Abstract
Phospholipids are an integral part of the cellular membrane structure and can be produced by a de novo biosynthetic pathway and, alternatively, by the Kennedy pathway. Studies in several yeast species have shown that the phospholipid phosphatidylserine (PS) is synthesized from CDP-diacylglycerol and serine, a route that is different from its synthesis in mammalian cells, involving a base-exchange reaction from preexisting phospholipids. Fungal-specific PS synthesis has been shown to play an important role in fungal virulence and has been proposed as an attractive drug target. However, PS synthase, which catalyzes this reaction, has not been studied in the human fungal pathogen Cryptococcus neoformans. Here, we identified and characterized the PS synthase homolog (Cn Cho1) in this fungus. Heterologous expression of Cn CHO1 in a Saccharomyces cerevisiae cho1Δ mutant rescued the mutant's growth defect in the absence of ethanolamine supplementation. Moreover, an Sc cho1Δ mutant expressing Cn CHO1 had PS synthase activity, confirming that the Cn CHO1 encodes PS synthase. We also found that PS synthase in C. neoformans is localized to the endoplasmic reticulum and that it is essential for mitochondrial function and cell viability. Of note, its deficiency could not be complemented by ethanolamine or choline supplementation for the synthesis of phosphatidylethanolamine (PE) or phosphatidylcholine (PC) via the Kennedy pathway. These findings improve our understanding of phospholipid synthesis in a pathogenic fungus and indicate that PS synthase may be a useful target for antifungal drugs.
Keywords: phosphatidylserine, phospholipid metabolism, inositol phospholipid, fungi, microbial pathogenesis, antifungal drug, Cryptococcus neoformans, mitochondria, phosphatidylserine synthase, reactive oxygen species, virulence
Introduction
Cryptococcus neoformans is a human fungal pathogen and the leading cause of life-threatening fungal meningoencephalitis, especially in immunocompromised individuals (1, 2). It is an environmental organism that infects humans via the respiratory tract to cause pulmonary cryptococcosis, followed by homogeneous dissemination to the central nervous system to cause meningitis (3). Despite its clinical significance, treatment options for invasive cryptococcosis are extremely limited. Currently, an acute infection is commonly treated with amphotericin B or azoles in combination with 5-flucytosine (4). However, both treatment courses have serious drawbacks: amphotericin B produces toxic side effects, whereas triazoles are fungistatic, which can promote the emergence of drug resistance. The main therapeutic challenge in developing new antifungal agents is that both fungi and their mammalian hosts are eukaryotes and therefore contain similar cellular machinery. Hence, identification and characterization of fungal-specific enzymes that are important for fungal survival, growth, or virulence could identify valuable potential drug targets.
Phospholipids are an integral part of eukaryotic cell membrane and are asymmetrically distributed across the bilayer. In general, phosphatidylserine (PS)2 and phosphatidylethanolamine (PE) are mostly restricted to the cytoplasmic leaflet of the membrane, whereas phosphatidylcholine (PC) and sphingolipids are mainly concentrated in the exocytoplasmic leaflet (5, 6). The lipid asymmetry creates two distinct membrane surfaces with very different adhesive properties. Among them, PS is the most abundant negatively charged phospholipid in eukaryotic membranes. Its covalent attachment of serine to the phosphate gives PS a net negative charge on the head group (7, 8). A high PS concentration in the cytoplasmic leaflet of the plasma membrane in mammals is essential for targeting and function of several intracellular signaling proteins and activation of specific kinases, such as protein kinase C (6, 9). Translocation of PS to the exocytoplasmic leaflet of the membrane could be detrimental to cell viability, because macrophages recognize cell-surface PS as a phagocytic signal to engulf cells by binding to the exposed PS (8, 10, 11). In mammals and some parasites (e.g. Trypanosoma brucei), PS is generated by exchanging serine for choline in PC and for ethanolamine in PE by the activity of PS synthases PSS1 and PSS2, respectively (12, 13). In yeast, however, PS is synthesized from CDP-diacylglycerol (CDP-DAG) and serine by the fungal-specific PS synthase enzyme Cho1 (14–16).
Thus far, the role of Cho1 has been investigated in Saccharomyces cerevisiae and Candida albicans. The S. cerevisiae cho1 mutant (Sc cho1Δ) is viable, but because it is unable to synthesize PS, which serves as a substrate for the de novo synthesis of PE and PC, the Sc cho1Δ mutant can only grow if ethanolamine or choline is exogenously supplied, enabling PE and PC synthesis via the Kennedy pathway (Fig. 1) (17). The Sc CHO1 gene expression is negatively regulated by inositol and choline availability (18). The C. albicans cho1Δ/Δ (Ca cho1Δ/Δ) mutant can only grow when supplemented with ethanolamine (15), but it cannot be rescued by choline on solid medium and displays poor growth when supplemented with choline in liquid medium. In addition, the Ca cho1Δ/Δ mutant is avirulent in a mouse model of systemic candidiasis, suggesting that the PS synthase homolog in C. albicans is necessary for virulence and therefore represents a potential new drug target (15). However, the function and importance of PS synthase in C. neoformans development and pathogenesis have not been analyzed yet.
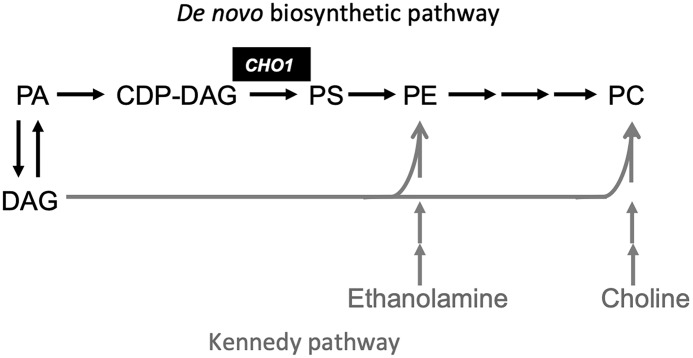
Figure 1.
Model of fungal phospholipid synthesis. In this model, PE and PC can be synthesized either by utilizing ethanolamine and choline or by converting PS into PE. PS, however, can only be synthesized from CDP-DAG and serine by using the PS synthase Cho1. PA, phosphatidate.
Our previous study revealed that C. neoformans lipid flippase, which translocates certain phospholipids (PS and PE) from the exocytoplasmic leaflet to the cytoplasmic leaflet to maintain the asymmetrical distribution of phospholipids, is essential for virulence in a murine model of cryptococcosis. It is also required for the inherent resistance of this fungus to echinocandin drugs. Mutants of the lipid flippase regulatory subunit Cdc50 showed accumulation of PS on the outer layer of the plasma membrane, were hypersensitive to macrophage killing, and showed reduced virulence (19). We hypothesized that loss of Cdc50 prevents PS translocation, leading to increased PS exposure on the cell surface, which may promote macrophage recognition and killing of such cells, because PS accumulation on the cell surface has been shown to serve as a phagocytic signal for macrophages (20). The overall reduced phospholipid level in the cdc50Δ mutant was also observed in a separate study (21). Therefore, we were interested in further exploring the role of PS in fungal development and virulence.
In this study, we characterized the only Cho1 homolog in C. neoformans (Cn Cho1) and confirmed that it functions as a PS synthase. We were unable to generate a deletion mutant of Cn CHO1, revealing that Cn Cho1 is essential for C. neoformans viability, despite the presence of an active Kennedy pathway in this fungus. We further investigated the mechanism of Cn Cho1 essentiality and revealed that PS is likely an essential phospholipid for this fungus. The essentiality of the fungal-specific PS synthase in C. neoformans warrants further investigation of this enzyme as a potential drug target.
Results
Cho1 homolog in C. neoformans is a PS synthase
Our previous study showed that deletion of the regulatory subunit of the lipid flippase Cdc50 leads to the accumulation of PS on the outer leaflet of the bilayer membrane in Cryptococcus, which appears to contribute to the loss of fungal virulence (19). Therefore, we tried to generate a mutant strain lacking PS to better understand the role of PS in fungal virulence. This led us to identify the only homolog of the S. cerevisiae PS synthase Cho1 protein.
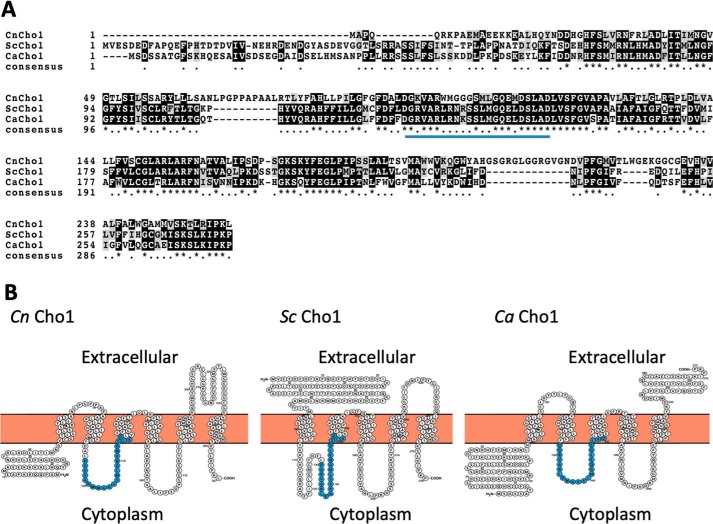
The C. neoformans Cho1 (Cn Cho1) protein shares high amino acid sequence identity with the known PS synthases in S. cerevisiae (Sc Cho1) and C. albicans (Ca Cho1). All of them share the conserved sequence of CDP-diacylglycerol–serine O-phosphatidyltransferase (DGX2ARX7,8GX3DX3D). Protein secondary structure analysis also indicated that all three are integral membrane proteins with multiple transmembrane domains, albeit with some differences in the number and the topology of potential transmembrane regions (Fig. 2).
Figure 2.
Sequence analysis for Cn Cho1, Sc Cho1, and Ca Cho1. A, sequence alignment of Cho1 homologs from C. neoformans (Cn Cho1), S. cerevisiae (Sc Cho1), and C. albicans (Ca Cho1) using ClustalW. B, protein secondary structural prediction of Cn Cho1 based on HMMTOP2.0 suggests it has six transmembrane domains. The predicted enzyme activation sites are highlighted in blue.
To confirm the localization of Cn Cho1 protein in C. neoformans, we generated a Cn Cho1:mCherry fusion construct and expressed this fusion protein in C. neoformans WT H99. Our results showed that the fluorescent signal mostly localized to the ER membrane (Fig. 3A and Fig. S1). We then purified the membrane fraction by centrifugation and treated it with either PBS, Triton X-100, or 1 m NaCl to determine whether Cn Cho1 is an integral membrane protein or a membrane-binding protein (Fig. 3B). Our results clearly showed that Cn Cho1 was solubilized by Triton X-100, but not by high salt, indicating that it is an integral membrane protein.
Figure 3.
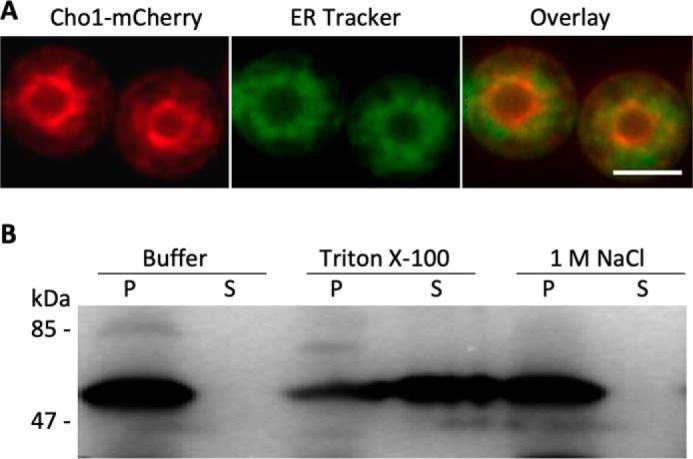
Cn Cho1 is an integral membrane protein that primarily localizes to the endoplasmic reticulum. A, Cho1 protein in C. neoformans localizes to the ER membrane in a similar manner to Cho1 from other yeasts. In this strain, the CHO1 gene is fused with an mCherry tag at its C terminus, and its expression is under the control of the C. neoformans actin promoter. ER tracker was used to visualize the ER membrane. Among over 100 cells analyzed, ∼99% showed co-localization of Cho1 protein with the ER tracker. Additional data were shown in Fig. S1. Scale bar, 10 μm. B, membrane protein separation assay confirms that Cho1 is an integral membrane protein. As described under “Experimental procedures,” a total membrane fraction obtained from cells expressing Cho1 was extracted with 1× PBS buffer, 1% Triton X-100, or salt (1 m NaCl). Following ultracentrifugation, equivalent amounts of the insoluble pellet (P) and the supernatant (S) were analyzed by Western blotting using RFP-tag antibody.
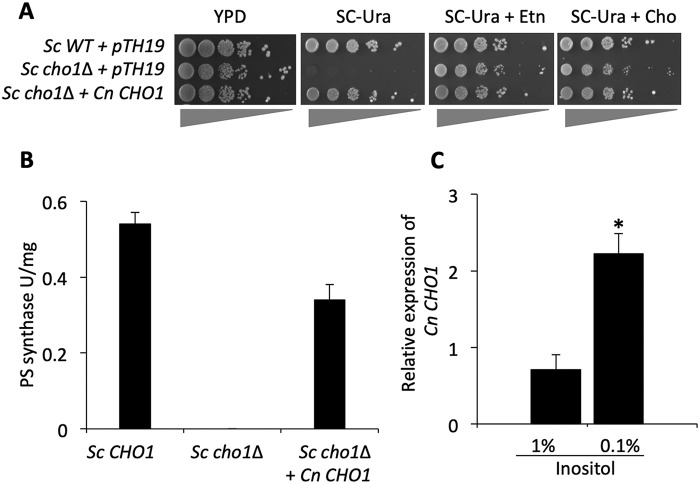
To confirm the Cn Cho1 protein is a PS synthase, we expressed Cn CHO1 gene in a S. cerevisiae strain carrying a deletion of Sc CHO1 (Sc cho1Δ). As expected, the Sc cho1Δ mutant was an ethanolamine auxotroph and could not grow on SC medium lacking ethanolamine (Fig. 4A). We observed that heterologous expression of Cn CHO1 in this mutant background fully complemented its growth defect, indicating that Cn CHO1 is likely a PS synthase (Fig. 4A). We then measured the PS synthase activity in cell lysates directly using [3-3H]serine as described previously (16, 22, 23). Our results showed that the Sc cho1Δ mutant completely lacked PS synthase activity and that expressing Cn CHO1 in this mutant largely restored its PS synthase activity (Fig. 4B), supporting the conclusion that Cn Cho1 has PS synthase activity.
Figure 4.
C. neoformans Cho1 is a PS synthase, and its expression is regulated by inositol. A, expression of Cn CHO1 gene fully complements S. cerevisiae (Sc) cho1Δ mutant auxotrophic for ethanolamine (Etn). WT S. cerevisiae can grow well in the absence of ethanolamine unlike the Sc cho1Δ mutant transformed with pTH19 (empty vector), which is ethanolamine auxotrophic. Sc cho1Δ expressing Cn CHO1 restores growth in the absence of ethanolamine, indicating that C. neoformans CHO1 gene can fully complement Sc CHO1 gene function. B, enzymatic analysis of Saccharomyces strains for PS synthase activity. Cho1 enzyme activity in WT S. cerevisiae was absent in Sc cho1Δ strain. This activity was successfully restored when the Sc cho1Δ strain was transformed with Cn CHO1 gene indicating that it is a PS synthase. The assay was performed in duplicate and repeated twice. C, Cn CHO1 gene expression is regulated by inositol. CHO1 gene expression is suppressed in the presence of 1% inositol as compared with its expression in the presence of 0.1% inositol indicating that phospholipid precursor inositol negatively regulates CHO1 gene expression. The analysis of CHO1 gene expression was performed in triplicate, and the two-tail t test was used for statistical analysis (* indicates p < 0.001).
In S. cerevisiae, extracellular inositol negatively regulates Sc CHO1 expression (18). Thus, we examined Cn CHO1 gene expression in the presence of various inositol concentrations to determine whether it is also regulated by inositol. Indeed, our results indicated that Cn CHO1 transcription is repressed by a high level of inositol and induced or derepressed as the inositol level decreases (Fig. 4C). Although we have not been able to identify C. neoformans homologs of S. cerevisiae inositol-dependent transcriptional regulators, our results indicate that the Cn CHO1 gene is regulated by inositol similarly to Sc CHO1.
CHO1 is an essential gene in C. neoformans
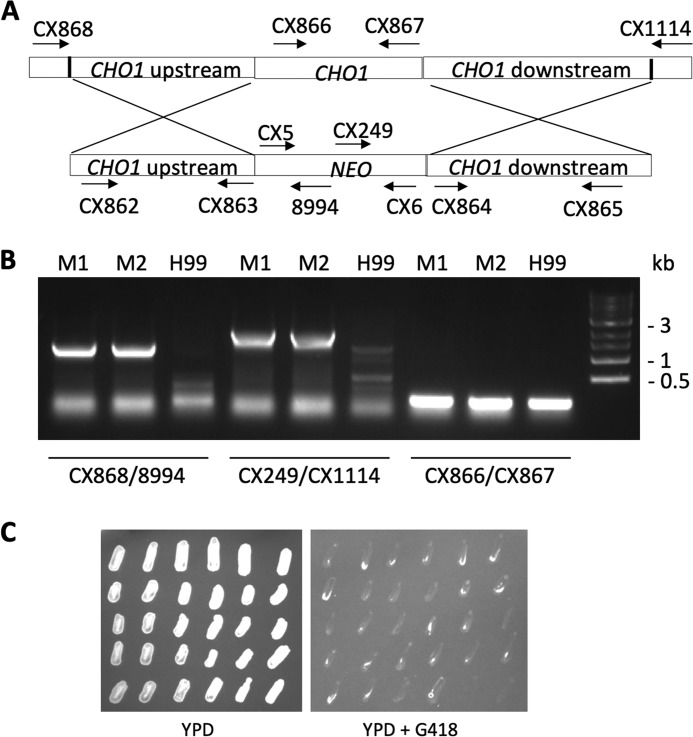
To examine the role of PS synthase in C. neoformans, we attempted to generate a deletion mutant for Cn CHO1 in the H99 background, but were unsuccessful despite the extensive effort of screening over 200 transformants (data not shown). We then deleted one allele of Cn CHO1 from a diploid C. neoformans strain AI187 (24), replacing it with a neomycin (NEO) resistance cassette (Fig. 5A). The heterozygous Cn CHO1/cho1Δ strain was viable and grew normally on YPD. We incubated the strain on a V8 mating medium to induce mating and sporulation, dissected over 20 spores, and patched them on YPD with or without G418. All of the tested spores grew on YPD, but none of them grew on YPD with G418, suggesting that all viable spores contain the WT Cn CHO1 allele (Fig. 5C) and that Cn Cho1 is likely essential for fungal viability. We also repeated the experiment by supplementing the medium with 1 mm ethanolamine or 1 mm choline, but we still did not obtain any viable haploid Cn cho1Δ strains (data not shown).
Figure 5.
Cn Cho1 is an essential protein for fungal viability. A Cn CHO1/cho1Δ heterozygous mutant was generated by deleting one Cn CHO1 allele in a diploid strain AI187 and undergoing sporulation on mating medium. Spores were isolated and grown on YPD or YPD + G418. Among the 28 spores isolated, none of them grew on YPD containing G418, indicating that they are all WT alleles. A, schematic of Cn CHO1 deletion strategy. CX numbers indicate primers used to screen the mutants. Detailed description of each primer can be found in Table S1. B, PCR results of the mutant screen. M1, mutant 1; M2, mutant 2. C, growth of progeny colonies on YPD and YPD containing G418 after 3 days of incubation.
To better understand the potential essentiality of Cn Cho1, we used homologous recombination to replace the Cn CHO1 native promoter with an inducible CTR4 promoter (PCTR4-CHO1). The activity of the CTR4 promoter can be suppressed by the addition of CuSO4 and ascorbic acid and induced by the addition of the copper chelator bathocuproinedisulfonic acid (BCS) (25). First, we tested cell growth by adding 25 μm CuSO4 that has been shown to significantly suppress the CTR4 promoter in previous studies (25, 26). However, we did not observe a significant growth defect between WT and the PCTR4-CHO1 strain (Fig. S3). By testing the growth assay using media containing different CuSO4 concentrations, we found that when 1 mm CuSO4 was used, the strain grew very poorly (Fig. 6, B and C). As a control, WT strain treated with 1 mm CuSO4 only showed modest growth defect (Fig. 6D), which is consistent with a previous report using such a CuSO4 concentration (27). qRT-PCR analysis of CHO1 expression under the control of the CTR4 promoter showed that although 25 μm CuSO4 significantly inhibited the gene expression, adding 1 mm CuSO4 led to even further inhibition (Fig. 6A). From these results, we concluded that although 25 μm CuSO4 can significantly reduce gene expression, the remaining low level of PS synthase activity is sufficient to support fungal growth. The further gene suppression due to addition of 1 mm CuSO4 may be sufficient to abolish the Cho1 enzyme activity, leading to growth arrest.
Figure 6.
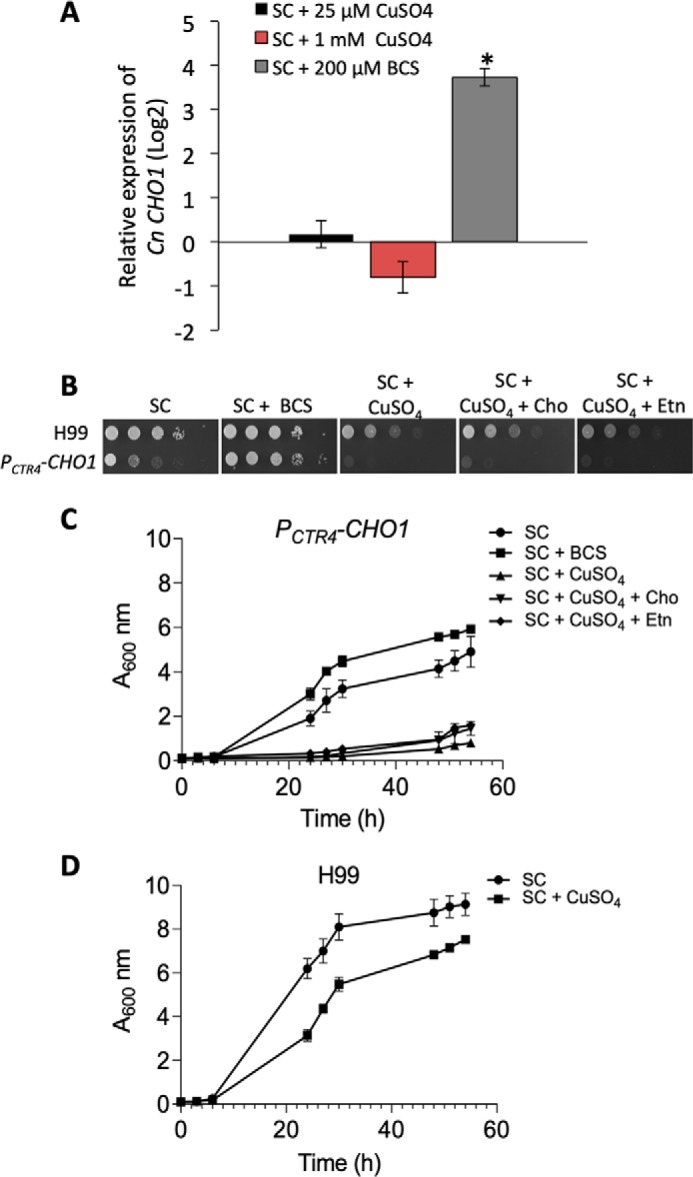
Inhibiting CHO1 expression leads to growth arrest independent of the Kennedy pathway. A, qRT-PCR analysis of PCTR4-CHO1 expression under different medium conditions. CHO1 gene expression was performed in triplicate, and two-tail t test was used for statistical analysis (* indicates p < 0.0001). B, growth rates of C. neoformans WT H99 and PCTR4-CHO1 cells on SC agar medium with indicated supplements. Addition of 1 mm ethanolamine or 1 mm choline did not rescue cell growth. C, growth of PCTR4-CHO1-expressing cells in SC liquid medium with indicated supplements. D, growth of H99 in SC medium in the presence or absence of 1 mm CuSO4. Each growth assay was done in triplicate and repeated at least twice.
The Kennedy pathway in C. neoformans is active
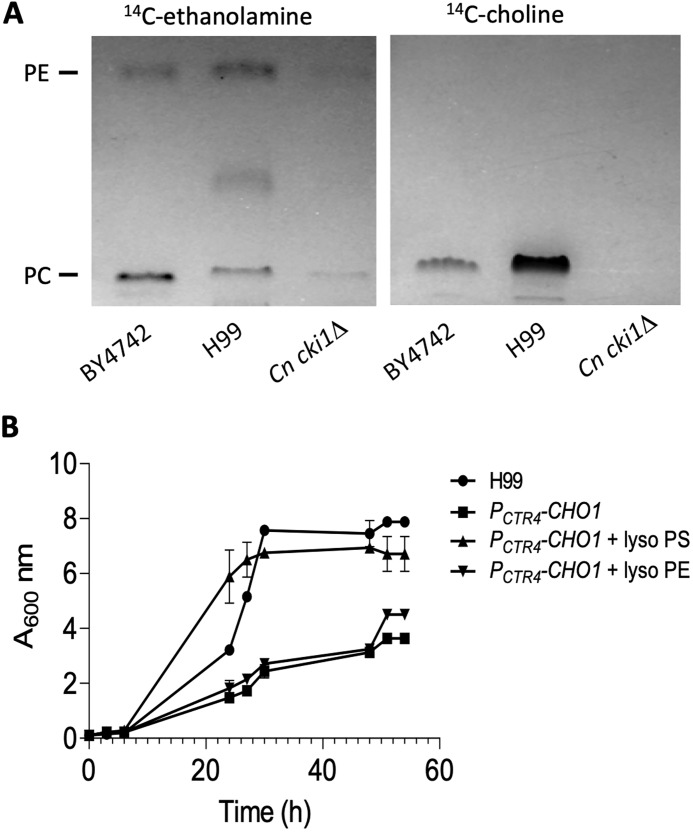
In S. cerevisiae, phospholipids such as PE and PC can be produced either by decarboxylation of PS or by utilizing ethanolamine and choline via the Kennedy pathway (Fig. 1) (17). Therefore, S. cerevisiae cho1Δ mutant is ethanolamine and choline auxotrophic and is still viable when supplemented with ethanolamine or choline. We investigated whether ethanolamine or choline could also rescue the growth arrest of the PCTR4-CHO1 strain under conditions that repressed CHO1. Interestingly, we found that adding 1 mm ethanolamine or 1 mm choline did not rescue the growth of the PCTR4-CHO1 strain under inhibition conditions (Fig. 6, B and C). One possibility for this lack of rescue is that the Kennedy pathway may be inactive in C. neoformans. To test this possibility, we monitored PE and PC production by using [14C]ethanolamine and [14C]choline in a 1D-TLC analysis. Our data showed that both C. neoformans WT H99 and S. cerevisiae WT BY4742 could produce PE and PC from ethanolamine and choline, respectively, indicating that the Kennedy pathway is active in C. neoformans (Fig. 7A). However, it remains unclear as to how much PE and PC is made using this pathway in C. neoformans. The C. neoformans mutant of the CKI1/EKI1 homolog (CNAG_04408) could still produce PE but could not produce PC (Fig. 7A), consistent with a role of this protein in the PC-producing branch of the Kennedy pathway.
Figure 7.
Kennedy pathway is active, and PS is essential in C. neoformans. A, cultures of S. cerevisiae WT strain BY4742, C. neoformans WT strain H99, and its Cn cki1Δ (predicted Sc CKI1 homolog) were cultured in SC medium with supplement of 10 μCi [14C]ethanolamine or [14C]choline. The production of PE and PC was detected by the radioactive signal in the 1D-TLC analysis. Purified PE and PC standards were purchased from Avanti Lipids. Positions of PE and PC were determined using iodine staining. 1D-TLC assay was repeated multiple times with identical results. B, lyso-PS rescued the growth arrest of PCTR4-CHO1 strain under inhibitory conditions. H99 and PCTR4-CHO1 strains were cultured in SC medium containing 1 mm CuSO4 and 1% Nonidet P-40, in the presence or absence of 50 μm lyso-PE or lyso-PS for 60 h at 30 °C with shaking. Growth rate was determined by A600 nm at different time points. Each growth assay was done in a triplicate and repeated at least twice.
PS is essential for Cryptococcus cell viability
Another possibility for Cn CHO1 essentiality is that PS is essential for viability in C. neoformans. Although PE and PC can be produced by both the de novo PS synthetic pathway and the Kennedy pathway, PS can only be produced via PS synthase (Cho1). Therefore, we supplemented the growth medium with 1 mm lyso-PS and examined whether lyso-PS (derivative of phosphatidylserine with one fatty acid chain that rapidly incorporates into the cells and is converted to PS) could rescue the growth arrest induced by CuSO4. Indeed, we observed significant rescue of growth defect by lyso-PS but not by lyso-PE (Fig. 7B). This result indicates that PS is essential for Cryptococcus viability.
Cho1 is essential for normal mitochondrial function
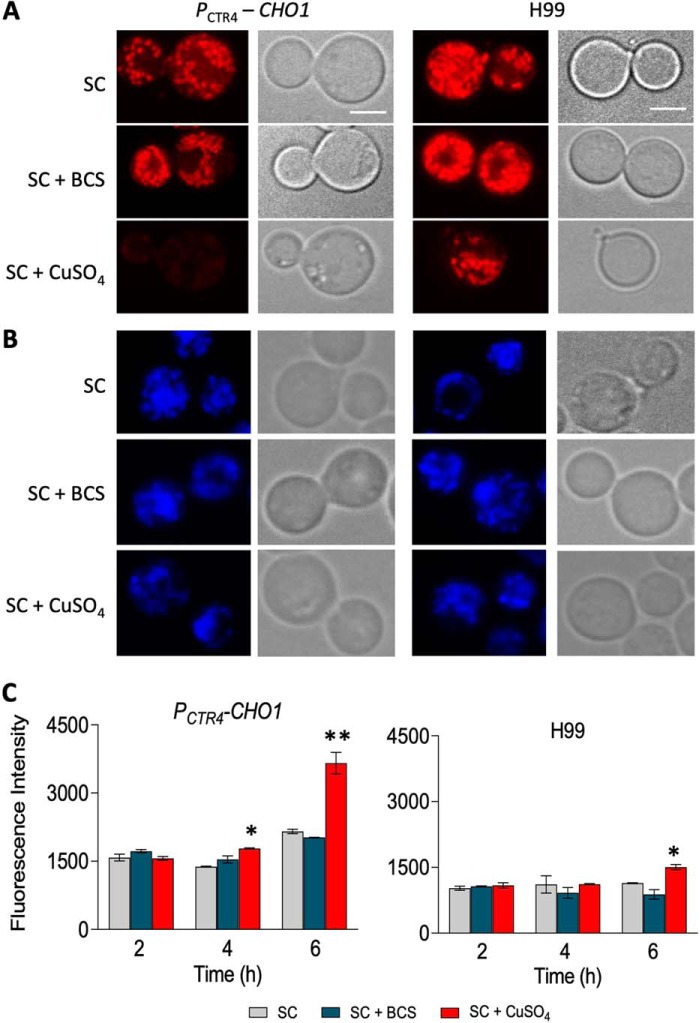
Mitochondria are essential organelles as they supply the cell with energy. Furthermore, mitochondria protect the cell against reactive oxygen species (ROS), as disruption of the electron transport chain leads to an increase in ROS, which in turn damages nucleic acids, proteins, and lipids, eventually leading to cell death (28, 29). Because the mitochondrial membrane is composed of lipids, including PS, PE, and PC, and Sc Cho1 has been shown to play a role in mitochondria function (30, 31), we hypothesized that Cn CHO1 may play an important role in mitochondrial function by contributing to lipid homeostasis in the mitochondrial membrane. To test our hypothesis, we first used MitoTracker Red FM to examine whether reduced levels of Cn CHO1 expression perturb mitochondrial membrane potential. The results by confocal microscopy showed that in the presence of CuSO4, PCTR4-CHO1 strain was unable to retain the MitoTracker Red FM, whereas in the presence of BCS or SC medium alone, this dye accumulated in the mitochondria (Fig. 8A and Fig. S4). To determine whether PCTR4-CHO1 strain lacked mitochondria, yeast mitochondrial DNA (mtDNA) was examined using DAPI. Mitochondrial DNA staining using DAPI indicated that this mitochondrial dysfunction was not due to the lack of mitochondria or damage to mtDNA, as PCTR4-CHO1 stain treated with CuSO4 still showed the mtDNA signal (Fig. 8B and Fig. S4). We also determined the cell viability after CuSO4 treatment for 0, 2, and 4 h by measuring the yeast CFU of treated cells, and we found that the majority of the cell populations were still viable (Table S2). These data support our hypothesis that Cho1 is essential to maintain healthy mitochondria by helping maintain mitochondrial membrane integrity.
Figure 8.
Cho1 is required for mitochondrial function. C. neoformans WT H99 and PCTR4-CHO1 strains were cultured in SC medium alone and in the presence of 1 mm CuSO4 or 200 μm BCS for 6 h. Cells were then incubated for 10 min in the presence of MitoTracker Red FM at a concentration 0.2 μm and DAPI at a concentration 1 μg before analyzing the mitochondria and mtDNA under the microscope. Scale bar, 10 μm. Among all the cells examined, 99% of them showed identical co-localization. Images with more cells were shown in Fig. S4. A, reduced CHO1 gene expression in the presence of copper prevents MitoTracker Red FM accumulation into the mitochondria. B, mitochondrial DNA stained with DAPI. C, reduced CHO1 gene expression leads to higher levels of intracellular ROS generation. H99 and PCTR4-CHO1 strains were grown to log phase at 30 °C, loaded with H2DCFDA, and exposed to BCS and CuSO4 for 2, 4, and 6 h. Quantification of flow cytometry indicates that there is a significant increase in ROS generation in the PCTR4-CHO1 strain in the presence of CuSO4 as compared with BCS or SC medium alone at hour 4 and at hour 6. ROS generation is also significantly higher in PCTR4-CHO1 as compared with H99 when treated with CuSO4 at hour 4 and at hour 6. * indicates p < 0.05 and **indicates p < 0.01 based on two-tail t test.
Perturbed mitochondrial respiratory chain may lead to increased generation of ROS (29). Therefore, we used the ROS indicator dye H2DCFDA to monitor ROS generation. After a 2-h incubation in the presence of CuSO4, BCS, or SC medium alone, there was no significant change in ROS generation in PCTR4-CHO1 and H99 strain. However, the ROS level significantly increased in the PCTR4-CHO1 strain as compared with H99 after 4 and 6 h of incubation (Fig. 8C). These data demonstrate that Cn Cho1 plays an important role in C. neoformans mitochondrial function, and mitochondrial dysfunction likely contributes to the lethality of Cn Cho1.
Discussion
In this study, we have identified and characterized the Cn Cho1 protein, the sole homolog of PS synthase homolog in C. neoformans. We have shown that heterologous expression of Cn CHO1 in the S. cerevisiae cho1Δ mutant fully complements the PS synthase activity, confirming that Cn Cho1 is a PS synthase. We further found that the Cryptococcus PS synthase is essential for fungal viability, despite the existence of an active Kennedy pathway to produce PE and PC. Our results demonstrated that PS is essential for fungal cell viability, and although the main reason for Cn Cho1 essentiality is not fully understood, we did show that it contributes to mitochondrial function.
Fungal Cho1 homologs have been shown to play an important role in cell signaling and synthesis of major cellular phospholipids, but this is the first report indicating that Cho1 is essential for cell viability in a fungus. In S. cerevisiae, the cho1Δ mutant is viable but auxotrophic for ethanolamine. The Sc cho1Δ mutant grows normally in the presence of ethanolamine or choline, suggesting that the Kennedy pathway can support lipid production in this organism (32). The cho1Δ/cho1Δ null mutant is also viable in C. albicans (15). Interestingly, although the in vitro growth of this mutant can be rescued by addition of ethanolamine, Cho1 is essential for Candida virulence in a murine infection model and has thus been proposed as a potential drug target (15, 33). A recent study further demonstrated that the mutant cells might not be able to acquire sufficient ethanolamine from the host, leading to the avirulent phenotype (34). Why Cho1 is essential in C. neoformans remains unclear. Our 1D-TLC analysis showed that PE and PC can be produced by cells supplemented with choline or ethanolamine in culture medium, indicating that the Kennedy pathway remains active in C. neoformans. These data suggest that the essentiality of Cho1 in C. neoformans is not due to a lack of PE and PC production via the Kennedy pathway, although we do not know how much of these lipids are synthesized through this pathway in this fungus.
Using the CTR4-inducible promoter system, we were able to knock down the PS synthase activity to a level that prevents cell growth. The growth arrest of the PCTR4-CHO1–expressing strain in the presence of CuSO4 was rescued by the addition of lyso-PS, but not lyso-PE, indicating that PS is essential for C. neoformans and that the inability to produce PS is the reason for Cn cho1Δ mutant lethality. This is consistent with the conclusion that C. neoformans can make PE and PC via the Kennedy pathway but that PS has to be synthesized using PS synthase Cho1. PS can be converted to PE by PS decarboxylases Psd1 and Psd2. This step is generally considered irreversible in fungi such as S. cerevisiae and is likely to be irreversible in C. neoformans as well.
PS plays several important cellular roles. Besides being a key component of the lipid bilayer membrane, PS also functions as a phagocytosis-inducing signal for macrophages and a signal to trigger inflammatory activity during infection (8, 10, 11). Because the Cn cho1Δ mutant is inviable, we could not test the function of Cho1 in host–pathogen interactions. Based on our results, it is likely that PS is an essential part of the cell membrane in C. neoformans. The importance of PS in C. neoformans is also underscored by the phenotype of the lipid flippase cdc50Δ mutant, which shows PS accumulation on the extracytoplasmic leaflet of the membrane. This mutant is also defective for fungal virulence and shows increased phagocytosis and killing by macrophages in vitro (19).
Another potential role of Cho1 is to maintain mitochondrial integrity and function. The inner and outer yeast mitochondrial membranes are composed of various lipids, including PC, PE, PS, phosphatidylinositol, phosphatidic acid, phosphatidylglycerol, and cardiolipin. Two abundant phospholipids, PC and PE, are mainly synthesized from PS (29–31). Although distributions of these lipids in the inner and outer mitochondrial membranes differ, a change in mitochondrial lipid homeostasis can lead to mitochondrial respiration defects (31). In S. cerevisiae, the loss of PS or PE biosynthesis leads to the formation of petite mutants, which cannot utilize nonfermentable carbon sources such as glycerol due to mitochondrial defects (29, 31). In contrast, C. albicans is a nonpetite forming yeast, indicating that it cannot grow without functional mitochondria (15). In C. neoformans, mutants directly involved in mitochondrial function display disturbed mitochondrial action potential and increased levels of intracellular ROS (28). Functional mitochondria are required for the survival of C. neoformans, especially under low-oxygen conditions. Our results indicate that Cho1 plays an important role in maintaining the integrity of the mitochondrial membrane, which likely contributes to C. neoformans viability.
In summary, in this study, we demonstrated that the only homolog of PS synthase in C. neoformans, Cho1, has PS synthase activity and is essential for viability. Because humans use different mechanisms to produce PS as compared with fungi and there is no Cho1 in mammalian cells, the essentiality of the PS biosynthetic pathway represents this pathway and Cho1 as an excellent potential target for antifungal drug development.
Experimental procedures
Strains and media
C. neoformans and S. cerevisiae strains used in this study are listed in Table 1. Strains were grown at 30 °C using yeast peptone dextrose (YPD) or SC medium. The H99 strain containing the CHO1 gene controlled by the CTR4 promoter (PCTR4-CHO1) was grown in SC medium supplemented with 25 μm or 1 mm CuSO4 and 1 mm ascorbic acid or in the presence of 200 μm BCS.
Table 1.
Strains used in this study
| Genotype | Source/Ref. | |
|---|---|---|
| C. neoformans strains | ||
| H99 | MATα wildtype | 43 |
| AI187 | MATa/α diploid wildtype | 24 |
| CUX663 | AI187 cho1Δ::NEO CHO1 | This study |
| CUX664 | MATα PACT-CHO1:mCherry-NAT | This study |
| CUX1081 | MATα cho1Δ::NEO PCTR4-CHO1-NAT | This study |
| CUX1082 | MATα cki1Δ::NAT | UCSF 2015 deletion collection |
| S. cerevisiae strains | ||
| BY4741 | MATa his3Δ1 leu2Δ0 ura3Δ0 | ATCC deletion collection |
| W303-1A | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | 44 |
| JSY94A | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 cho1Δ::TRP1 | 41 |
| YUX104 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 [pTH19] | This study |
| YUX105 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 cho1Δ::TRP1 [pTH19] | This study |
| YUX106 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 cho1Δ::TRP1 [pTH19-Cn CHO1] | This study |
Growth assay
The WT H99 and H99-expressing PCTR4-CHO1 strains were grown overnight in YPD medium at 30 °C. Cells were harvested by centrifugation at 350 × g, washed twice with sterile water, and inoculated at A600 nm = 0.1 in SC medium containing either 200 μm BCS or CuSO4 with 1 mm ascorbic acid in the presence or absence of 1 mm ethanolamine and 1 mm choline. The growth of each strain in various conditions was determined by measuring A600 nm every 3 h for 3 days. Growth assay was performed in triplicate and repeated at least twice.
Heterologous expression of Cn Cho1 into Sc cho1Δ mutant
C. neoformans Cn CHO1 cDNA (CNAG_01469) was cloned into yeast expression vector pTH19 (PADH1 URA3 2μ) (35) and transformed in S. cerevisiae cho1 mutant (Sc cho1Δ) background, in which the expression of the Cn CHO1 gene was under the control of the ADH1 promoter. Cultures of S. cerevisiae strains WT W303-A1, Sc cho1Δ-expressing empty vector, and Sc cho1Δ-expressing Cn CHO1 gene (Sc cho1Δ + Cn CHO1) (Table 1) were prepared with serial dilutions on SC-Ura medium with and without 1 mm ethanolamine. The results were photographed after 48 h of incubation at 30 or 37 °C.
Generation of the cho1Δ mutant strain
Generation of a CHO1 deletion mutant in haploid WT H99 and diploid strain AI187 backgrounds was attempted following the strategy illustrated in Fig. 5A. The cho1Δ mutant was generated by overlap PCR and biolistic transformation as described previously (36). The 5′- and 3′-flanking regions of the CHO1 gene were amplified from H99 genomic DNA with primers CX862/CX863 and CX864/CX865, respectively (Table S1). The dominant selectable marker (NEO) was amplified with the M13 primers (M13F and M13R) from plasmid pJAF1 (37). Each target gene replacement cassette was generated by overlap PCR with primers CX862/CX865 (Table S1). Purified overlap PCR products were precipitated onto 10-μl gold microcarrier beads (0.6 μm; Bio-Rad), and biolistically transformed in H99 and AI187 as described previously (38). Stable transformants were selected on YPD medium containing G418 (200 mg/liter). To screen for mutants of the CHO1 gene, diagnostic PCR was performed by analyzing the removal of Cn CHO1 ORF with primers CX866/CX867 (Table S1). Positive transformants identified by the PCR screen were further confirmed with primers CX868/JH8994.
Generation of the PCTR4-CHO1–expressing strain
The native CHO1 promoter was replaced by the CTR4-inducible promoter following the strategy illustrated in Fig. S2. The fragment containing CHO1 ORF and the downstream terminal region was amplified from H99 genomic DNA with primers CX1269/CX1270 (Table S1). Amplified PCR product was cloned into the BamHI sites of the pCTR4-2 vector (kindly provided by Dr. Tamara Doering laboratory, Washington University in St. Louis (25)) using an In-Fusion HD cloning kit (Takara, CA). The CHO1 upstream and selectable marker (NAT) fragments were amplified using primers CX1271/CX1272 and CX5/CX1273, respectively (Table S1). Overlap PCR product (CHO1 upstream-NAT fragment) was cloned into NdeI sites of CHO1 ORF pCTR4-2 vector by In-Fusion HD cloning. The constructed vector was biolistically transformed into WT H99. Positive transformants identified by PCR using CX1306/JH8994 and CX1307/CX1276 primers (Table S1) were further confirmed by Southern blotting and gene sequencing.
RNA isolation and cDNA synthesis
H99 and H99-expressing PCTR4-CHO1 strains were grown in 50 ml of YPD medium at 30 °C overnight. Cells were then centrifuged, washed twice with sterile water, and transferred into 50 ml of SC medium containing either 200 μm BCS for inducing condition or 25 μm or 1 mm CuSO4 with 1 mm ascorbic acid for suppressing condition. Cell cultures were incubated at 30 °C for 20 h. RNAs were isolated using the TRIzol method followed by phenol/chloroform extraction. The genomic DNA was eliminated using rDNase and RNA purification kit (Takara, CA). The quality of RNA was confirmed by measuring the A260/280 m ratio on a spectrophotometer (Bio-Rad) and visualizing on an RNA gel. For cDNA synthesis, RNA samples were reverse-transcribed using SMARTScribe reverse transcriptase (Takara, CA).
To determine CHO1 gene expression in H99 under various inositol concentrations, cells were grown in 50 ml of YPD medium at 30 °C overnight. Cells were then centrifuged, washed twice with sterile water, and transferred into 50 ml of SC supplemented with 0.1 or 1% inositol (w/v), respectively. Cells were collected at two time points, 4 h after incubation at 30 °C, and RNA was isolated as described above.
Real-time PCR
The real-time PCR was performed using 2× master mix SYBR Green on an Mx4000 quantitative PCR system (Stratagene, CA). The amplification conditions consist of 1 cycle at 95 °C for 10 min, 40 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and 1 cycle at 95 °C for 1 min, 55 °C for 30 s, and 95 °C for 30 s. The results were normalized to GAPDH and analyzed using 2−ΔΔCt method (39).
Protein extraction and Western blot analysis
A previously described method with minor modification was used to determine whether CHO1 is an integral membrane protein (40). C. neoformans strain expressing CHO1:mCherry (Table 1) was grown in 50 ml of YPD to early mid-log phase before harvesting by centrifugation at 1500 × g for 5 min at 4 °C. Cells were washed with cold water and centrifuged at 1500 × g for 5 min. Cells were then resuspended in 20 ml of Tris-HCl/EDTA buffer (0.1 m Tris-HCl, pH 7.5, 0.5 m EDTA) with 1 mm DTT and incubated at 30 °C for 2 h. Cell pellets were washed with 1 m sorbitol and incubated in 1 m sorbitol with lyticase (1 μg/μl) and chitinase (30 mg/ml) at 30 °C for 3 h. One gram of spheroplasts was resuspended in homogenizing buffer (150 mm NaCl, 20 mm Tris-HCl, pH 8) and lysed using a beads beater (8 times for 40 s each cycle). Cells were centrifuged at 13,000 × g for 10 min at 4 °C. The insoluble pellet was resuspended in 8 ml of either buffer alone (150 mm NaCl, 20 mm Tris, pH 8.0, 5 mm MgCl, 1 mm phenylmethylsulfonyl fluoride), buffer with 1% Triton X-100, or buffer with 1 m NaCl. Extracts were incubated on ice for 30 min and then separated into a soluble and pellet fraction by ultracentrifugation at 226,000 × g for 60 min at 4 °C. Equivalent amounts from the pellet and the supernatant were analyzed by Western blotting and SDS-PAGE. The antibodies were used at the following dilutions: RFP-tag antibody 1:1000 (GenScript) and anti-rabbit 1:1000 (GenScript).
Lipid extraction, separation, and identification
Lipid isolation followed a previously reported method with modifications (41, 42). S. cerevisiae WT strain BY4742, C. neoformans WT strain H99, and its mutant of the EKI/CKI kinase homolog (Cn cki1Δ) strains (Table 1) were grown in YPD overnight to A600 nm = 5. Cells were centrifuged, washed twice in sterile water, and incubated at a starting A600 nm = 0.1 in 2 ml of SC medium with 10 μCi of [14C]ethanolamine and 10 μCi of lsqb]14C]choline overnight at 30 °C. Labeled cells were harvested by centrifugation, washed twice with sterile water, and incubated in 1 ml of spheroplast buffer (1 m sorbitol, 0.05 m sodium phosphate monobasic, 0.1% 2-mercaptoethanol (v/v)) with 100 μg/ml chitinase for 1 h at 30 °C. Cells were collected by centrifugation, washed with sterile water, and resuspended in chloroform/methanol (2:1). Glass beads were added, and cells were lysed using a beads beater (8 times for 40 s each cycle). Lysate was transferred into new 1.5-ml tubes and incubated on a shaker at room temperature for 30 min. To separate the organic phase containing phospholipids from the aqueous phase containing nonlipid cellular material, 0.2 volume of water was added. Mixture was vortexed and centrifuged at 1000 × g for 1 min. Eighteen microliters of lipids were loaded onto HPTLC silica gel (Millipore, MA). Solvent used in the mobile phase consisted of chloroform/methanol/acetic acid (65:25:10). Lipids were visualized using 99.99% iodide and autoradiography film (Denville Scientific, NJ).
ER tracker staining
C. neoformans overnight cultures were washed twice in Hanks' balanced salt solution (HBSS) buffer (1.26 mm CaCl2, 5.33 mm KCl, 0.44 mm KH2PO4, 0.5 mm MgCl2·6H2O, 0.44 mm MgSO4·7H2O, 138 mm NaCl, 4 mm NaHCO3, 0.3 mm Na2HPO4, and 5.6 mm d-glucose) before being fixed with 3.7% formaldehyde for 5 min at 30 °C. Fixed cells were washed with HBSS buffer three times and stained with 1 μm ER tracker (Life Technologies, Inc.) for 30 min at 30 °C before being observed under the fluorescence microscope (Nikon).
Mitochondria and DNA staining
C. neoformans overnight cultures were resuspended in SC medium containing either 200 μm BCS or 1 mm CuSO4 and 1 mm ascorbic acid. Cells were incubated at 30 °C for 6 h and then washed twice in 1× PBS and resuspended in 1× PBS with 0.2 μm MitoTracker Red FM (Invitrogen) and 1 μg of DAPI (ThermoFisher Scientific). Mixture was incubated for 10 min at 30 °C. Cells were then washed twice with 1× PBS and observed under a fluorescence microscope (Nikon).
ROS measurement
ROS detection technique followed a previously reported method (28). Cells were grown overnight in YPD medium at 30 °C. The following day, cells were diluted in SC medium and allowed to grow until A600 nm reached 0.5. H2DCFDA (Invitrogen) at a final concentration of 10 μm was added, and cells were incubated for an additional 2 h. Cells were then washed to remove the excess dye and resuspended in SC, SC with 200 μm BCS, and SC with 1 mm CuSO4 to further incubate for 2, 4, and 6 h. Cells were then harvested, washed with 1× PBS, and resuspended in 1 ml of PBS. Fluorescence signal was analyzed using Accuri Flow Cytometer (BD Biosciences). Two-tail t test was used for statistical analysis, where * represents p < 0.05, and ** represents p < 0.01.
PS synthase activity assay
All assays were conducted at 30 °C in a total volume of 0.1 ml. PS synthase activity was measured following the incorporation of water-soluble [3-3H]serine (10,000 cpm/nmol) into chloroform-soluble [3-3H]PS (16, 22, 23). The enzyme reaction contained 50 mm Tris-HCl, pH 8.0, 0.6 mm MnCl2, 4 mm Triton X-100, 0.2 mm CDP-DAG, and 0.5 mm serine (41). The enzyme assays were conducted in triplicate, and the average S.D. of the assays was ±5%. The reactions were linear with time and protein concentration. A unit of PS synthase activity was defined as the amount of enzyme that catalyzed the formation of 1 nmol of product/min.
Statistical analysis
Means with standard deviations were calculated using the t test for multiple comparisons. The p value of <0.05 was considered a significant difference.
Author contributions
P. K., Y. W., G.-S. H., K. J. G., and C. X. data curation; P. K., Y. W., G.-S. H., K. J. G., Y.-G. G., and C. X. formal analysis; P. K., Y. W., and C. X. validation; P. K., Y. W., G.-S. H., K. J. G., Y.-G. G., and C. X. investigation; P. K., Y. W., and C. X. visualization; P. K., Y. W., G.-S. H., K. J. G., and C. X. methodology; P. K. and C. X. writing-original draft; P. K., Y.-G. G., G. M. C., and C. X. project administration; P. K., G.-S. H., Y.-G. G., G. M. C., and C. X. writing-review and editing; K. J. G., Y.-G. G., and C. X. software; G. M. C. and C. X. conceptualization; G. M. C. and C. X. supervision; G. M. C. and C. X. funding acquisition.
Supplementary Material
Acknowledgments
We thank Erika Shor for critical reading and editing of the manuscript and valuable comments for the study. We thank Dr. Xilin Zhao for valuable comments and material support for the ROS experiments. We thank Dr. Todd Reynolds for valuable comments on the study. We thank Dr. Tamara Doering for the pCTR4-2 plasmid. We also acknowledge use of the C. neoformans genome sequences at FungiDB.
This work was supported in part by National Institutes of Health Grants R01AI123315 (to C. X.) and R37GM028140 (to G. M. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4 and Tables S1–S2.
- PS
- phosphatidylserine
- PE
- phosphatidylethanolamine
- PC
- phosphatidylcholine
- DAG
- diacylglycerol
- SC
- synthetic complete
- H2DCFDA
- dichlorodihydrofluorescein diacetate
- DAPI
- 4′,6-diamidino-2-phenylindole
- ROS
- reactive oxygen species
- 1D-TLC
- one-dimensional thin-layer chromatography
- ER
- endoplasmic reticulum
- qRT
- quantitative RT
- BCS
- bathocuproinedisulfonic acid.
References
- 1. Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., and Chiller T. M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530 10.1097/QAD.0b013e328322ffac [DOI] [PubMed] [Google Scholar]
- 2. Rajasingham R., Smith R. M., Park B. J., Jarvis J. N., Govender N. P., Chiller T. M., Denning D. W., Loyse A., and Boulware D. R. (2017) Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17, 873–881 10.1016/S1473-3099(17)30243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heitman J., Kozel T. R., Kwon-Chung K. J., Perfect J. R., and Casadevall A. (2011) Cryptococcus from Human Pathogen to Model Yeast. American Society for Microbiology, Washington, D. C. [Google Scholar]
- 4. Saag M. S., Graybill R. J., Larsen R. A., Pappas P. G., Perfect J. R., Powderly W. G., Sobel J. D., and Dismukes W. E. (2000) Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30, 710–718 10.1086/313757 [DOI] [PubMed] [Google Scholar]
- 5. Panatala R., Hennrich H., and Holthuis J. C. (2015) Inner workings and biological impact of phospholipid flippases. J. Cell Sci. 128, 2021–2032 10.1242/jcs.102715 [DOI] [PubMed] [Google Scholar]
- 6. Leventis P. A., and Grinstein S. (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 39, 407–427 10.1146/annurev.biophys.093008.131234 [DOI] [PubMed] [Google Scholar]
- 7. Kay J. G., Koivusalo M., Ma X., Wohland T., and Grinstein S. (2012) Phosphatidylserine dynamics in cellular membranes. Mol. Biol. Cell 23, 2198–2212 10.1091/mbc.e11-11-0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birge R. B., Boeltz S., Kumar S., Carlson J., Wanderley J., Calianese D., Barcinski M., Brekken R. A., Huang X., Hutchins J. T., Freimark B., Empig C., Mercer J., Schroit A. J., Schett G., and Herrmann M. (2016) Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 23, 962–978 10.1038/cdd.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newton A. C., and Keranen L. M. (1994) Phosphatidyl-l-serine is necessary for protein kinase C's high-affinity interaction with diacylglycerol-containing membranes. Biochemistry 33, 6651–6658 10.1021/bi00187a035 [DOI] [PubMed] [Google Scholar]
- 10. Wu Y., Tibrewal N., and Birge R. B. (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 16, 189–197 10.1016/j.tcb.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 11. Segawa K., and Nagata S. (2015) An apoptotic 'Eat Me' signal: phosphatidylserine exposure. Trends Cell Biol. 25, 639–650 10.1016/j.tcb.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 12. Farine L., Niemann M., Schneider A., and Bütikofer P. (2015) Phosphatidylethanolamine and phosphatidylcholine biosynthesis by the Kennedy pathway occurs at different sites in Trypanosoma brucei. Sci. Rep. 5, 16787 10.1038/srep16787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vance J. E. (2015) Phospholipid synthesis and transport in mammalian cells. Traffic 16, 1–18 10.1111/tra.12230 [DOI] [PubMed] [Google Scholar]
- 14. Letts V. A., Klig L. S., Bae-Lee M., Carman G. M., and Henry S. A. (1983) Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc. Natl. Acad. Sci. U.S.A. 80, 7279–7283 10.1073/pnas.80.23.7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y. L., Montedonico A. E., Kauffman S., Dunlap J. R., Menn F. M., and Reynolds T. B. (2010) Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol. Microbiol. 75, 1112–1132 10.1111/j.1365-2958.2009.07018.x [DOI] [PubMed] [Google Scholar]
- 16. Bae-Lee M. S., and Carman G. M. (1984) Phosphatidylserine synthesis in Saccharomyces cerevisiae. Purification and characterization of membrane-associated phosphatidylserine synthase. J. Biol. Chem. 259, 10857–10862 [PubMed] [Google Scholar]
- 17. Carman G. M., and Kersting M. C. (2004) Phospholipid synthesis in yeast: regulation by phosphorylation. Biochem. Cell Biol. 82, 62–70 10.1139/o03-064 [DOI] [PubMed] [Google Scholar]
- 18. Kelley M. J., Bailis A. M., Henry S. A., and Carman G. M. (1988) Regulation of phospholipid biosynthesis in Saccharomyces cerevisiae by inositol. Inositol is an inhibitor of phosphatidylserine synthase activity. J. Biol. Chem. 263, 18078–18085 [PubMed] [Google Scholar]
- 19. Huang W., Liao G., Baker G. M., Wang Y., Lau R., Paderu P., Perlin D. S., and Xue C. (2016) Lipid flippase subunit Cdc50 mediates drug resistance and virulence in Cryptococcus neoformans. MBio 7, e00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shor E., Wang Y., Perlin D. S., and Xue C. (2016) Cryptococcus flips its lid-membrane phospholipid asymmetry modulates antifungal drug resistance and virulence. Microb. Cell 3, 358–360 10.15698/mic2016.08.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu G., Caza M., Bakkeren E., Kretschmer M., Bairwa G., Reiner E., and Kronstad J. (2017) A P4-ATPase subunit of the Cdc50 family plays a role in iron acquisition and virulence in Cryptococcus neoformans. Cell Microbiol. 19, e12718 10.1111/cmi.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carman G. M., and Bae-Lee M. (1992) Phosphatidylserine synthase from yeast. Methods Enzymol. 209, 298–305 10.1016/0076-6879(92)09037-4 [DOI] [PubMed] [Google Scholar]
- 23. Cousminer J. J., and Carman G. M. (1981) Solubilization of membrane-associated phosphatidylserine synthase from Clostridium perfringens. Can. J. Microbiol. 27, 544–547 10.1139/m81-080 [DOI] [PubMed] [Google Scholar]
- 24. Idnurm A. (2010) A tetrad analysis of the basidiomycete fungus Cryptococcus neoformans. Genetics 185, 153–163 10.1534/genetics.109.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ory J. J., Griffith C. L., and Doering T. L. (2004) An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21, 919–926 10.1002/yea.1139 [DOI] [PubMed] [Google Scholar]
- 26. Liu T. B., and Xue C. (2014) Fbp1-mediated ubiquitin-proteasome pathway controls Cryptococcus neoformans virulence by regulating fungal intracellular growth in macrophages. Infect. Immun. 82, 557–568 10.1128/IAI.00994-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding C., Festa R. A., Chen Y. L., Espart A., Palacios Ò., Espín J., Capdevila M., Atrian S., Heitman J., and Thiele D. J. (2013) Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 13, 265–276 10.1016/j.chom.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ingavale S. S., Chang Y. C., Lee H., McClelland C. M., Leong M. L., and Kwon-Chung K. J. (2008) Importance of mitochondria in survival of Cryptococcus neoformans under low oxygen conditions and tolerance to cobalt chloride. PLoS Pathog. 4, e1000155 10.1371/journal.ppat.1000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grant C. M., MacIver F. H., and Dawes I. W. (1997) Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 410, 219–222 10.1016/S0014-5793(97)00592-9 [DOI] [PubMed] [Google Scholar]
- 30. Kohlwein S. D., Kuchler K., Sperka-Gottlieb C., Henry S. A., and Paltauf F. (1988) Identification of mitochondrial and microsomal phosphatidylserine synthase in Saccharomyces cerevisiae as the gene product of the CHO1 structural gene. J. Bacteriol. 170, 3778–3781 10.1128/jb.170.8.3778-3781.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simbeni R., Pon L., Zinser E., Paltauf F., and Daum G. (1991) Mitochondrial membrane contact sites of yeast. Characterization of lipid components and possible involvement in intramitochondrial translocation of phospholipids. J. Biol. Chem. 266, 10047–10049 [PubMed] [Google Scholar]
- 32. Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., and Fogel S. (1980) Yeast mutants auxotrophic for choline or ethanolamine. J. Bacteriol. 141, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cassilly C. D., and Reynolds T. B. (2018) PS, it's complicated: the roles of phosphatidylserine and phosphatidylethanolamine in the pathogenesis of Candida albicans and other microbial pathogens. J. Fungi 4, E28 10.3390/jof4010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis S. E., Tams R. N., Solis N., Wagner A. S., Chen T., Jackson J. W., Hasim S., Montedonico A. E., Dinsmore J., Sparer T. E., Filler S. G., and Reynolds T. B. (2018) Candida albicans cannot acquire sufficient ethanolamine from the host to support virulence in the absence of de novo phosphatidylethanolamine synthesis. Infect. Immun. 86, IAI.00815-17 10.1128/IAI.00815-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harashima T., and Heitman J. (2002) The Gα protein Gpa2 controls yeast differentiation by interacting with kelch repeat proteins that mimic Gβ subunits. Mol. Cell 10, 163–173 10.1016/S1097-2765(02)00569-5 [DOI] [PubMed] [Google Scholar]
- 36. Davidson R. C., Blankenship J. R., Kraus P. R., de Jesus Berrios M., Hull C. M., D'Souza C., Wang P., and Heitman J. (2002) A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148, 2607–2615 10.1099/00221287-148-8-2607 [DOI] [PubMed] [Google Scholar]
- 37. Fraser J. A., Subaran R. L., Nichols C. B., and Heitman J. (2003) Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2, 1036–1045 10.1128/EC.2.5.1036-1045.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davidson R. C., Cruz M. C., Sia R. A., Allen B., Alspaugh J. A., and Heitman J. (2000) Gene disruption by biolistic transformation in serotype D strains of Cryptococcus neoformans. Fungal Genet. Biol. 29, 38–48 10.1006/fgbi.1999.1180 [DOI] [PubMed] [Google Scholar]
- 39. Xue C., Liu T., Chen L., Li W., Liu I., Kronstad J. W., Seyfang A., and Heitman J. (2010) Role of an expanded inositol transporter repertoire in Cryptococcus neoformans sexual reproduction and virulence. MBio 1, e00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siniossoglou S., Santos-Rosa H., Rappsilber J., Mann M., and Hurt E. (1998) A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J. 17, 6449–6464 10.1093/emboj/17.22.6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi H. S., Han G. S., and Carman G. M. (2010) Phosphorylation of yeast phosphatidylserine synthase by protein kinase A: identification of Ser46 and Ser47 as major sites of phosphorylation. J. Biol. Chem. 285, 11526–11536 10.1074/jbc.M110.100727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atkinson K., Fogel S., and Henry S. A. (1980) Yeast mutant defective in phosphatidylserine synthesis. J. Biol. Chem. 255, 6653–6661 [PubMed] [Google Scholar]
- 43. Perfect J. R., Schell W. A., and Rinaldi M. G. (1993) Uncommon invasive fungal pathogens in the acquired immunodeficiency syndrome. J. Med. Vet. Mycol. 31, 175–179 10.1080/02681219380000211 [DOI] [PubMed] [Google Scholar]
- 44. Wallis J. W., Chrebet G., Brodsky G., Rolfe M., and Rothstein R. (1989) A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58, 409–419 10.1016/0092-8674(89)90855-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.