Abstract
A significant portion of the clinical phenotype observed in Alzheimer’s disease (AD) occurs through nicotinic acetylcholine receptors (nAChRs). Degeneration of cholinergic neurons, combined with aberrant nAChR expression and activation partially through amyloid-beta peptide (Aβ)–nAChR leads to upregulation of pro-inflammatory pathways and subsequently the progressive cognitive decline of AD. Interestingly, the cholinergic anti-inflammatory pathway is also mediated through nAChR particularly α7 nAChR. Thus, agonists of these receptors will likely exert pro-cognitive benefits through multiple mechanisms including stimulating the cholinergic pathway, modulating inflammation, and buffering the effects of amyloid. Despite this promising theoretical use, trials thus far have been complicated by adverse effects or minimal improvement. This review will provide an update on several pharmacological nAChR agonists tested in clinical trials and reasons that further investigation of nAChR agonists is merited.
Implications
nAChRs have consistently presented a promising theoretical use in the treatment of AD; however, trials thus far have been complicated by adverse effects or minimal improvement. This review will provide an update on several pharmacological nAChR agonists trialed and reasons that further investigation of nAChR agonists is merited.
Introduction
The significance of cholinergic systems cannot be overstated in understanding the pathology and progression of Alzheimer’s dementia.1 Neurons in the basal forebrain, including the neurons that form the nucleus basalis of Meynert, hippocampus, and cerebral neocortex are lost in the disease and contribute to the symptomatic development of dementia. Two of the main histopathological markers of Alzheimer disease are the accumulation of amyloid-beta peptide (Aβ) and misfolded hyperphosphorylated tau forming neurofibrillary tangles.2 Alzheimer’s disease (AD) is also accompanied by cholinergic dysfunction as evidenced by the reduction of choline acetyltransferase, vesicular acetylcholine (ACh) transporter,3,4 release of ACh, and nicotinic receptors.5 The loss of cholinergic innervation in early AD is most prominent in the cortex and hippocampus, and the mainstay of AD symptomatic control relies on acetylcholinesterase inhibitors (AChEIs; donepezil and galantamine), which exert positive cholinergic effects mediated by indirectly increasing activity at nicotinic acetylcholine receptors (nAChRs).6,7 The importance of the cholinergic system in progression of AD is summarized in the cholinergic hypothesis proposed by Perry et al. in 1999, which is strengthened by the phenocopying of cognitive deficits in rodent models with antagonism of acetylcholine receptors (AChRs).1,8,9 More specifically, animal lesion studies that target cholinergic input to the neocortex or hippocampus from the basal forebrain and incidental human disruptions via arterial aneurysms, arterial venous malformations, and resections can reproduce similar cognitive deficits observed in AD.10,11 Thus, the basal forebrain and rostral forebrain cholinergic pathways including projections to the thalamus are critical for awareness, attention, and working memory.12,13
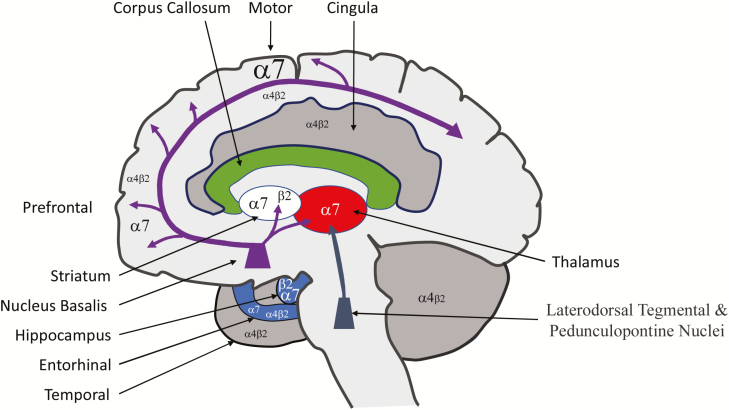
Degeneration of these pathways results in the development of cognitive decline.14 Accordingly, AChRs (nicotine and muscarinic) have been posited to play a central role in mediating AD pathology, which might offer potential treatment targets. They are cys-loop ligand-gated ion channels composed of five heteromeric or homomeric subunits that form an ionic pore.15,16 These channels have been implicated in the development of memory, locomotion, attention, and anxiety.4 In the mammalian brain, 11 different subunits are known to exist, alpha (α2–α7, α9, and α10) and beta (β2–β4), that pentamerize to form a multimeric ligand-gated ion channel. The α and β distinction is based on the presence of adjacent cysteine groups in the extracellular domain found only in the α subunits. The unique anatomical distribution of each nAChR subtype within the structures involved in cognition (the basal forebrain, hippocampus, and cerebral cortex) implicates receptors composed of α7, α4, and β2 subunits as important potential targets of pharmacological intervention (Figure 1).17–20 As discussed by Wu and Lukas21 and numerous others,22–31 the receptor subunit composition confers different functional and pharmacological properties governed by subunit composition. Despite the greater than 50 000 permutations that can be achieved with 11 subunits forming a pentameric channel, the α7 nAChR and α4β2 nAChR are predominant with differing functional properties.3,16,32 As described by Dineley et al., the α7 homomeric receptor when compared to the α4β2 nAChR has rapid activation and desensitization as well as high calcium permeability.4
Figure 1.
Cholinergic circuitry of the nucleus basalis with relative expression of α7 and α4β2. (Based on data presented in Mesulam18 and Paterson and Nordberg.40)
The α4β2 nAChR and α7 nAChR localize presynaptically and somatodendritically on hippocampal principal glutamatergic neurons, and GABAergic interneurons that modulate principal cell activity. This modulation is thought to occur through several mechanisms, including direct large Ca2+ conductance through α7 nAChRs, by depolarization with subsequent activation of voltage-gated Ca2+ channels, and Ca2+-mediated release from intracellular stores.33 Furthermore, in vivo studies demonstrated that activation of α7 nAChRs potentiated hippocampal-prefrontal cortex synapses.34,35 Interestingly, the gene encoding the α7 subunit harbors a high degree of variability and polymorphism.36 A study conducted by Carson et al. identified a single nucleotide polymorphism affecting the α7 nAChR promoter and 5ʹUTR as protective against AD. This finding led to the conclusion that this haplotype may result in increased α7 nAChR expression with improved cholinergic neurotransmission and ultimately decreased susceptibility to AD.37
The nAChRs are widely distributed throughout the central nervous system (CNS) and are expressed in both neurons and glia.38–40 Neuroinflammation mediated by the neuroimmune system, mainly microglia, is hypothesized to play a central and complex role in the progression of AD.41 On the one hand, microglia clear Aβ, likely to serve a protective role during early stages of AD.42–47 However, in aged brains and later stages of AD, microglia lose their protective ability and become overactive resulting in uncontrolled inflammation.48–51 A role of the α7 nAChR as a potent mediator of anti-inflammatory pathways was reviewed by Campbell52 and Egea et al.53 who described the engagement of the Jak2/STAT3 pathway to potentiate subsequent inhibition of nuclear factor-κB nuclear translocation and activation of the master regulator of oxidative stress, Nrf2/HO-1, following α7 nAChR activation. They concluded that α7 nAChR activation may be a potential therapeutic target for AD treatment because oxidative stress and neuroinflammation are currently thought to be the most important pathological mechanisms in neurodegenerative diseases such as AD.
Physiology of nAChR and Aβ in AD
The discovery of a direct Aβ–nAChR interaction led to a significant expansion in the molecular understanding of their role in AD.54 This interaction has been observed in rodent experimental models, as well as postmortem AD brains.54,55 Interestingly, Aβ enriches in regions abundant in α4β2 and α7 nAChRs; this observation may provide important insight into the selectivity of hippocampal and neocortical toxicity in AD.56–58 Under normal conditions, low levels of the α7 nAChRs–Aβ complex are thought to play a physiological role by enhancing learning and memory.59,60 However, at elevated levels, the α7 nAChRs–Aβ complex leads to disruption of the cholinergic phenotype, synaptic plasticity, and ultimately cognitive dysfunction.60–62
Inflammation and AD
It has been shown that nAChRs play a central role in the cholinergic anti-inflammatory pathway, demonstrated in blood-borne macrophages.63 In non-neuronal cells of the nervous system, an important observation regarding the role of nicotine-mediated anti-inflammatory effects is demonstrated by the reduction of lipopolysaccharide-induced tumor necrosis factor-α release by microglia. In these studies, activation of the α7 nAChR is thought to reduce inflammation by an increase of cyclooxygenase 2 expression and the synthesis of prostaglandin E2.32 Furthermore, blockade of ionic flux through the receptor did not alter tumor necrosis factor-α release, suggesting an alternate metabotropic mechanism. This mechanism has been shown to be crucial for neuroprotection in both in vitro and in vivo models of lipopolysaccharide-mediated neuronal death; in particular, nicotine has been demonstrated to prevent neuronal death in the substantia nigra after the injection of lipopolysaccharide.64 Nicotine induces the expression of a wide plethora of genes in human microglia such as TGF-β1, IL4, CX3CL1, CCR2, CXCR6, and has potent anti-inflammatory effects by reducing tumor necrosis factor-α and interleukin-1β release.
nAChR agonists are being developed as symptomatic treatments for Alzheimer’s dementia (summarized in Table 1).
Table 1.
Summary of Investigational Drugs Targeting Nicotinic Acetylcholine Receptors (nAChRs) in AD
| Compound/Clinicaltrials.gov ID | Mechanism of action | Participant’s characteristic | Status |
|---|---|---|---|
| Encenicline | Partial agonist of the α7 nAChR, with long half-life | Mild-to-moderate AD | Some improvement from baseline; suspended after phase 3 trial due to serious complications |
| Pozanicline (ABT-089) | Partial agonist with high affinity for α4β2, and low affinity for α6β2, not α7, and α3β4 | Mild-to-moderate AD | No improvement, futility criteria met |
| ABT-418 | Bioisostere of nicotine; agonist binding with high affinity to the α4β2, α7/5-HT3, and α2β2 | Moderate AD (small sample size) | Robust improvement in verbal learning and recall |
| WAY-317538/SEN-12333 | α7 agonist | Rodent model | Improved cognition in experimental model |
| RG3487 | α7 agonist and 5-HT3 antagonist | Mild-to-moderate AD | Press release with cognitive improvements, drug has been discontinued, reason unknown |
| Nelonicline (ABT-126) | α7 allosteric modulator | Mild-to-moderate AD | No significant improvement |
| AZD-3480 (TC-1734, ispronicline) | α4β2 partial agonist | Mild-to-moderate AD | Similar to donepezil, development halted |
| Varenicline | Α4β2, α3β4, α7, 5-HT3 partial agonist | Mild-to-moderate AD | No improvement in cognition, psychiatric, and gastrointestinal side effects |
Cholinergic dysfunction is a key feature in the pathology of AD. The degeneration of cholinergic neurons, a decrease in ACh-mediated signaling via reduction in choline acetyltransferase, and loss of cholinergic receptors form the cornerstone of our current understanding of AD and therapy. The role of the nAChRs has been evaluated extensively in human AD as well as numerous animal models of the disease. Here we discuss the role of nAChR in AD as well as the potential pharmacological interventions that may serve in the ameliorating and possibly reversing AD progression.
The history of AChEIs, including physostigmine,65 tacrine,66,67 tetrahydroaminoacridine,68 donepezil,69,70 metrifonate,71 and others, showed some promise in improving memory and cognitive deficits seen with AD. Xanomeline, an orthosteric muscarinic agonist, has also been investigated.72 However, there is less research available on nAChR agonists. In the past two decades, there have been nAChR agonists developed for symptomatic AD dementia, including encenicline, pozanicline, ABT-418, and WAY-317538.
The selective partial agonist of the α7 nAChR, encenicline, a member of the quinuclidines with high specificity for α7 nAChR, demonstrated procognitive effects at low nanomolar concentration in animal and human subjects. Pharmacokinetic analysis demonstrated linear kinetics with increasing doses, with a long plasma half-life of approximately 60 hours and a high volume of distribution. Thus, it can be given in once-daily dosing. Encenicline was also found to improve neuropsychological test performance in a U-shaped dose–response manner and increased relative power of α waves (8–12 Hz) on electroencephalogram, a bandwidth known to be affected in AD and mild cognitive impairment.73,74 Thus, a clear pharmacodynamic effect was demonstrated on the CNS in favor of a potential treatment of cognitive impairment. However, a phase 3 study at doses of 3 mg/day versus 2 mg/day was started in 2014 that resulted in suspension of further studies of encenicline in the fall of 2015 due to a rare but serious gastrointestinal issue in elderly patients. No further details regarding the gastrointestinal presentation were made available.75
Pozanicline (ABT-089) is a partial agonist that binds with high affinity to the α4β2 nAChR and has partial agonism to the α6β2 subtype, but not the α7 and α3β4 subtypes. A phase 2 double-blind adaptive study evaluating safety and efficacy for pozanicline when combined with AChEIs was conducted. The study (which included randomization of 337 patients) was stopped when futility criteria were met. There were no clinically meaningful differences among the treatment groups for any of the demographics or baseline characteristics in regard to AD when administered as adjunctive therapy to AChEIs.76,77
ABT-418 acts as an agonist binding with high affinity to the α4β2, α7/5-HT3, and α2β2 receptors. It has been studied in both attention deficit hyperactivity disorder and AD. One study found that the most robust effects after ABT-418 administration were seen in verbal learning and recall. This initial study was published in 1999, and no follow-up studies regarding AD are currently available. There are mixed results regarding adverse effects, though some studies noted patients experienced nausea.78–80
WAY-317538/SEN-12333 is a potent, selective, small α7 nAChR agonist that has also been investigated for use in AD. It was shown to have excellent brain penetration and oral bioavailability with a favorable pharmacokinetic profile. Initial results in a structure–activity relationship study and biological evaluation showed procognitive efficacy in multiple behavioral cognition assays.81 Despite these initial results, there are currently no trials to further investigate this agonist as a therapeutic medication.
RG3487 is a novel α7 nAChR selective partial agonist having 5-HT3 receptor antagonist properties. Initially tested in age-impaired rats, RG3487 improved object recognition memory and reversed spatial learning deficit and impairments in executive function tasks.82,83 Initially, RG3487 was tested in cognitive symptoms of schizophrenia. When further assessed in 2014 in patients with schizophrenia, cognitive symptoms did not improve, though negative symptoms associated with schizophrenia did improve.84 In 2006, Memory Pharmaceuticals announced positive preliminary data from a phase 1 trial.85 In 2007, they announced positive phase 2a results from a proof-of-concept trial in 80 patients with AD that ran over 8 weeks. The primary endpoint of the trial looked at change from baseline in the Quality of Episodic Secondary Memory factor score of the Cognitive Drug Research battery. Results for the trial noted that subjects receiving 5 and 15 mg of RG3487 demonstrated a statistically significant effect on the Quality of Episodic Secondary Memory compared to placebo.86 In 2009 and 2010, a phase 2 dose ranging study in 389 people with mild-to-moderate AD compared 1, 5, or 15 mg/day of RG3487 to placebo. Results of this phase 2 study have not been announced to the public. Development of this drug has been discontinued.85
Nelonicline (ABT-126) is an α7 nAChR allosteric modulator developed to treat cognitive deficits in schizophrenia and AD. For AD, a phase 2 study in 2009 in 274 subjects with mild-to-moderate AD compared 5–25 mg of ABT-126 to placebo and to donepezil. Results showed good tolerability for ABT-126 with side effects similar to donepezil.87 In 2012, AbbVie started two 24-week phase 2b trials with 400 patients each, comparing doses from 25 to 75 mg. The first of the two trials compared ABT-126 combined with an AChEI to placebo. The second trial compared ABT-126 as a monotherapy to placebo. The studies found no efficacy at these higher doses was insufficient to continue further development.88,89 Each trial offered a 28-week, open-label extension to participants who completed the blinded portion of the study. These studies were terminated with formal results showing insufficient efficacy to continue development.87,90
AZD-3480 (TC-1734, ispronicline) is an α4β2 nAChR partial agonist. It showed memory-enhancing properties in rodents. Two phase 1 studies were performed evaluating pharmacokinetics, safety profile, and bioavailability. The first study was a double-blind, placebo-controlled crossover design with a rising single-dose scheme, whereas the second used a double-blind, placebo-controlled, parallel group design with a rising multiple-dose scheme.91 Adverse events were generally mild with dizziness and headache being reported most frequently.91–93 A phase 2b monotherapy trial was performed on 293 patients with mild-to-moderate AD. However, the trial failed to show superiority when compared to donepezil at 52 weeks and development as a pharmaceutical drug was halted.94
Varenicline (Chantix) is a partial α4β2 nAChR agonist that also acts on α3β4 and α7 nAChRs. It is most commonly prescribed to aid smoking cessation. It also acts as an agonist at 5-HT3 serotonin receptors, which may explain its effects on mood. In 2009 and 2010, a phase 2 trial comparing varenicline to placebo was performed.95 This 6-week trial involving 66 patients with mild-to-moderate AD measured for improvement in cognition. The results showed that varenicline did not improve cognition and patients developed worsening neuropsychiatric state and gastrointestinal side effects. Development of varenicline for AD was discontinued.96
Discussion
The nAChRs are widely dispersed throughout the CNS, making them a reasonable target when treating degenerative diseases. This is particularly true in patients with AD as targeted treatment would be both disease and target specific. AD is well documented to result from cholinergic deficit, with loss of production of ACh and loss of cholinergic neurons, specifically the α4β2 nAChR subtype. Despite this increasing understanding of the pathophysiology behind AD, there has been limited success with nAChR agonists in clinical trials as outlined earlier. Although this may create some hesitation to continue to pursue nAChR agonists as a potential treatment option, we contend that there are several reasons that this endeavor should not be abandoned.
There is evidence that the cholinergic pathway can alter the course of AD. This has been demonstrated by two decades of AChEIs use. As nAChR agonists develop, there should be further investigation exploring the synergy between AChEIs and nAChR agonists. This would need to be evaluated cautiously to avoid any potentiating side effects, particularly gastrointestinal, that can arise from increased ACh in the body.
There is a growing consensus among experts that clinical trials with AD must enroll patients during the earliest stages of disease. A central issue to date in studies evaluating nAChR agonists is that patient selection often involves subjects with moderate-to-severe disease. As advances in reliable diagnostic tools become available (biomarkers, brain imaging, and cognitive testing), patients can be treated earlier and potentially alter the course of their disease.97,98 There are many ongoing trials regarding early diagnosis including identifying mild cognitive impairment due to AD prodrome. These patients, if willing, must be identified and enrolled in these studies. Along a similar theme, as there is early loss of the α4β2 receptor subtype, there have been advances to attempt to detect early loss of these receptors. This includes single photon emission computed tomography and positron emission tomography radioligands as potential diagnostic tools and biomarkers. As this early identification improves, so too may the disease-modifying potential of these medications.
Although researchers continue to develop nAChR agonists, it is possible that a more specific pharmaceutical that targets the α4β2 or α7 nAChRs would minimize non-CNS effects and have a safer side effect profile.16
Future drug development might continue to explore targeting the α7 nAChR with the aim of mitigating inflammation and amyloid toxicity due the well-described α7 nAChR–Aβ interaction as well as the modulation of inflammatory pathways.
In addition to being widely distributed throughout the peripheral nervous system and CNS, the nAChRs are also found within the immune system. The inflammatory response mediated by the immune system within the CNS leads to the classical maladaptive features of stroke, neurodegenerative disease, spinal cord injury, and autoimmune-mediated disease such as multiple sclerosis. Activation of inflammatory pathways serves many important functions in the human body such as tissue remodeling, repair, and metabolic adaptations.99 The neuroimmune system, represented by microglia, a type of macrophage that drives neuroinflammation, has a beneficial effect on scavenging cellular debris, tissue healing, and repair. However, chronic activation of microglia leads to the noxious effect on neurons and thus contributing to the pathophysiology of neurodegenerative diseases. Thus, continuing to pursue the cholinergic pathway as a potential therapeutic target in AD will likely be efficacious if implemented during the early prodromal stage prior to symptom onset.
Funding
This work was supported by NIA P30 AG091610, the Barrow Neurological Foundation, and the Keep Memory Alive Foundation. JLH and YA-H received no funding.
Declaration of Interests
JLH and YA-H declare no conflict of interest.
Acknowledgments
Justin L. Hoskin and Yazan Al-Hasan contributed equally to the study.
References
- 1. Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness?Trends Neurosci. 1999;22(6):273–280. [DOI] [PubMed] [Google Scholar]
- 2. Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez CM, Dineley KT. α7 nicotinic acetylcholine receptors in Alzheimer’s disease: neuroprotective, neurotrophic or both?Curr Drug Targets. 2012;13(5):613–622. [DOI] [PubMed] [Google Scholar]
- 4. Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36(2):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nyakas C, Granic I, Halmy LG, Banerjee P, Luiten PG. The basal forebrain cholinergic system in aging and dementia. Rescuing cholinergic neurons from neurotoxic amyloid-β42 with memantine. Behav Brain Res. 2011;221(2):594–603. [DOI] [PubMed] [Google Scholar]
- 6. Arias E, Alés E, Gabilan NH, et al. Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46(1):103–114. [DOI] [PubMed] [Google Scholar]
- 7. Arias E, Gallego-Sandín S, Villarroya M, García AG, López MG. Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells: role of nicotinic receptors. J Pharmacol Exp Ther. 2005;315(3):1346–1353. [DOI] [PubMed] [Google Scholar]
- 8. Terry AV Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306(3):821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 9. Selkoe DJ. Preventing Alzheimer’s disease. Science. 2012;337(6101):1488–1492. [DOI] [PubMed] [Google Scholar]
- 10. Morris MK, Bowers D, Chatterjee A, Heilman KM. Amnesia following a discrete basal forebrain lesion. Brain. 1992;115:1827–1847. [DOI] [PubMed] [Google Scholar]
- 11. Damasio AR, Graff-Radford NR, Eslinger PJ, Damasio H, Kassell N. Amnesia following basal forebrain lesions. Arch Neurol. 1985;42(3):263–271. [DOI] [PubMed] [Google Scholar]
- 12. Wagner MT, Spangenberg KB, Bachman DL, O’Connell P. Unawareness of cognitive deficit in Alzheimer disease and related dementias. Alzheimer Dis Assoc Disord. 1997;11(3):125–131. [DOI] [PubMed] [Google Scholar]
- 13. Lopez OL, Becker JT, Somsak D, Dew MA, DeKosky ST. Awareness of cognitive deficits and anosognosia in probable Alzheimer’s disease. Eur Neurol. 1994;34(5):277–282. [DOI] [PubMed] [Google Scholar]
- 14. Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996;40(3):399–410. [DOI] [PubMed] [Google Scholar]
- 15. Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137(1):22–54. [DOI] [PubMed] [Google Scholar]
- 16. Yu LF, Zhang HK, Caldarone BJ, Eaton JB, Lukas RJ, Kozikowski AP. Recent developments in novel antidepressants targeting α4β2-nicotinic acetylcholine receptors. J Med Chem. 2014;57(20):8204–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quik M, Wonnacott S. α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev. 2011;63(4):938–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J Comp Neurol. 2013;521(18):4124–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies P, Feisullin S. Postmortem stability of alpha-bungarotoxin binding sites in mouse and human brain. Brain Res. 1981;216(2):449–454. [DOI] [PubMed] [Google Scholar]
- 20. Sugaya K, Giacobini E, Chiappinelli VA. Nicotinic acetylcholine receptor subtypes in human frontal cortex: changes in Alzheimer’s disease. J Neurosci Res. 1990;27(3):349–359. [DOI] [PubMed] [Google Scholar]
- 21. Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol. 2011;82:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89(1):73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol. 2015;124:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fasoli F, Gotti C. Structure of neuronal nicotinic receptors. Curr Top Behav Neurosci. 2015;23:1–17. [DOI] [PubMed] [Google Scholar]
- 25. Lewis AS, Picciotto MR. High-affinity nicotinic acetylcholine receptor expression and trafficking abnormalities in psychiatric illness. Psychopharmacology (Berl). 2013;229(3):477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–246. [DOI] [PubMed] [Google Scholar]
- 27. McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74(8):1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. [DOI] [PubMed] [Google Scholar]
- 29. Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014;89(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Unwin N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynaptic membranes. Q Rev Biophys. 2014;46(4):283–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96:302–311. [DOI] [PubMed] [Google Scholar]
- 32. Millar NS, Harkness PC. Assembly and trafficking of nicotinic acetylcholine receptors (review). Mol Membr Biol. 2008;25(4):279–292. [DOI] [PubMed] [Google Scholar]
- 33. Placzek AN, Zhang TA, Dani JA. Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta Pharmacol Sin. 2009;30(6):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stoiljkovic M, Kelley C, Nagy D, Hurst R, Hajós M. Activation of α7 nicotinic acetylcholine receptors facilitates long-term potentiation at the hippocampal-prefrontal cortex synapses in vivo. Eur Neuropsychopharmacol. 2016;26(12):2018–2023. [DOI] [PubMed] [Google Scholar]
- 35. Matsuyama S, Matsumoto A, Enomoto T, Nishizaki T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur J Neurosci. 2000;12(10):3741–3747. [DOI] [PubMed] [Google Scholar]
- 36. Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behav Brain Res. 2000;113(1–2):43–56. [DOI] [PubMed] [Google Scholar]
- 37. Carson R, Craig D, McGuinness B, et al. α7 nicotinic acetylcholine receptor gene and reduced risk of Alzheimer’s disease. J Med Genet. 2008;45(4):244–248. doi: 10.1136/jmg.2007.052704. [DOI] [PubMed] [Google Scholar]
- 38. Parada E, Egea J, Buendia I, et al. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. 2013;19(11):1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119(4):965–977. [DOI] [PubMed] [Google Scholar]
- 40. Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61(1):75–111. doi: 10.1016/S0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 41. Solito E, Sastre M. Microglia function in Alzheimer’s disease. Front Pharmacol. 2012;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wyss-Coray T, Loike JD, Brionne TC, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9(4):453–457. [DOI] [PubMed] [Google Scholar]
- 43. Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2(12):679–689. [DOI] [PubMed] [Google Scholar]
- 44. Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response?Nat Med. 2006;12(9):1005–1015. [DOI] [PubMed] [Google Scholar]
- 45. Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304(1):1–7. [DOI] [PubMed] [Google Scholar]
- 46. Frautschy SA, Yang F, Irrizarry M, et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152(1):307–317. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1858113&tool=pmcentrez&rendertype=abstract. Accessed April 24, 2018. [PMC free article] [PubMed] [Google Scholar]
- 47. Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273(49):32730–32738. [DOI] [PubMed] [Google Scholar]
- 48. Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28(33):8354–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jimenez S, Baglietto-Vargas D, Caballero C, et al. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28(45):11650–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mrak RE, Griffin WST. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8(4):369–375. http://www.ncbi.nlm.nih.gov/pubmed/16556968. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 51. Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campbell IL. Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res Brain Res Rev. 2005;48(2):166–177. [DOI] [PubMed] [Google Scholar]
- 53. Egea J, Buendia I, Parada E, Navarro E, León R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol. 2015;97(4):463–472. [DOI] [PubMed] [Google Scholar]
- 54. Parri HR, Hernandez CM, Dineley KT. Research update: alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer’s disease. Biochem Pharmacol. 2011;82:931–942. [DOI] [PubMed] [Google Scholar]
- 55. Dineley KT. Beta-amyloid peptide–nicotinic acetylcholine receptor interaction: the two faces of health and disease. Front Biosci. 2007;12:5030–5038. [DOI] [PubMed] [Google Scholar]
- 56. Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275(8):5626–5632. [DOI] [PubMed] [Google Scholar]
- 57. Pym L, Kemp M, Raymond-Delpech V, Buckingham S, Boyd CAR, Sattelle D. Subtype-specific actions of beta-amyloid peptides on recombinant human neuronal nicotinic acetylcholine receptors (alpha7, alpha4beta2, alpha3beta4) expressed in Xenopus laevis oocytes. Br J Pharmacol. 2005;146(7):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu J, Kuo YP, George AA, Xu L, Hu J, Lukas RJ. beta-Amyloid directly inhibits human alpha4beta2-nicotinic acetylcholine receptors heterologously expressed in human SH-EP1 cells. J Biol Chem. 2004;279(36):37842–37851. [DOI] [PubMed] [Google Scholar]
- 59. Puzzo D, Privitera L, Leznik E, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28(53):14537–14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chu LW, Ma ES, Lam KK, Chan MF, Lee DH. Increased alpha 7 nicotinic acetylcholine receptor protein levels in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2005;19(2–3):106–112. [DOI] [PubMed] [Google Scholar]
- 61. Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. [DOI] [PubMed] [Google Scholar]
- 62. Wang HY, Stucky A, Liu J, Shen C, Trocme-Thibierge C, Morain P. Dissociating beta-amyloid from alpha 7 nicotinic acetylcholine receptor by a novel therapeutic agent, S 24795, normalizes alpha 7 nicotinic acetylcholine and NMDA receptor function in Alzheimer’s disease brain. J Neurosci. 2009;29(35):10961–10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sopori ML, Kozak W, Savage SM, et al. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology. 1998;23(2):189–204. [DOI] [PubMed] [Google Scholar]
- 64. Park HJ, Lee PH, Ahn YW, et al. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26(1):79–89. [DOI] [PubMed] [Google Scholar]
- 65. Thal LJ, Schwartz G, Sano M, et al. A multicenter double-blind study of controlled-release physostigmine for the treatment of symptoms secondary to Alzheimer’s disease. Physostigmine Study Group. Neurology. 1996;47(6):1389–1395. [DOI] [PubMed] [Google Scholar]
- 66. Raskind MA, Sadowsky CH, Sigmund WR, Beitler PJ, Auster SB. Effect of tacrine on language, praxis, and noncognitive behavioral problems in Alzheimer disease. Arch Neurol. 1997;54(7):836–840. http://www.ncbi.nlm.nih.gov/pubmed/9236571. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 67. Knopman D, Schneider L, Davis K, et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality, Tacrine Study Group. Neurology. 1996;47(1):166–177. [DOI] [PubMed] [Google Scholar]
- 68. Sahakian BJ, Owen AM, Morant NJ, et al. Further analysis of the cognitive effects of tetrahydroaminoacridine (THA) in Alzheimer’s disease: assessment of attentional and mnemonic function using CANTAB. Psychopharmacology (Berl). 1993;110(4):395–401. [DOI] [PubMed] [Google Scholar]
- 69. Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. The Donepezil Study Group. Dementia. 1996;7(6):293–303. [DOI] [PubMed] [Google Scholar]
- 70. Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50(1):136–145. [DOI] [PubMed] [Google Scholar]
- 71. Becker RE, Colliver JA, Markwell SJ, Moriearty PL, Unni LK, Vicari S. Double-blind, placebo-controlled study of metrifonate, an acetylcholinesterase inhibitor, for Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10(3):124–131. http://www.scopus.com/inward/record.url?eid=2-s2.0-0029792078&partnerID=40&md5=acb9ded09a7a1dc40a4715151fee5fab. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 72. Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–473. [DOI] [PubMed] [Google Scholar]
- 73. Bhattacharya BS, Coyle D, Maguire LP. Alpha and theta rhythm abnormality in Alzheimer’s disease: a study using a computational model. Adv Exp Med Biol. 2011;718:57–73. [DOI] [PubMed] [Google Scholar]
- 74. Prinz PN, Vitiello MV. Dominant occipital (alpha) rhythm frequency in early stage Alzheimer’s disease and depression. Electroencephalogr Clin Neurophysiol. 1989;73(5):427–432. [DOI] [PubMed] [Google Scholar]
- 75. Barbier AJ, Hilhorst M, Van Vliet A, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin Ther. 2015;37(2):311–324. [DOI] [PubMed] [Google Scholar]
- 76. Lenz RA, Pritchett YL, Berry SM, et al. Adaptive, dose-finding phase 2 trial evaluating the safety and efficacy of ABT-089 in mild to moderate Alzheimer disease. Alzheimer Dis Assoc Disord. 2015;29(3):192–199. [DOI] [PubMed] [Google Scholar]
- 77. Rueter LE, Anderson DJ, Briggs CA, et al. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev. 2004;10(2):167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Decker MW, Brioni JD, Sullivan JP, et al. (S)-3-methyl-5-(1-methyl-2-pyrrolidinyl)isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: II. In vivo characterization. J Pharmacol Exp Ther. 1994;270(1):319–328. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7913497. Accessed April 24, 2018. [PubMed] [Google Scholar]
- 79. Arneric SP, Sullivan JP, Briggs CA, et al. (S)-3-methyl-5-(1-methyl-2-pyrrolidinyl) isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: I. In vitro characterization. J Pharmacol Exp Ther. 1994;270(1):310 LP–318. http://jpet.aspetjournals.org/content/270/1/310.abstract. Accessed April 24, 2018. [PubMed] [Google Scholar]
- 80. Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer’s disease. Psychopharmacology (Berl). 1999;142(4):334–342. [DOI] [PubMed] [Google Scholar]
- 81. Haydar SN, Ghiron C, Bettinetti L, et al. SAR and biological evaluation of SEN12333/WAY-317538: novel alpha 7 nicotinic acetylcholine receptor agonist. Bioorg Med Chem. 2009;17(14):5247–5258. doi: 10.1016/j.bmc.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 82. Rezvani AH, Kholdebarin E, Brucato FH, Callahan PM, Lowe DA, Levin ED. Effect of R3487/MEM3454, a novel nicotinic α7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuro-Psychopharmacology Biol Psychiatry. 2009;33(2):269–275. [DOI] [PubMed] [Google Scholar]
- 83. Wallace TL, Callahan PM, Tehim A, et al. RG3487, a novel nicotinic α7 receptor partial agonist, improves cognition and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2011;336(1):242–253. [DOI] [PubMed] [Google Scholar]
- 84. Umbricht D, Keefe RS, Murray S, et al. A randomized, placebo-controlled study investigating the nicotinic α7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39(7):1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. RG3487 | ALZFORUM. Available from: https://www.alzforum.org/therapeutics/rg3487. Accessed April 7, 2018. [Google Scholar]
- 86. Memory Pharmaceuticals Announces Positive Phase 2a Results for MEM 3454 in Alzheimer’s Disease—Drugs.com MedNews. Available from: https://www.drugs.com/clinical_trials/memory-pharmaceuticals-announces-positive-phase-2a-results-mem-3454-alzheimer-s-2538.html. Accessed April 7, 2018. [Google Scholar]
- 87. Gault LM, Lenz RA, Ritchie CW, et al. ABT-126 monotherapy in mild-to-moderate Alzheimer’s dementia: randomized double-blind, placebo and active controlled adaptive trial and open-label extension. Alzheimers Res Ther. 2016;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gault LM, Meier A, Florian H, et al. A phase 2 trial of the efficacy and safety of the alpha7 Agonist ABT-126 as an add-on treatment in mild-to-moderate Alzheimer’s dementia. Alzheimer’s Dement. 2014;10(4):P917–P918. [Google Scholar]
- 89. Othman A, Meier A, Ritchie CW, Florian H, Gault LM, Tang Q. Efficacy and safety of the alpha7 agonist ABT-126 as a monotherapy treatment in mild-to-moderate Alzheimer’s dementia: results of a phase 2B trial. Alzheimer’s Dement. 2014;10(4):P137. [Google Scholar]
- 90. Florian H, Meier A, Gauthier S, et al. Efficacy and safety of ABT-126 in subjects with mild-to-moderate Alzheimer’s disease on stable doses of acetylcholinesterase inhibitors: a randomized, double-blind, placebo-controlled study. J Alzheimers Dis. 2016;51(4):1237–1247. [DOI] [PubMed] [Google Scholar]
- 91. Dunbar G, Boeijinga PH, Demazières A, et al. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology (Berl). 2007;191(4):919–929. [DOI] [PubMed] [Google Scholar]
- 92. Dunbar G, Demazières A, Monreal A, et al. Pharmacokinetics and safety profile of ispronicline (TC-1734), a new brain nicotinic receptor partial agonist, in young healthy male volunteers. J Clin Pharmacol. 2006;46(7):715–726. [DOI] [PubMed] [Google Scholar]
- 93. Gatto GJ, Bohme GA, Caldwell WS, et al. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects. CNS Drug Rev. 2004;10(2):147–166. http://www.ncbi.nlm.nih.gov/pubmed/15179444. Accessed April 24, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Targacept Drops Development of Alzheimer’s Drug—WSJ. Available from: https://www.wsj.com/articles/targacept-drops-development-of-alzheimers-drug-1405367773. Accessed April 8, 2018. [Google Scholar]
- 95. Varenicline | ALZFORUM. Available from: https://www.alzforum.org/therapeutics/varenicline. Accessed April 8, 2018. [Google Scholar]
- 96. Kim SY, Choi SH, Rollema H, et al. Phase II crossover trial of varenicline in mild-to-moderate Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37(3–4):232–245. [DOI] [PubMed] [Google Scholar]
- 97. Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Skok M, Lykhmus O. The role of α7 nicotinic acetylcholine receptors and α7-specific antibodies in neuroinflammation related to Alzheimer disease. Curr Pharm Des. 2016;22(14):2035–2049. [DOI] [PubMed] [Google Scholar]