus ostreatus in the process of biosorption of copper ions (II) Aplicação de biomassa fúngica de Pleurotus ostreatus em processo de biossorção de íons cobre (II) Eng Sanit Ambient. 2012:413–420. [Google Scholar]
- 20.Peng Q., Liu Y., Zeng G., Xu W., Yang C., Zhang J. Biosorption of copper(II) by immobilizing Saccharomyces cerevisiae on the surface of chitosan-coated magnetic nanoparticles from aqueous solution. J. Hazard. Mater. 2010;177:676–682. doi: 10.1016/j.jhazmat.2009.12.084. [DOI] [PubMed] [Google Scholar]
- 21.Luiza L.I.A., Clark M. Universidade Federal de Minas Gerais; 2010. Remoção de fenilalanina por adsorvente produzido a partir da torta prensada de grãos defeituosos de café.http://www.bibliotecadigital.ufmg.br/dspace/bitstream/handle/1843/URMR-87QMYW/dissertacaofinal.pdf?sequence=1 [Google Scholar]
- 22.Zago S., Maria E. Adsorção/Dessorção do explosivo tetril em turfa e em argissolo vermelho amarelo. Quim. Nova. 2004;27:849–854. [Google Scholar]
- 23.Cambuim K.B. Universidade Federal da Paraíba; 2009. Carvão de endocarpo de coco da baía ativado quimicamente com H3PO4 e fisicamente com vapor d’ água: produção, caracterização e aplicações. [Google Scholar]
- 24.Paganini P.P. 2007. Síntese e caracterização de trocadores iônicos inorgânicos a base de óxidos mistos estanho-titânio para utilização na recuperação de cádmio e níquel e estudos fotuluminescentes. [Google Scholar]
- 25.Ying B.Xe F.C.Z., Naili Y., Juan D., Limin D. Mechanism of Pb (II) biosorption by Saccharomyces cerevisiae. Conf. Environ. Sci. Inf. Appl. Technol. 2009:712–715. [Google Scholar]
- 26.Dorobantu L.S., Goss G.G., Burrell R.E. Atomic force microscopy: a nanoscopic view of microbial cell surfaces. Micron. 2012;43:1312–1322. doi: 10.1016/j.micron.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Chen C., Wen D., Wang J. Cellular surface characteristics of Saccharomyces cerevisiae before and after Ag(I) biosorption. Bioresour. Technol. 2014;156:380–383. doi: 10.1016/j.biortech.2014.01.065. [DOI] [PubMed] [Google Scholar]