Abstract
Objective
This study was designed to develop a nomogram for assessing the survival of patients with Ewing sarcoma (ES).
Methods
Data from patients diagnosed with ES between 2004 and 2013 were collected from the Surveillance, Epidemiology, and End Results (SEER) database. Based on patient registration, the primary cohort was divided into a training set (n = 479, data from 17 cancer registries) and a validation set (n = 137, data from 1 cancer registry). Then, the prognostic effects of variables were analyzed using Kaplan–Meier method and Cox proportional hazard model. Moreover, nomograms were established for estimating 3- and 5-year overall survival (OS) and cancer-special survival (CSS) based on Cox regression model. Last, nomogram was validated by training set and validation set.
Results
According to the multivariate analysis of training set, nomogram which combined age, race, stage, tumor site, tumor size and chemotherapy was identified. The internal bootstrap resampling approach suggested the nomogram had sufficient discriminatory power with the C-index of OS: 0.754 (95% CI, 0.705–0.802) and CSS: 0.759 (95% CI, 0.700–0.800). The calibration plots also demonstrated good consistence between the prediction and the observation.
Conclusion
Our nomogram is a reliable and powerful tool for distinguishing and predicting the survival of ES patients, thus helping to better select medical examinations and optimize treatment options in collaboration with medical oncologists and surgeons.
Keywords: Ewing sarcoma, SEER, Nomogram
1. Introduction
Ewing sarcoma (ES), first proposed by James Ewing in 1921 [1], is a small round cell highly malignant sarcoma. It is the second most common primary malignant osseous sarcoma in children and adolescents [2]. With the progress of the multi-model treatment, including chemotherapy, surgery and radiotherapy, the 5-year overall survival rate for local disease improved from approximately 10% to 55–65% [3], [4], [5], [6].
Previous studies revealed that clinically relevant prognostic factors and clinical features including age [7], [8], tumor site [8], [9], tumor size [9], [10], the use of surgery [7], [11], radiotherapy [8], [9] and chemotherapy [12] had been declared as independent prognostic factors for patients with ES. However, the survival rate is influenced by many factors, and no one single thing can accurately predict the survival of ES. As a result, to build an prognostic prediction model, it can integrate all significant prognostic factors to accurately predict the survival of ES.
Nomograph is a simple predictive tool, which has been constructed in several tumors and proven to be effective [13], [14], [15]. Convenient, in the form of the nomogram, graphical representation to allow easy and fast to get the forecast in practice. By integrating all sorts of important factors, the graph provides a personalized estimate of the probability of events, such as the incidence of individual disease or the probability of death. Thus, the nomograph has become a reliable instrument for predicting many cancer clinical results.
Clinical information of ES patients was obtained from the Surveillance, Epidemiology, and End Results (SEER) dataset between 2004 and 2013 that allowed detailed analyses of survival of ES. The SEER database comprises 18 population-based cancer registries and represents 28% of US population [16]. To our knowledge, this study was the first attempt to build a prognostic nomogram for ES based on the clinicopathological data of 616 patients, to determine whether this model provides accurate prediction of patient survival and which clinicopathological characteristics are independently associated with survival of ES patients.
2. Methods
2.1. Patient selection
All the data were collected from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER database comprises 18 population-based cancer registries and represents 28% of US population [16].
Data for patients diagnosed with ES were obtained from the SEER Program. The inclusion criteria were as follows: (1) diagnosed as ES with ICD-O-3/WHO 2008 morphology codes 9260; (2) diagnosed between 2004 and 2013; (3) primary site was selected as C400-419, described in previous study [17]; (4) complete follow-up. The exclusion criteria were as follows: (1) survival months less than one months; (2) unknown tumor size, SEER stage and race; (3) exclude multiple primary cancers.
2.2. Prognostic variables
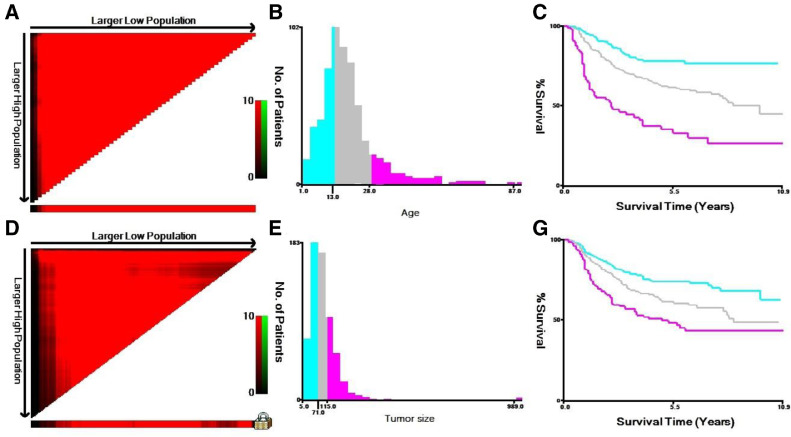
Data were assembled from the SEER program on patient age, gender, race, ethnicity, tumor stage, tumor site, tumor size, surgical use, radiotherapy, chemotherapy, and survival time. Patient age and tumor size were stratified into three groups, using the X-tile program to get the best cut-off points (Fig. 1). Then we divided tumor size into three groups, <71 mm, 71–115 mm and >115 mm. And the age was divided into three groups, <13 years, 13 to 28 years, and >28 years. Also, the SEER database did not record the exact location of the bone. Therefore, we classified patients involved “the upper and lower limbs as appendicular”, while patients coded with "the pelvis, spine, ribs, or scapula" as axial. SEER stage was categorized as localized, regional, and distant with the use of SEER Program Coding and Staging Manual [18] for bone sarcomas. Localized disease was defined if a tumor was confined to the periosteum, and regional disease was defined if a tumor extended beyond the periosteum without distant metastasis. And distant stage involved distant and further contiguous extension metastasis.
Fig. 1.
(A-F) The graphs show defining the optimal cutoff values of age and tumor size via X-tile analysis. (A, D) The black dot indicates that optimal cutoff values of age and tumor size have been identified. (B, E) A histogram and (C, F) Kaplan–Meier were constructed based on the identified cutoff values. Optimal cutoff values of age and tumor size were identified as 13 years and 28 years, 71 mm and 115 mm based on survival, respectively.
2.3. Nomogram construction and validation
We extracted the following variables into this research: age, gender, race, ethnicity, tumor stage, tumor site, tumor size, surgical use, radiotherapy and chemotherapy. These demographics and disease features of the different group of patients were compared using the chi-square test, as needed. Survival curves were applied using the Kaplan–Meier method, and the log-rank test was used to test differences between the groups. By the forward stepwise in the Cox proportional hazards regression model, all the independent risk factors were identified. Then, based on the data of the training set, a nomogram model is established. Ground on the results of the multivariate analyses, a nomogram was constructed that combined all independent prognostic factors to predict OS and CSS for 3 and 5 years. To validate the nomogram, we measured both internally (from 17 cancer registries) and externally (from 1 cancer registry) by discrimination and calibration. An index of concordance (C-index) between predicted probability and observed outcome was determined to evaluate the predictive performance. Comparisons between nomograms and SEER stage were performed using the rcorrp.cens package in Hmisc in R and were evaluated by the C-index. We constructed a calibration plot to determine whether the predicted survival is consistent with the actual survival. The best cut-off points were identifed by X-tile X-tile program (Yale University,New Haven, CT, USA). The SEER database was analyzed via SEER*Stat software (Version 8.3.5; NCI, Bethesda, USA). All statistical analyses were performed by the statistical software package SPSS for Windows, version 22.0 (IBM Corporation, Armonk, NY, USA) and the R software version 3.13 (http://www.r-project. org/). P-value of <0.05 was expected as statistically significant.
3. Results
3.1. Patient characteristics
According to the inclusion criteria, 616 patients with ES were assembled from the SEER database (2004–2013) and were assigned to the training cohort (n = 479) and the validation cohort (n = 137). Table 1 summarized the sociodemographic and clinicopathological features of patients in the training and validation cohort. There was no statistical difference between the two sets.
Table 1.
Clinicopathological variables for patients.
| Characteristic | Training cohort | Validaton cohort | Total | P |
|---|---|---|---|---|
| N= | N= | N= | ||
| No. (%) | No. (%) | No. (%) | ||
| Age | 0.990 | |||
| <13 | 132 (27.6%) | 38 (27.7%) | 170 (27.6%) | |
| 13–28 | 279 (58.2%) | 79 (57.7%) | 358 (58.1%) | |
| >28 | 68 (14.2%) | 20 (14.6%) | 88 (14.3%) | |
| Gender | 0.714 | |||
| Male | 303 (64.0%) | 89 (65.0%) | 392 (63.6%) | |
| Female | 176 (36.7%) | 48 (35.0%) | 224 (36.4%) | |
| Ethnicity | 0.781 | |||
| Spanish-Hispanic-Latino | 106 (22.1%) | 52 (38.0%) | 158 (25.6%) | |
| Non-Spanish-Hispanic-Latino | 373 (77.9%) | 85 (62.0%) | 458 (74.4%) | |
| Race | 0.901 | |||
| White | 432 (90.2%) | 122 (89.1%) | 554 (89.9%) | |
| Black | 14 (2.9%) | 4 (2.9%) | 18 (2.9%) | |
| other | 33 (6.9%) | 11 (8.0%) | 44 (7.1%) | |
| Stage | 0.083 | |||
| Localized | 143 (29.9%) | 29 (21.2%) | 172 (27.9%) | |
| Regiinal | 192 (40.1%) | 56 (40.9%) | 248 (40.3%) | |
| Distant | 144 (30.1%) | 52 (38.0%) | 192 (31.8%) | |
| Tumorsite | 0.721 | |||
| Axial | 160 (33.4%) | 48 (35.0%) | 294 (33.8%) | |
| Appendix | 319 (66.6%) | 89 (65.0%) | 322 (66.2%) | |
| Tumorsize | 0.687 | |||
| <71 | 198 (41.3%) | 51 (37.2%) | 249 (40.4%) | |
| 71–115 | 159 (33.2%) | 49 (35.8%) | 208 (33.8%) | |
| >115 | 122 (25.5%) | 37 (27.0%) | 159 (25.8%) | |
| Surgery | 0.798 | |||
| Yes | 309 (64.5%) | 90 (65.7%) | 399 (64.8%) | |
| No | 170 (35.5%) | 47 (34.3%) | 217 (35.2%) | |
| Radiotherapy | 0.481 | |||
| Yes | 122 (25.5%) | 39 (28.5%) | 161 (26.1%) | |
| No | 357 (74.5%) | 98 (71.5%) | 455 (73.9%) | |
| Chemotherapy | 0.999 | |||
| Yes | 465 (97.1%) | 133 (97.1%) | 598 (97.1%) | |
| No | 14 (2.9%) | 4 (2.9%) | 18 (2.9%) |
Other, American Indian/AK Native, Asian/Pacifc Islander; NSHL, Nonspanish-Hispanic-Latino.
3.2. Prognostic nomograms for OS and CSS
For the training cohort, 479 patients were enrolled in univariate and multivariate analyses to identify risk factors of OS and CSS. As shown in Tables 2 and 3, age, gender, race, stage, tumor site, tumor size, surgery and chemotherapy were found to be associated with OS and CSS in univariate analyses using the Kaplan–Meier method and were then compared using the log-rank test (p <0.05). To control potential confounding variables, the multivariate analysis showed that four variables were independent prognostic factors for CSS, including age, race, stage, tumor site, tumor size and chemotherapy (Table 2). Five variables were also independent predictive factors for OS, including age, race, stage, tumor size and chemotherapy (Table 3).
Table 2.
Univariate and multivariate analyses of CSS in training cohort.
| Characteristic | Univariate analysis |
Multivariate analysis |
|
|---|---|---|---|
| P | HR (95%CI) | P | |
| Age | <0.001 | ||
| <13 | Reference | ||
| 13–28 | 1.410 (0.939–2.118) | 0.097 | |
| >28 | 4.079 (2.589–6.427) | <0.001 | |
| Gender | 0.025 | ||
| Male | Reference | ||
| Female | 0.844 (0.617–1.155) | 0.288 | |
| Ethnicity | 0.832 | ||
| Spanish-Hispanic-Latino | |||
| Non-Spanish-Hispanic-Latino | |||
| Race | 0.004 | ||
| White | 2.783 (1.496–5.178) | 0.001 | |
| Black | 1.418 (0.865–2.325) | 0.166 | |
| other | |||
| Stage | <0.001 | ||
| Localized | Reference | ||
| Reginal | 1.550 (0.961–2.499) | 0.072 | |
| Distant | 3.670 (2.307–5.841) | <0.001 | |
| Tumor site | <0.001 | ||
| Axial | Reference | ||
| Appendix | 0.732 (0.537–0.996) | 0.047 | |
| Tumor size | <0.001 | ||
| <71 | |||
| 71–115 | 1.400 (0.978 −2.005) | 0.066 | |
| >115 | 1.988 (1.376–2.871) | <0.001 | |
| Surgery | <0.001 | ||
| Yes | Reference | ||
| No | 1.243 (0.905–1.708) | 0.179 | |
| Radiotherapy | <0.001 | ||
| Yes | Reference | ||
| No | 0.823 (0.610–1.110) | 0.201 | |
| Chemotherapy | <0.001 | ||
| Yes | Reference | ||
| No | 3.288 (1.797–6.016) | <0.001 | |
Other, American Indian/AK Native, Asian/Pacifc Islander; NSHL, Nonspanish-Hispanic-Latino.
Table 3.
Univariate and multivariate analyses of OS in training cohort.
| Characteristic | Univariate analysis |
Multivariate analysis |
|
|---|---|---|---|
| P | HR (95%CI) | P | |
| Age | <0.001 | ||
| <13 | Reference | ||
| 13–29 | 1.481 (0.988–2.219) | 0.057 | |
| >29 | 4.467 (2.854–6.992) | <0.001 | |
| Gender | 0.035 | ||
| Male | Reference | ||
| Female | 0.866 (0.638–1.176) | 0.357 | |
| Ethnicity | 0.980 | ||
| Spanish-Hispanic-Latino | |||
| Non-Spanish-Hispanic-Latino | |||
| Race | 0.002 | ||
| White | Reference | ||
| Black | 2.901 (1.599–5.263) | <0.001 | |
| other | 1.344 (0.821–2.200) | 0.240 | |
| Stage | <0.001 | ||
| Localized | Reference | ||
| Reginal | 1.522 (0.960–2.414) | 0.074 | |
| Distant | 3.507 (2.239–5.495) | <0.001 | |
| Tumor site | <0.001 | ||
| Axial | Reference | ||
| Appendix | 0.750 (0.555–1.015) | 0.062 | |
| Tumor size | <0.001 | ||
| <71 | Reference | ||
| 71–115 | 1.472 (1.937–2.090) | 0.030 | |
| >115 | 2.020 (1.406–2.901) | <0.001 | |
| Surgery | <0.001 | ||
| Yes | |||
| No | 1.221 (0.894–1.667) | 0.208 | |
| Radiotherapy | <0.001 | ||
| Yes | Reference | ||
| No | 0.797 (0.595–1.069) | 0.129 | |
| Chemotherapy | <0.001 | ||
| Yes | Reference | ||
| No | 3. 506 (1.960–6.272) | <0.001 | |
Other, American Indian/AK Native, Asian/Pacifc Islander; NSHL, Nonspanish-Hispanic-Latino.
3.3. Construction and validation of the nomograms
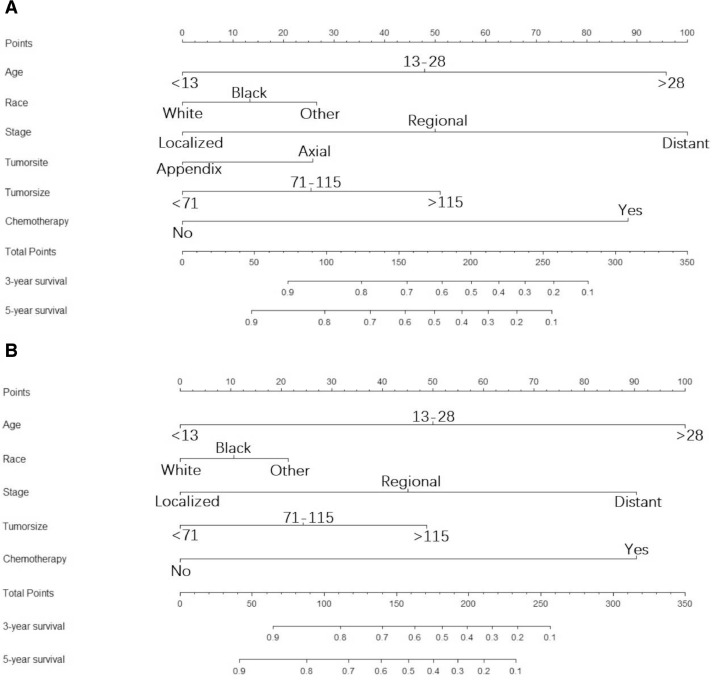
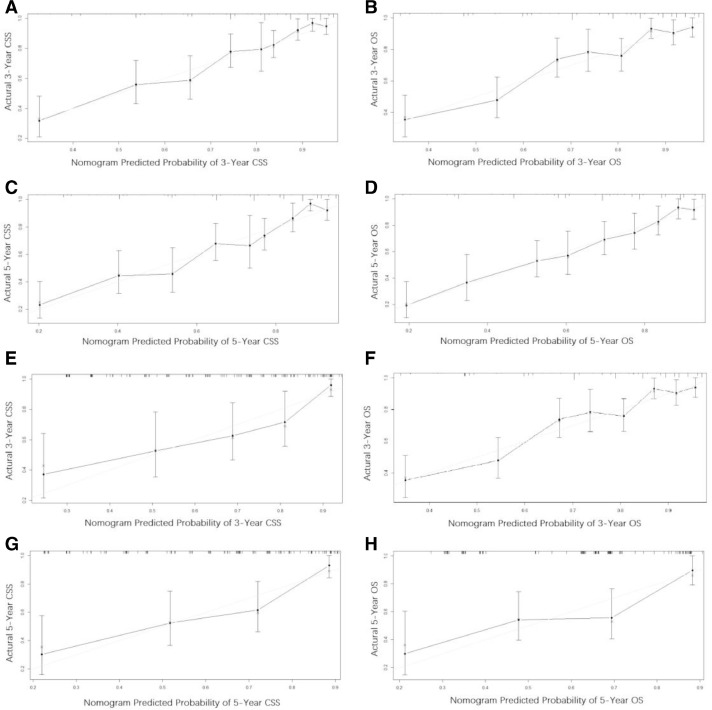
Throughout the study cohort, all independent predictors of OS and CSS were integrated into the nomogram. Age, race, stage, tumor site, tumor size and chemotherapy were included as prognostic predictors in the nomograms (Fig. 2). By adding the scores for each selected variable, the probability of patient individual survival can be easily calculated. Analysis of the internal validation cohort showed C-indices values (OS: 0.754, 95% CI, 0.705–0.802; CSS: 0.759, 95% CI, 0.700–0.820) and external validation (OS:0.715, 95% CI, 0.634–0.795; CSS: 0.731, 95% CI, 0.650–0.812). Internal and external calibration curves demonstrated prominent accordance between predicted and observed values of 3-, and 5-year OS and CSS (Fig. 3). In addition, the nomograms built in current study also revealed more powerful efficiency of discrimination for both OS and CSS prediction contrasted with the SEER stage (Table 4). As an example, a patient with ES a 5-year survival rate of 40% estimated with a nomogram with a C-index of 0.759 needs to be explained in this way; “at 5 years, you have a 40% survival rate, using a method that can tell alive versus death 75.9% of the time”.
Fig. 2.
Nomograms for predicting the 3-, and 5-year overall survival (A) and cancer-specific (B) survival of ES patients. Description using nomograms: First, each feature point of the patient is assigned by plotting a vertical line to a point scale from the variable. Then, sum all the points and draw a vertical line from the total point scale to the liver metastasis axis to obtain the probability.
Fig. 3.
(A–H) The graphs show the calibration plots for internal validation of (A) actual 3-year cancer special survival and (B) 3-year overall survival; (C) actual 5-year cancer special survival and (D) actual 5-year overall survival; and external validation of (E) actual 3-year cancer special survival and (F) 3-year overall survival; and (G) actual 5-year cancer special survival and (H) 5-year overall survival. The 45-degree line represents an ideal match between the actual survival (Y-axis) and nomogram-predicted survival (X-axis). The perpendicular line means 95% confidence intervals. Closer distances from the points to the dashed line indicate higher prediction accuracy.
Table 4.
C-indexes for the nomograms and seer stage in patients with ES.
| Survival | Training set |
Validation set |
|||||
|---|---|---|---|---|---|---|---|
| C-index | 95%CI | P | C-index | 95%CI | P | ||
| CSS | <0.001 | <0.001 | |||||
| Nomogram | 0.759 | 0.700–0.820 | 0.731 | 0.650–0.812 | |||
| SEER stage | 0.679 | 0.618–0.741 | 0.649 | 0.568–0.731 | |||
| OS | <0.001 | <0.001 | |||||
| Nomogram | 0.754 | 0.705–0.802 | 0.715 | 0.634–0.795 | |||
| SEER stage | 0.672 | 0.623–0.721 | 0.653 | 0.572–0.734 | |||
HR: hazard ratio, CI: confidence interval.
4. Discussion
The present study was undertaken to update our current knowledge about the survival of ES. We need to establish an effective prediction model that can be used as tools for individualized prediction of patient's survival outcome. Nomogram is a graph of mathematical models, and it incorporates biology and clinical variables to determine the probability of clinical events. We identified several independent prognostic factors of ES patients and built a nomogram to effectively and visually predict the OS and CSS. The nomograms which came out of a retrospective collection of data from the SEER dataset of 616 patients, showing favorable differentiation and calibration. It is noteworthy that the study population was composed of patients diagnosed with ES between 2004 and 2013 from the SEER program, not all patients from 2013 have a full 5 years. We have a shorter observation time for those patients and these subjects may or may not experience the event in that short stipulated time. Therefore, the Kaplan–Meier estimate was taken to computing the survival over time in spite of all these difficulties associated with subjects or situations [19].
Age was identified as an important survival factor for OS and CSS of patients with ES in several studies [7], [8], [11], although the precise mechanism remains unclear. This can be partly explained more prone to elderly patients with metastatic disease, and due to low tolerability accept lower doses of chemotherapy [20]. Other possible reason [7] may be that they have not been studied, such as getting care, delaying expression and level of care, and explaining why older people are worse than young people. In the present study, we indicated that patients older than 28 years have a lower OS and CSS than patients younger than 13 years old. It is noteworthy that there was no significant survival difference between patients <13 years and 13–28 years of age. Our study uses 13 and 29 years of age may be not the optimal cut-off points to find a clear association between age and survival.
In comparison to previous studies [8], [21], [22], we also have found a positive association among large tumor size, axial involvement and metastatic disease. Our multivariate analysis revealed that larger size (>115 mm) and the axial confers a poor prognosis. And a clear association between OS and primary tumor location could not be found. Leavey et al. [23] reported that poor prognosis is observed for large tumors defined by >80 mm in diameter and recurrent ES tumors are more likely to bigger size, however measured and whatever the treatment. Larger tumor size and axial primaries may often be associated with metastatic disease, both of which have proven to be risk factors for reduced survival [10]. Cotterill et al. [4] analyzed that poor prognostic factors were concluded to tumor size of 100 mL or more, axial primaries and metastasis at diagnosis, and they also draw a conclusion that pelvic primaries or larger tumors had a high association with metastatic disease. In addition, metastasis at diagnosis has been shown to have a direct impact on overall and cancer-specific mortality [24]. Indeed, Ewing sarcomas are an invasive type of tumors with high local recurrence and distant metastasis [25]. Approximately 20–32% of patients present with metastases at diagnosis that are often resistant to intensive therapy [26], [27]. The most common first metastatic site is the lung (70–80%), followed by bone (40–45%) [28]. Although the current protocol has been improved, the 5-year OS range for patients with metastatic ES is 20–35% [29], [30]. This conclusion was similar to that of our study which showed that patients with metastasis at diagnosis were more likely to have poor OS and Ewing-specific survival. At the time of diagnosis, approximately 31% of ES patients have metastatic disease.
ES is currently treated in a multidisciplinary manner involving chemotherapy, surgery, and radiotherapy. Treatment in relation with survival was evaluated by several studies which showed discordant results. Radiation therapy has been controversial, because current knowledge is based on conflicting results from observational studies [31], [32], [33]. Lee et al. [7] specifically demonstrated that patients who accept radiotherapy have a better OS compared to who don't accept. Other studies [8], [34] investigated that radiotherapy versus no radiotherapy could not confirm a clear relation between the use of radiotherapy and OS. In current study, we also could not draw a conclusion that radiotherapy was not found to be independently associated with OS or CSS. The tumor origin, site, size as well as patients’ age and sex might be possible reasons for this controversy. The other thing to consider was that the radiosensitivity of ES, which is noted based on the surrounding structures [30]. Also, the radiation therapy caused a high incidence of local recurrence [35] and an increased risk of late effects [36]. Today, radiotherapy was only advised for inoperable lesions, with a recommended dose of 54 to 55 Gy to the tumor with a 2-cm security margin [37]. Moreover, the previous studies [7], [11] found that compared with patients who did not undergo surgery, patients received surgery for local treatment have a better OS. These results were however not confirmed in two other studies [28], [38]. The relationship between surgical treatment and survival in ES patients was still inconclusive [12]. In our multivariate model, surgical intervention including excision and limb salvage procedures lost significance when compared with no surgery. For patients diagnosed with Ewing's sarcoma of bone, chemotherapy is the standard of care for initial treatment [3], [20], [39]. We found that the overall survival of the chemotherapy group increased by six months compared with the non-chemotherapy group and multivariate analysis indicated that chemotherapy was an independent prognostic factor. The actual evidence showed that in patients with Ewing's sarcoma of bone following neo-adjuvant chemotherapy has greatly improved 5-year overall survival rates from about 25% to 60%, with reported recurrence rates ranging from 15% to 30% [40], [41], [42] in patients with localized disease. Over the years, many trials address the efficacy of multiple drugs, including doxorubicin, vincristine, cyclophosphamide, ifosfamide, etoposide, and actinomycin, as well as established prognostic factors that are now used in customized therapies [30]. Granowetter et al. [43] confirmed that the addition of ifosfamide and etoposide to vincristine, doxorubicin and cyclophosphamide significantly improved event-free and overall survival in patients with nonmetastatic ES of bone. Unfortunately, it had much less effect on the survival of patients with metastases at diagnosis [38]. Therefore, chemotherapy can be recommended as the primary treatment for ES patients or as part of a multidisciplinary approach to operable patients. These findings should be taken into account in the formulation of treatment options and support the role of chemotherapy.
Based on the risk function of multivariate analysis, we established the first nomogram to predict survival in ES patients. Based on multivariate analysis, we established the first nomogram to predict the 3- and 5-year OS and CSS for ES patients. The nomogram was assessed by C index, which ranged from 0.5 to 1.0, indicating that the predicted survival rate was more consistent with the actual survival rate [13]. The C-indices for CSS and OS prediction in the training cohort were 0.759 and 0.754, respectively. C-indices for OS and CSS prediction in the validation cohort were 0.755 and 0.713, respectively. Calibration plots revealed a good agreement between prediction and observation. In addition, our nomogram displays better discrimination power for predicting OS and CSS than do the SEER stage.
The study currently has the following limitations. First, information on the serum levels of lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and carcinoembryonic antigen (CEA), which is the most widely used sarcoma marker, as well as some positive variables associated with prognoses, such as surgical margin status and detailed plan of radiotherapy and chemotherapy, was unavailable in the SEER dataset. Second, considering that the study was retrospective, some patient data was inevitably lost. This may reduce the number of qualified cases and it may lead to the risk of potential selection bias. Third,
If another model of nomograph used independent large-scale data set for external validation, our results will be more reliable; this would verify whether our results are universally applicable. Despite these limitations, our prognostic nomograms are an important and effective model for accurately predicting individual survival outcomes in patients with ES.
5. Conclusion
We comprehensively identified independent prognostic factors for ES, including age, race, seer stage, tumor size, and chemotherapy. Based on these variables, nomograms were validated as a useful tool for risk assessment and survival prediction in ES patients. We hope that our results will facilitate advances in individual treatment by quantitatively analyzing survival predictors.
Footnotes
No conflicts of interests or disclosures.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100223.
Appendix. Supplementary materials
References
- 1.Ewing J. Diffuse endothelioma of bone. CA: Cancer J. Clin. 1972;22(2):95–98. doi: 10.3322/canjclin.22.2.95. [DOI] [PubMed] [Google Scholar]
- 2.Pan Y., Lu L., Chen J., Zhong Y., Dai Z. Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER Registry from 1973 to 2014. J. Orthop. Surg. Res. 2018;13(1):76. doi: 10.1186/s13018-018-0784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacci G., Ferrari S., Bertoni F., Rimondini S., Longhi A., Bacchini P. Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J. Clin. Oncol. 2000;18(1):4. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Cotterill S., Ahrens S., Paulussen M., Jurgens H., Voute P., Gadner H. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J. Clin. Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G., Longhi A., Ferrari S., Mercuri M., Versari M., Bertoni F. Prognostic factors in non-metastatic Ewing's sarcoma tumor of bone: an analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol. 2006;45(4):469–475. doi: 10.1080/02841860500519760. [DOI] [PubMed] [Google Scholar]
- 6.Esiashvili N., Goodman M., Marcus R.B., Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J. Pediatr. Hematol. Oncol. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 7.Lee J., Hoang B.H., Ziogas A., Zell J.A. Analysis of prognostic factors in Ewing sarcoma using a population‐based cancer registry. Cancer. 2010;116(8):1964–1973. doi: 10.1002/cncr.24937. [DOI] [PubMed] [Google Scholar]
- 8.Miller B.J., Gao Y., Duchman K.R. Does surgery or radiation provide the best overall survival in Ewing's sarcoma? A review of the national cancer database. J. Surg. Oncol. 2017;116(3):384–390. doi: 10.1002/jso.24652. [DOI] [PubMed] [Google Scholar]
- 9.Biswas B., Rastogi S., Khan S., Shukla N., Deo S., Agarwala S. Developing a prognostic model for localized Ewing sarcoma family of tumors: a single institutional experience of 224 cases treated with uniform chemotherapy protocol. J. Surg. Oncol. 2015;111(6):683–689. doi: 10.1002/jso.23861. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez‐Galindo C., Liu T., Krasin M.J., Wu J., Billups C.A., Daw N.C. Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children's Research Hospital studies. Cancer. 2007;110(2):375–384. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 11.Verma V., Denniston K.A., Lin C.J., Lin C. A comparison of pediatric vs. adult patients with the Ewing sarcoma family of tumors. Front. Oncol. 2017;7:82. doi: 10.3389/fonc.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosma S., Ayu O., Fiocco M., Gelderblom H., Dijkstra P. Prognostic factors for survival in Ewing sarcoma: a systematic review. Surg. Oncol. 2018;27(4):603–610. doi: 10.1016/j.suronc.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Bianco F.J. Nomograms and medicine. Eur. Urol. 2006;50(5):884–886. doi: 10.1016/j.eururo.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Shariat S.F., Karakiewicz P.I., Suardi N., Kattan M.W. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin. Cancer Res. 2008;14(14):4400–4407. doi: 10.1158/1078-0432.CCR-07-4713. [DOI] [PubMed] [Google Scholar]
- 15.Albert J.M., Liu D.D., Shen Y., Pan I.-W., Shih Y.-C.T., Hoffman K.E. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J. Clin. Oncol. 2012;30(23):2837. doi: 10.1200/JCO.2011.41.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health NIo. National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer Fact Sheets: Thyroid Cancer, 2014. https://seer.cancer.gov/statfacts/html/thyro.html. Accessed 2014 December 17.
- 17.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz A.G., Ries L.A.G., Hurlbut A.A., Young J., Jr, Roffers S. National Cancer Institute Cancer Statistics Branch; 2001. SEER Summary Staging Manual-2000 Codes and Coding Instructions. [Google Scholar]
- 19.Goel M.K., Khanna P., Kishore J. Understanding survival analysis: Kaplan–Meier estimate. Int. J. Ayurveda Res. 2010;1(4):274. doi: 10.4103/0974-7788.76794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman D.N., Chastain K., Chou J.F., Moskowitz C.S., Adsuar R., Wexler L.H. Morbidity and mortality after treatment of Ewing sarcoma: a single‐institution experience. Pediatr. Blood Cancer. 2017;64(11):e26562. doi: 10.1002/pbc.26562. [DOI] [PubMed] [Google Scholar]
- 21.Bacci G., Ferrari S., Longhi A., Donati D., Barbieri E., Forni C. Role of surgery in local treatment of Ewing's sarcoma of the extremities in patients undergoing adjuvant and neoadjuvant chemotherapy. Oncol. Rep. 2004;11(1):111–120. [PubMed] [Google Scholar]
- 22.Albergo J., Gaston C., Laitinen M., Darbyshire A., Jeys L., Sumathi V. Ewing's sarcoma: only patients with 100% of necrosis after chemotherapy should be classified as having a good response. Bone Joint J. 2016;98(8):1138–1144. doi: 10.1302/0301-620X.98B8.37346. [DOI] [PubMed] [Google Scholar]
- 23.Leavey P.J., Mascarenhas L., Marina N., Chen Z., Krailo M., Miser J. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi‐modality therapy: a report from the Children's Oncology Group. Pediatr. Blood Cancer. 2008;51(3):334–338. doi: 10.1002/pbc.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunetto A.L., Castillo L.A., Petrilli A.S., Macedo C.D., Boldrini E., Costa C. Carboplatin in the treatment of Ewing sarcoma: results of the first Brazilian collaborative study group for Ewing Sarcoma Family Tumors—EWING1. Pediatr. Blood Cancer. 2015;62(10):1747–1753. doi: 10.1002/pbc.25562. [DOI] [PubMed] [Google Scholar]
- 25.Siegel R.D., Ryan L.M., Antman K.H. Adults with Ewing's sarcoma. An analysis of 16 patients at the Dana-Farber Cancer Institute. Am. J. Clin. Oncol. 1988;11(6):614–617. [PubMed] [Google Scholar]
- 26.Grünewald T.G., Cidre-Aranaz F., Surdez D., Tomazou E.M., de Álava E., Kovar H. Ewing sarcoma. Nat. Rev. Dis. Prim. 2018;4(1):5. doi: 10.1038/s41572-018-0003-x. [DOI] [PubMed] [Google Scholar]
- 27.Duchman K.R., Gao Y., Miller B.J. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39(2):189–195. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Arpaci E., Yetisyigit T., Seker M., Uncu D., Uyeturk U., Oksuzoglu B. Prognostic factors and clinical outcome of patients with Ewing's sarcoma family of tumors in adults: multicentric study of the Anatolian Society of Medical Oncology. Med. Oncol. 2013;30(1):469. doi: 10.1007/s12032-013-0469-z. [DOI] [PubMed] [Google Scholar]
- 29.Ahrens S., Hoffmann C., Jabar S., Braun‐Munzinger G., Paulussen M., Dunst J. Evaluation of prognostic factors in a tumor volume‐adapted treatment strategy for localized Ewing sarcoma of bone: the CESS 86 experience. Med. Pediatr. Oncol.: Off. J. SIOP—Int. Soc. Pediatr. Oncol. 1999;32(3):186–195. doi: 10.1002/(sici)1096-911x(199903)32:3<186::aid-mpo5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Gaspar N., Hawkins D.S., Dirksen U., Lewis I.J., Ferrari S., Le Deley M.-C. Ewing sarcoma: current management and future approaches through collaboration. J. Clin. Oncol. 2015;33(27):3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 31.Schuck A., Ahrens S., Paulussen M., Kuhlen M., Könemann S., Rübe C. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int. J. Radiat. Oncol. Biol. Phys. 2003;55(1):168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 32.Bacci G., Forni C., Longhi A., Ferrari S., Donati D., De Paolis M. Long-term outcome for patients with non-metastatic Ewing's sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur. J. Cancer. 2004;40(1):73–83. doi: 10.1016/j.ejca.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Shankar A., Pinkerton C., Atra A., Ashley S., Lewis I., Spooner D. Local therapy and other factors influencing site of relapse in patients with localised Ewing's sarcoma. Eur. J. Cancer. 1999;35(12):1698–1704. doi: 10.1016/s0959-8049(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 34.Obata H., Ueda T., Kawai A., Ishii T., Ozaki T., Abe S. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: the Japanese Musculoskeletal Oncology Group cooperative study. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2007;109(4):767–775. doi: 10.1002/cncr.22481. [DOI] [PubMed] [Google Scholar]
- 35.DuBois S.G., Krailo M.D., Gebhardt M.C., Donaldson S.S., Marcus K.J., Dormans J. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer. 2015;121(3):467–475. doi: 10.1002/cncr.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardes J., Von Eiff C., Streitbuerger A., Balke M., Budny T., Henrichs M.P. Reduction of periprosthetic infection with silver‐coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010;101(5):389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 37.Donaldson S.S., Torrey M., Link M.P., Glicksman A., Gilula L., Laurie F. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG# 8346. Int. J. Radiat. Oncol. Biol. Phys. 1998;42(1):125–135. doi: 10.1016/s0360-3016(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 38.Koohbanani B., Han G., Reed D., Zhao Q., Yi D., Henderson-Jackson E. Ethnicity and age disparities in Ewing sarcoma outcome. Fetal Pediatr. Pathol. 2013;32(4):246–252. doi: 10.3109/15513815.2012.721480. [DOI] [PubMed] [Google Scholar]
- 39.Phillips R.F., Higinbotham N.L. The curability of Ewing's endothelioma of bone in children. J. Pediatr. 1967;70(3):391–397. doi: 10.1016/s0022-3476(67)80136-7. [DOI] [PubMed] [Google Scholar]
- 40.Jawad M.U., Cheung M.C., Min E.S., Schneiderbauer M.M., Koniaris L.G., Scully S.P. Ewing sarcoma demonstrates racial disparities in incidence‐related and sex‐related differences in outcome: an analysis of 1631 cases from the SEER database, 1973–2005. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2009;115(15):3526–3536. doi: 10.1002/cncr.24388. [DOI] [PubMed] [Google Scholar]
- 41.Guerra J.L.L., Márquez-Vega C., Ramírez-Villar G.L., Cabrera P., Ordóñez R., Praena-Fernández J.M. Prognostic factors for overall survival in paediatric patients with Ewing sarcoma of bone treated according to multidisciplinary protocol. Clin. Transl. Oncol. 2012;14(4):294–301. doi: 10.1007/s12094-012-0798-y. [DOI] [PubMed] [Google Scholar]
- 42.Miller B.J., Lynch C.F., Buckwalter J.A. Conditional survival is greater than overall survival at diagnosis in patients with osteosarcoma and Ewing's sarcoma. Clin. Orthop. Relat. Res. 2013;471(11):3398–3404. doi: 10.1007/s11999-013-3147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granowetter L., Womer R., Devidas M., Krailo M., Wang C., Bernstein M. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J. Clin. Oncol. 2009;27(15):2536. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.