Abstract
Introduction:
The diagnosis of endogenous Cushing syndrome is often challenging and requires multiple repeated blood, urine and saliva tests to detect elevated cortisol levels. Hair cortisol concentration has been described as a marker of long-term exposure to systemic cortisol in patients with Cushing syndrome. Like hemoglobin A1c is used to detect serum glucose exposure over months, segmental hair cortisol can help identify patients with milder forms of and/or periodic or cyclical Cushing syndrome, which may reduce time and costs associated with collection of urine, salivary, and serum cortisol.
Areas covered:
Success of hair cortisol in detection of Cushing syndrome will be discussed in context of current literature, including differences between total or segmental hair cortisol in accurately determining timeline of cortisol exposure. Optimal methods of hair collection, storage, processing, and analysis and efforts towards standardization will be a major focus.
Expert commentary:
Recent evidence suggests sensitivity and specificity of hair cortisol in detecting Cushing syndrome is similar to current initial tests for hypercortisolemia. Future guidelines should consider this test as a routine part of the repertoire of screening tests for Cushing syndrome. Possible confounders to explain discrepant results in the literature will be discussed.
Keywords: Hair, Cortisol, Hair Cortisol, Cushing syndrome, Cushing disease, Hypercortisolemia
1. Introduction
The diagnosis of endogenous Cushing syndrome is often difficult and requires multiple repeated blood, urine and saliva tests to detect elevated cortisol levels [1]. Clinicians and patients find themselves frustrated with the complexity of diagnosis, given that some of the tests may have to be repeated a number of times. While some patients with Cushing syndrome have classical features of the disease (obesity, buffalo hump, stretch marks, hyperglycemia), many have fewer, transient or cycling hypercortisolemic peaks [2,3] with diagnosis achieved only after several years and consultations [4]. Given this unpredictable pattern, physicians often are not able to detect elevated cortisol levels, since most current tests (24 h urine free cortisol, 1-mg overnight or 2-mg 48 h dexamethasone suppression tests, late night salivary cortisol) are not able to provide retrospective analysis of hypercortisolism.
Hair cortisol concentration has been described as a retrospective marker of long-term exposure to systemic cortisol in patients with Cushing syndrome [5–9]. Measurement of hair cortisol may provide valuable historical calendar-like information on the development of hypercortisolism [5–7] as far back as several months prior to collection, but recent data suggest that it is best correlated with a more recent cortisol exposure [8]. The combination of objective data provided by hair cortisol levels and subjective symptoms of Cushing syndrome may help clinicians make a more definitive diagnosis prior to subjecting patients to major imaging tests and even more invasive procedures.
Hair sample collection is easy, non-invasive, and does not require special training [8]. A pencil-width selection of strands of hair from the vertex of the scalp is usually enough to provide valuable information about dynamic systemic cortisol exposure [6]. Because hair samples can be easily and conveniently collected, stored at room temperature, and sent via regular mail, hair cortisol analysis is an attractive alternative to current, less efficient methods for serum and urinary cortisol samples.
The goal of this review is to describe hair cortisol testing in evaluating Cushing syndrome, review the methodology for hair cortisol collection and processing, and to discuss areas of further research.
2. Utility of hair cortisol in patients with Cushing syndrome
Hair cortisol has been shown to correlate with the timeframe of symptoms and treatment response in patients with Cushing syndrome and identify monthly exposure to circulating cortisol. Thomson and colleagues found that nine patients with Cushing syndrome had higher hair cortisol levels than healthy controls and that hair cortisol decreased after medical or surgical intervention [5]. Similarly, Manenschijn, et al., identified separate hair cortisol values in Cushing syndrome patients and healthy controls [6,7], and like Thomson, et al., they analyzed segmental hair in relation to symptoms and interventions. Manenschijn, et al. expanded the possibility of hair cortisol to track monthly cortisol exposure more specifically to capture periodic hypercortisolemia in Cushing syndrome [6]. Further studies have confirmed that patients with Cushing syndrome had higher hair cortisol than patient controls [8,10]. Using a cutoff 31.1 pg cortisol/mg hair, reported hair cortisol performs very well (sensitivity=93% and specificity=90% for healthy controls and 91% for patient controls), similar to current biochemical testing [10]. Table 1 summarizes reported performance of hair cortisol in diagnosis of Cushing syndrome.
Table 1.
Comparison of hair cortisol performance in the evaluation for Cushing syndrome. Proximal 1 cm refers to the 1 cm segment of hair closest to the scalp, and proximal 3 cm refers to the 3 cm segment of hair closest to the scalp. All studies referenced above quantitated hair cortisol concentration using enzyme immunoassay kits, which were developed initially for salivary cortisol measurement.
| Reference | Segment of hair analyzed | Healthy controls’ HCC (pg cortisol/mg hair) | Non-healthy non-CS patients’ HCC (pg cortisol/mg hair) | CS patients’ HCC (pg cortisol/mg hair) | Cutoff value (pg cortisol/mg hair) and sensitivity and specificity of HCC in CS vs all non- CS subjects |
|---|---|---|---|---|---|
| Thomson et al [5] | Proximal 1 cm | 32 healthy subjects: Median and range = 116 (26-204) | None | 6 patients with CS*: Median and range = 679 (279-2500) |
Cutoff value:
221 Sensitivity and specificity not provided |
| Manenschijn et al. [6] | Proximal 1-3 cm | 96 healthy subjects: Average = 27.3 (95% CI: 24.6-30.4) | 73 overweight or obese patients’ HCC values not provided. | 14 patients with CS: Average = 399.7 (95% CI: 171.8-930.0) |
Cutoff value: 75.9¶ Sensitivity= 86% Specificity=98% |
| Manenschijn et al. [7] | Proximal 3 cm | 195 healthy subjects: Average = 24.27-29.44 | None | 9 patients with CS: HCC values not provided | Not provided |
| Hodes et al. [8] | Proximal 1 cm§ | None | 6 patient controls: Average and SD= 38.9 ± 25.3 | 30 patients with CS: Average and SD = 266.6 ± 738.4 | Not provided |
| Wester et al. [10] | Proximal 1-3 cm | 174 healthy controls: Geometric mean = 8.4 (95% CI: 7.0-10.0) | 35 patient controls: Geometric mean= 12.7 (95% CI: 8.6-18.6) | Geometric mean: 26 CD = 82.6 (95% CI: 53.0-128.6) 10 adrenal CS = 80.5 (95% CI: 39.2-164.3) |
Cutoff value:
31.1 Sensitivity= 93% Specificity= 90-91% |
5 patients had endogenous Cushing syndrome, and 1 patient had iatrogenic Cushing syndrome.
The parameters only apply to non-overweight healthy controls.
Segmental and overall average HCC from the most proximal 3 cm of hair were analyzed, but only the most proximal 1 cm segment showed statistically significant differences in HCC between CS and non-CS patients.
CD=Cushing disease; 95% CI=95% confidence interval; CS=Cushing syndrome; HCC=hair cortisol concentration; SD=standard deviation.
Hair cortisol has been shown to directly correlate with urine and serum testing in healthy children, pregnant, and non-pregnant subjects [11–13]. Cortisol in the proximal 1 cm hair segment correlates well with urine free cortisol (r=0.5, p=0.005) and midnight serum cortisol (r=0.4, p=0.03) [8], and proximal 3 cm hair cortisol correlated with urine free cortisol (r=0.691, p<0.001), late-night salivary cortisol (r=0.761, p<0.001), and dexamethasone suppression testing (r=0.724, p<0.001) [10].
Like hemoglobin A1c is used to detect serum glucose exposure over months, segmental hair cortisol can help identify patients with periodic or cyclical Cushing syndrome, which can reduce anxiety, time, and costs associated with the limitations of urine, salivary, and serum cortisol. Segmental hair cortisol has helped in identifying cyclical Cushing syndrome [6], tracking symptoms in patients with Cushing syndrome before and after treatment [5,6], and correlating hypercortisolemic peaks over time in two patients with ectopic Cushing syndrome [10]. A significant direct correlation has been shown between monthly cumulative cortisol exposures in each 1 cm hair segments in healthy subjects [12,13] and patients with Cushing syndrome [8,10].
3. Methodological Considerations
Numerous factors affect the concentrations of cortisol measured in hair samples. These factors can be divided into four categories: hair characteristics; hair sample collection and storage procedures; sample processing and analytical methods; and characteristics of the sampled population (e.g., age, gender, ethnicity, health and disease, and medication use). Hair cortisol levels are generally higher in black compared to blond hair [25], but hair color and differences associated with age and ethnicity were obtained from studies of healthy populations. Relevant data on hair characteristics in patients with Cushing syndrome are not currently available. Hence, the focus of this section will be on the second and third categories that involve methodological considerations in hair cortisol measurement (Figure 1). Some of the recommended procedures for hair collection and processing have been published previously [9,14], but the key points are reviewed again here with updates if appropriate.
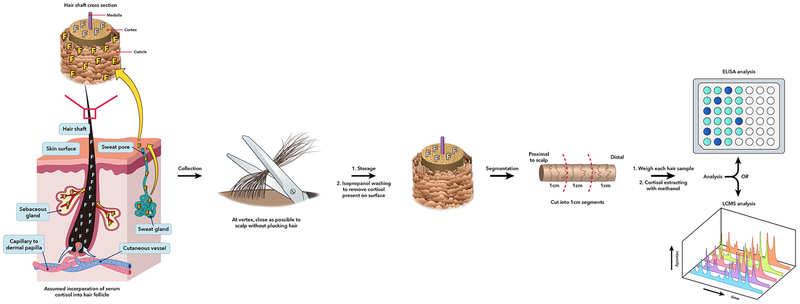
Figure 1.
Schematic of hair cortisol from collection to analysis. Systemic cortisol is currently assumed to diffuse from the dermal capillaries to the hair matrix and cortex. Cortisol can also be deposited on the hair cuticle via sweat. Hair is collected from the posterior vertex, as close to the scalp as possible, and then stored until processing. At the analyzing laboratory, hair is washed with isopropanol to remove the sweat-derived cortisol deposited on the hair cuticle. Hair is cut into 1 cm segments, weighed, minced or ground, and then incubated in methanol for cortisol extraction. Methanol solvent is separated from mixture, and then cortisol is obtained after methanol evaporation. Analysis of hair cortisol per 1 cm sample is performed with either enzyme-linked immunosorbent assay (ELISA) or liquid-chromatography/tandem mass spectroscopy (LC/MS-MS). This figure was created by Nichole Jonas and Jeremy Swanson using the program, Adobe Illustrator® CS6.
3.1. Hair collection and storage
Cortisol and other circulating analytes are assumed to be incorporated into the hair shaft during the anagen (actively growing) phase of the hair growth cycle (Figure 1). Consequently, samples for cortisol measurement should be obtained from the scalp, not from other parts of the body where hair turnover is much slower and growth ceases once the hair shaft has reached a certain length. The preferred region for sampling is the posterior vertex, the area of the scalp that shows the most consistent hair growth rate [15]. Hair should be cut as close to the scalp as possible using a clean scissors (Figure 1). It is important to avoid nicking the skin and contaminating the hair with blood, since blood cortisol concentrations are much higher than the concentrations in hair.
The length of the hair sample used for subsequent cortisol analysis is a critical factor influencing the results. Human scalp hair grows at an average rate of approximately 1 cm per month, although there is substantial individual variation [15]. Investigators generally use this figure to ascertain the period during which cortisol has been incorporated into the hair sample. Thus, the first centimeter proximal to the scalp is assumed to contain cortisol incorporated during the month prior to sampling, the second centimeter from the scalp would contain cortisol incorporated 2 months prior, and so forth. Such segmentation of hair samples into 1-cm-long pieces can, therefore, provide a retrospective calendar of cortisol incorporation during 1-month-long periods of time. However, LeBeau and colleagues [15] point out a few limitations of this approach as applied generally to segmental hair analyses. First, although (as mentioned earlier) there is significant individual variability around the mean growth rate of 1 cm per month, investigators almost never have information on the actual hair growth rate of each study participant. Second, it takes about 2 weeks for the growing hair shaft to reach the surface of the skin, in addition to which it is impossible using a scissors to cut the hair exactly at the skin’s surface without leaving a noticeable fragment of hair behind. For these reasons, the first centimeter of hair proximal to the scalp that is obtained using standard sampling methods actually contains cortisol that has been incorporated somewhat further back in time than the previous 1 month [15]. A third limitation specific to cortisol is the demonstrated reduction in hair cortisol concentrations with increasing distance from the scalp. This phenomenon was first reported by Kirschbaum and coworkers [16] and was more recently confirmed in a meta-analysis examining the factors influencing hair cortisol concentrations in humans [17].
Reduced cortisol concentrations in more distal hair segments appears to be caused by at least two factors, namely repeated exposure to water and shampoo from hair washing [18,19] and exposure to ultraviolet radiation, mainly from the sun [20]. Although hair treatments such as dying and bleaching could potentially also lead to cortisol loss, evidence for such an effect is relatively weak [17]. Loss of cortisol from the hair shaft becomes more pronounced beyond the first 3 cm from the scalp, leading to the recommendation that the most reliable results are obtained by restricting samples to no more than 3 cm in length [9,17].
Once the hair sample has been collected, it should be stored under dry and dark conditions using aluminum foil, a paper envelope, or a similar storage medium. Cortisol in hair is stable at room temperature for many months [21], but if very long (years) storage is intended, a reasonable recommendation is for samples to be stored frozen at −20°C or lower.
3.2. Hair processing
Review of the hair cortisol literature reveals numerous differences between laboratories in the details of sample processing and cortisol analysis. The most important differences in hair cortisol evaluation focus on sample washing, sample mincing or grinding, and analytical methodology. Cortisol excreted in sweat [22] and sebum has the potential to coat the outside of the hair shaft. This external cortisol, especially if deposited very recently, could interfere with the aim of measuring only cortisol that has been deposited within the interior of the shaft over a long period prior to sample collection. Therefore, hair samples should be washed carefully prior to cortisol extraction. The aim of washing is to remove most, if not all, of the external cortisol while minimally affecting cortisol bound to the interior of the hair shaft (Figure 1). Different laboratories have adopted a variety of wash solvents, including methanol, acetone, and water. Isopropanol is preferred in our laboratory and is a relatively mild solvent that, unlike methanol or water, does not cause hair swelling that could increase the risk of leaching cortisol from the interior of the shaft [9].
The second major difference in hair processing across laboratories is whether the sample is minced, ground to a powder, or used in its intact form for cortisol extraction. The Society for Hair Testing recommends that for measurement of analytes incorporated into hair, samples should either be cut into small pieces or milled to a powder [23]. The latter procedure, which may facilitate cortisol recovery by breaking up the keratin matrix and increasing the surface area for extraction is routinely used in our laboratory (Figure 1).
3.3. Hair analysis
The third key methodological factor is the analytical method selected to measure the sample’s cortisol content. Two major approaches are currently used: immunological methods, especially enzyme immunoassay (EIA), and liquid chromatography tandem mass spectrometry (LC-MS/MS) (Figure 1). EIAs have the advantage that many commercially manufactured cortisol kits are available relatively inexpensively, and that the needed equipment items (microplate reader and washer) are found in many laboratories. The disadvantages of EIA include the ability to measure only cortisol content of the sample (when a cortisol kit is being used), potential cross-reactivity of the antibody with other analytes present in the hair extract, curvilinear standard curves in most cases, and, depending on the kit, possibly insufficient sensitivity to measure extremely low levels of hair cortisol. LC-MS/MS methods have the advantage that they are highly specific compared to antibody-based methods, they can detect and quantify a panel of steroid hormones in the same extract, they produce linear standard curves over a wide concentration range, and they have high sensitivity. The disadvantages include the necessity of having access to and the expertise to manage very expensive and complex equipment, and the possible presence of matrix effects from the hair sample that either enhance or suppress the ion signal produced by the analyte(s) being quantified [24]. In pilot studies we observed a strong matrix effect in the form of ion suppression when hair extracts were analyzed for cortisol by LC-MS/MS (S. Pichette and J. Meyer, unpublished). Matrix effects can be accounted for in various ways, including sample cleanup by solid-phase extraction, but this entails an extra processing step, and it is necessary to ensure that the solid-phase extraction procedure does not produce a significant loss of analyte.
The diversity of procedures used for sample processing and cortisol analysis has made it difficult to establish a consensus reference range for human hair cortisol concentrations. Fortunately, the field is getting closer to that goal. For example, Binz and colleagues studied hair cortisol concentrations in healthy Swiss participants using an LC-MS/MS method reported a median value of 5.8 pg/mg in adults (median values are preferred over means because the distribution of values is skewed to the right) [25]. The researchers suggested a range of 4-15 pg/mg for “medium” cortisol levels, 0-4 pg/mg (0 meaning not detected in the assay) as the range for “low” levels, and >15 pg/mg representing “high” levels. Median values for toddlers (7 months to 3 years of age) and adolescents (14-17 years of age) were reported to be 10 pg/mg and 2.8 pg/mg respectively. Investigators reported a high correspondence of measured hair cortisol levels across four different European laboratories, all of which used LC-MS/MS [25]. The standard deviations for medium and high cortisol levels were all under 30%, indicating good analytical consistency. An earlier inter-laboratory comparison involving analysis of pooled hair samples by six different sites, each using either EIA or LC-MS/MS, also found high correlations between the cortisol values obtained by EIA versus LC-MS/MS; however, in this case the EIA values were consistently higher than the LC-MS/MS values [26]. Importantly, a second, currently ongoing inter-laboratory comparison in which we are participating has found much closer correspondence of hair cortisol concentrations across laboratories regardless of the analytical method (study in progress). This is an encouraging result for the field of hair cortisol analysis. Nevertheless, one should continue to exercise caution with respect to establishment and/or interpretation of hair cortisol reference ranges. Regardless of improvements in hair processing and analytical methodologies, there remains the possibility of distinct differences in the hair cortisol concentrations of healthy populations because of genetic background, living conditions, diet, and numerous other factors that have yet to be explored in a systematic way.
4. Conclusion
While rare, Cushing syndrome is associated with severe morbidity and mortality if untreated. In addition to the false positive and false negative rate associated with current initial biochemical tests, the maximum window of cortisol exposure is currently 24 hours, which may miss patients with cyclical or periodic hypercortisolemia. Hair cortisol can detect months’ worth of exposure to elevated cortisol levels and has been shown to stratify patients with and without Cushing syndrome. Hair cortisol is, to date, an experimental testing method in evaluating patients with Cushing syndrome.
5. Expert Commentary
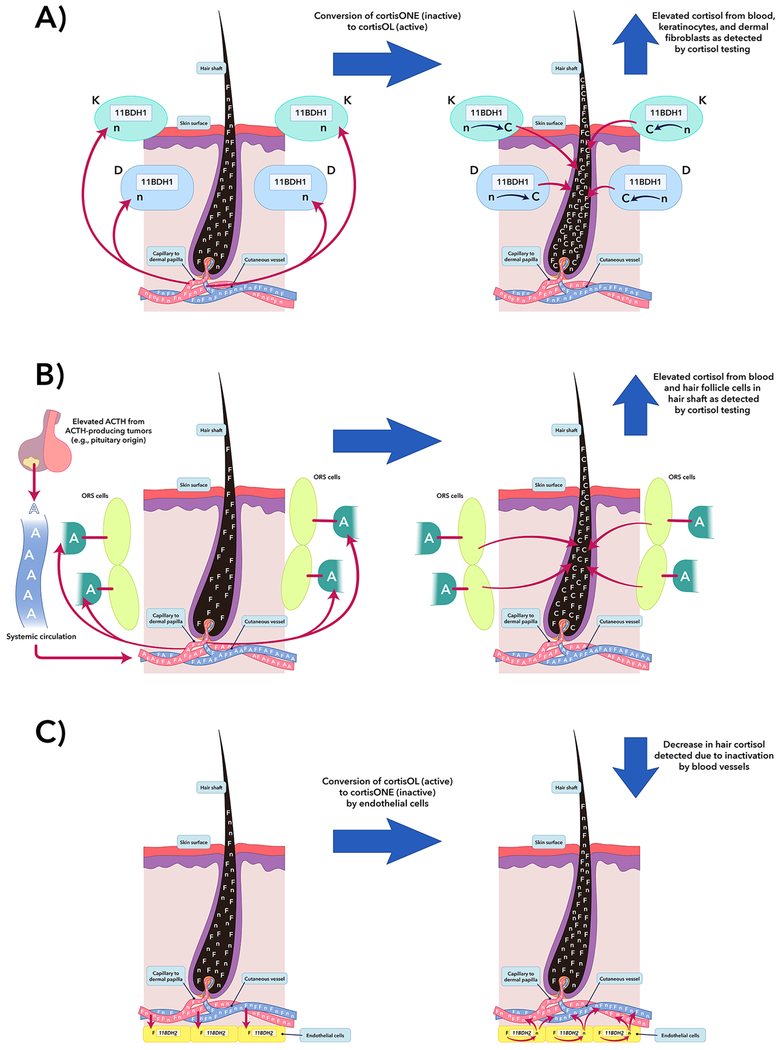
Results of Hodes and colleagues’ study on segmental hair cortisol in Cushing syndrome suggest that the current paradigm of passive diffusion of cortisol into hair follicles may be inadequate [8]. As a complex organ, skin has a two-way communication with the nervous system and endocrine glands to maintain homeostasis, serving as more than a simple physical barrier and synthesizer of vitamin D [27]. Skin and dermal appendages contain similar secretory and response elements seen in the hypothalamic-pituitary-adrenal axis [27,28], having the ability to autonomously generate glucocorticoids. Epidermal keratinocytes, dermal fibroblasts, and dermal blood vessels also can regulate local cortisol activation and deactivation via 11-beta-hydroxysteroid dehydrogenase (11BHD) types 1 and 2, respectively [29,30].
Given that the skin hypothalamic-pituitary-adrenal-like axis has elements that respond to adrenocorticotropin (ACTH) and cortisol, high serum ACTH should potentiate overall cortisol production in adrenals and skin and augment cortisol deposition in hair. Hair cortisol levels may therefore be highest in ectopic Cushing syndrome and Cushing disease. However, the relationship between hair cortisol and ACTH is limited, as seen in a subset of 20 patients with Cushing disease [8] (Figure 2).
Figure 2.
Potential confounding factors in hair cortisol analysis in Cushing syndrome patients. A) Human epidermal keratinocytes (K) and dermal fibroblasts (D) contain 11BDH type 1 and can convert cortisone to cortisol. Glucocorticoid exposure leads to increased expression of 11BDH type 1 in K and D cells [29]. Thus, K and D cells may have a role in increasing measured hair cortisol in Cushing syndrome patients. B) The outer root sheath (ORS) cells of the hair follicle in humans can synthesize cortisol under stimulation of ACTH [28]. In ACTH-dependent Cushing syndrome, it is suspected that these cells are contributing to cortisol deposited in hair matrix superimposed on systemic cortisol from elevated systemic glucocorticoid levels. In situations A and B, the measured hair cortisol concentration in final analysis should theoretically be a summation of systemically- and locally- produced cortisol. However, in a study by Hodes et al., serum ACTH and its relationships with UFC and serum midnight cortisol did not show a significant relationship [8], suggesting other confounders. C) One possible confounder that may diminish cortisol deposited in the hair matrix is dermal capillary 11BDH type 2 [30], which can convert cortisol to cortisone. This figure was modified by Nichole Jonas and Jeremy Swanson using the program, Adobe Illustrator® CS6. Reprinted by permission from SPRINGER NATURE, Endocrine, Hodes, et al., Hair cortisol in the evaluation of Cushing syndrome, 56(1):164-174, © 2017 [8]. F=systemically-produced cortisol, n=cortisone, C=locally-produced cortisol, and A=systemically-produced ACTH.
Even though ectopic Cushing syndrome exceeds adrenal Cushing syndrome and Cushing disease in serum and urinary cortisol measurements [31,32], differentiating between adrenal Cushing syndrome and Cushing disease based on hair cortisol remains a challenge. Chronic excessive systemic ACTH exposure has shown to suppress total body 11BHD type 2 activity [31], and ACTH and glucocorticoids have been shown to increase 11BHD type 1 activity and expression in epidermal and dermal cells in vitro [29]. Furthermore, 11BHD types 1 and 2 maintain homeostasis by activating cortisone to cortisol and inactivating cortisol to cortisone with cortisone concentration typically greater than cortisol in urine and hair in healthy individuals [32,33]. Therefore, evaluating the differences of hair cortisone and cortisol concentrations in patients with Cushing syndrome may help quantify the effects of systemic ACTH and cutaneous 11BHD types 1 and 2 on hair cortisol in Cushing syndrome. Further quantification of dermal ACTH production and 11BHD types 1 and 2 activities in vivo in Cushing syndrome may help explain differences seen hair cortisol measurements in ectopic and adrenal Cushing syndrome and Cushing disease and advance medical knowledge of the complex dermal HPA axis involved in health and disease.
6. Five-Year Review
Given its greater convenience for collection and fewer factors to affect measurement than current urine or salivary cortisol testing, hair cortisol measurement may be included in the evaluation for endogenous Cushing syndrome in the near future. Hair cortisol might serve a similar function in Cushing syndrome as hemoglobin A1c does in diagnosing diabetes mellitus. Further research into the hair cortisol metabolites may improve the precision of hair cortisol to differentiate between the various types of endogenous Cushing syndrome and can assist ongoing studies on skin’s cortisol metabolism.
Key Issues:
Hair cortisol has been shown to identify patients with hypercortisolemia and shows direct relationship with urine and serum cortisol.
Assuming an average hair growth rate of 1 cm per month, each 1 cm segment may represent approximately 1 month of cortisol exposure.
The most proximal 3 cm of hair should be segmented and analyzed for hair cortisol.
Collection and storage of hair is easy and convenient and can be done in any clinical setting, and analysis can be performed with LC/MS or standard cortisol EIA kits.
Shampooing and ultraviolet exposure may affect hair cortisol levels, but bleaching and dyeing hair reportedly have minimal effect.
Current standard hair cortisol levels have been identified in healthy adult and pediatric patients.
Future studies should address the effects that systemic ACTH and local 11BDH types 1 and 2 have on measured hair cortisol levels, which may help explain the difficulty in distinguishing adrenal Cushing syndrome and Cushing disease based on hair cortisol alone.
Acknowledgements
We thank Nichole Jonas and Jeremy Swan for assistance in creating and editing images for this manuscript. We thank Diane Cooper, MSLS, NIH Library, for assistance in writing this manuscript.
Funding
This research was supported in part by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), clinical trials NCT00005927 (Clinical and Molecular Analysis of ACTH-Independent Steroid Hormone Production in Adrenocortical Tissue) and NCT00001595 (A Clinical and Genetic Investigation of Pituitary Tumors and Related Hypothalamic Disorders
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Nieman LK, Biller BM, Findling JW, et al. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman TC, Ghods DE, Shahinian HK, et al. High Prevalence of Normal Tests Assessing Hypercortisolism in Subjects with Mild and Episodic Cushing’s Syndrome Suggests That the Paradigm for Diagnosis and Exclusion of Cushing’s Syndrome Requires Multiple Testing. Horm Metab Res. 2010;42(12):874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidambi S, Raff H, Findling JW. Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing’s syndrome. Eur J Endocrinol. 2007;157(6):725–731. [DOI] [PubMed] [Google Scholar]
- 4.Kreitschmann-Andermahr I, Psaras T, Tsiogka M, et al. From first symptoms to final diagnosis of Cushing’s disease: experiences of 176 patients. Eur J Endocrinol. 2015;172(6):X1. [DOI] [PubMed] [Google Scholar]
- 5.Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SHM. Hair Analysis Provides a Historical Record of Cortisol Levels in Cushing’s Syndrome. Exp Clin Endocrinol Diabetes. 2010;118(2):133–138.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manenschijn L, Koper JW, van den Akker EL, et al. A novel tool in the diagnosis and follow-up of (cyclic) Cushing’s syndrome: measurement of long-term cortisol in scalp hair. J Clin Endocrinol Metab. 2012;97(10):E1836–1843. [DOI] [PubMed] [Google Scholar]; ** This is a major study, but not the first, to show hair cortisol in detecting patients with periodic or cyclical Cushing syndrome, which can be elusive to diagnose.
- 7.Manenschijn L, Koper JW, Lamberts SWJ, Van Rossum EFC. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10–11):1032–1036. [DOI] [PubMed] [Google Scholar]
- 8.Hodes A, Lodish MB, Tirosh A, et al. Hair cortisol in the evaluation of Cushing syndrome. Endocrine. 2017;56(1):164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** First study to evaluate segmental hair cortisol in patients with Cushing syndrome and compare to urine and serum testing. Most proximal hair segment showed most significant relationship.
- 9.Meyer JS, Novak MA. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153(9):4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wester VL, Reincke M, Koper JW, et al. Scalp hair cortisol for diagnosis of Cushing’s syndrome. Eur J Endocrinol. 2017;176(6):695–703. [DOI] [PubMed] [Google Scholar]; ** Large study that evaluated hair cortisol in patients with Cushing syndrome and determine the sensitivity and specificity of hair cortisol in those patients.
- 11.D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011;104(2):348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanaelst B, Huybrechts I, Bammann K, et al. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49(8):1072–1081. [DOI] [PubMed] [Google Scholar]
- 13.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30(5):E183–E191. [DOI] [PubMed] [Google Scholar]
- 14.Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and Analysis of Cortisol from Human and Monkey Hair. J Vis Exp. 2013(83):e50882. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Detaileded description of hair cortisol collection and analysis is provided.
- 15.LeBeau MA, Montgomery MA, Brewer JD. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci Int. 2011;210:110–116. [DOI] [PubMed] [Google Scholar]; * This paper discusses the optimal approach of hair collection and the limitations of current assumed hair growth of 1 cm per month.
- 16.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–37. [DOI] [PubMed] [Google Scholar]
- 17.Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Xie Q, Gao W, et al. Time course of cortisol loss in hair segments under immersion in hot water. Clin Chim Acta. 2012;413(3-4):434–440. [DOI] [PubMed] [Google Scholar]
- 19.Xiang L, Sunesara I, Rehm KE, Marshall GD Jr., Hair Cortisol Concentrations Are Associated with Hair Growth Rate. Neuroimmunomodulation. 2016;23(5-6):287–294. [DOI] [PubMed] [Google Scholar]
- 20.Grass J, Miller R, Carlitz EH, et al. In vitro influence of light radiation on hair steroid concentrations. Psychoneuroendocrinology. 2016;73:109–116. [DOI] [PubMed] [Google Scholar]
- 21.Abell JG, Stalder T, Ferrie JE, et al. Assessing cortisol from hair samples in a large observational cohort: The Whitehall II study. Psychoneuroendocrinology. 2016;73:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grass J, Kirschbaum C, Miller R, Gao W, Steudte-Schmiedgen S, Stalder T. Sweat-inducing physiological challenges do not result in acute changes in hair cortisol concentrations. Psychoneuroendocrinology. 2015;53:108–116. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GA, Kronstrand R, Kintz P. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218(1-3):20–24. [DOI] [PubMed] [Google Scholar]
- 24.Liu HC, Lin DL, McCurdy HH. Matrix Effects in the Liquid Chromatography-Tandem Mass Spectrometry Method of Analysis. Forensic Sci Rev. 2013;25(1-2):65–78. [PubMed] [Google Scholar]
- 25.Binz TM, Rietschel L, Streit F, et al. Endogenous cortisol in keratinized matrices: Systematic determination of baseline cortisol levels in hair and the influence of sex, age and hair color. Forensic Sci Int. 2018;284:33–38. [DOI] [PubMed] [Google Scholar]
- 26.Russell E, Kirschbaum C, Laudenslager ML, et al. Toward standardization of hair cortisol measurement: results of the first international interlaboratory round robin. Ther Drug Monit. 2015;37(1):71–75. [DOI] [PubMed] [Google Scholar]
- 27.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology. 2018;159(5):1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review discusses the elements and significance of hypothalamic-pituitiary-adrenal-like axis in skin. This is an important topic of research in dermatological literature and may have implications in testing of hair cortisol.
- 28.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332–1334.* [DOI] [PubMed] [Google Scholar]
- 29.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011;131(1):30–36. [DOI] [PubMed] [Google Scholar]
- 30.Smith RE, Maguire JA, Stein-Oakley AN, et al. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues. J Clin Endocrinol Metab. 1996;81(9):3244–3248. [DOI] [PubMed] [Google Scholar]
- 31.Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617–3620. [DOI] [PubMed] [Google Scholar]
- 32.Palermo M, Shackleton CH, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11 beta-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf). 1996;45(5):605–611. [DOI] [PubMed] [Google Scholar]
- 33.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37(12):1105–1111.* [DOI] [PubMed] [Google Scholar]