Abstract
Background:
Myocardial infarction (MI) presentations are more common during winter months and morning hours. However, it is unknown whether MI with obstructive coronary artery disease (MI-CAD) and non-obstructive CAD (MINOCA) display similar patterns.
Methods:
We evaluated seasonal and circadian patterns of MI presentation by coronary artery disease (CAD) status and sex in patients with MI from 2007–2014 in the National Cardiovascular Data Registry (NCDR) Acute Coronary Treatment Intervention Outcomes Network (ACTION) Registry-Get With the Guidelines. Adult patients who underwent coronary angiography for MI were included. Patients with missing age, sex, or angiographic data, cocaine use, thrombolytic therapy prior to catheterization, or prior revascularization were excluded. Baseline demographics and characteristics of symptom onset, including season and time of day of presentation, were compared by CAD status and sex.
Results:
Among 322,523 patients, 112,547 were female (35%); 18,918 had MINOCA (5.9%). There was no seasonal pattern of MI overall. However, both men and women with MINOCA presented more often in the summer and fall while MI-CAD presentations were equally distributed across seasons. The most common time of presentation was 8am-2pm regardless of CAD status or sex. A secondary peak in women with MINOCA during late afternoon hours was also identified.
Conclusions:
Seasonal variation of MI differed between MINOCA and MI-CAD, with a small increase in MINOCA incidence in the summer and fall. MINOCA and MI-CAD most commonly occurred in the morning, with a secondary peak in late afternoon in women with MINOCA. These differences in presentation may relate to underlying MI pathophysiology.
Keywords: Myocardial infarction, epidemiology, nonobstructive coronary artery disease
Background:
Seasonal and circadian patterns associated with incidence of myocardial infarction (MI) have been documented but incompletely explained. Most studies have shown a peak in MI presentation during winter months and morning hours of the day.1–7 Among patients presenting with MI, 5–20% are found to have non-obstructive coronary artery disease (CAD) on angiography.8 Seasonal and circadian patterns in MI patients with non-obstructive CAD (MINOCA) have not been investigated specifically in comparison to those with obstructive CAD (MI-CAD). We sought to determine if there is a seasonal and circadian variation in MI presentation by CAD status (MI-CAD versus MINOCA) and sex among patients enrolled in a large national cardiovascular registry.
Methods:
Data were analyzed from the National Cardiovascular Data Registry (NCDR) Acute Coronary Treatment Intervention Outcomes Network Registry-Get With the Guidelines (ACTION Registry-GWTG). The registry contains prospectively collected data on all consecutive patients hospitalized with MI at > 750 participating hospitals in the United States (US).9
Patients in the registry who underwent coronary angiography for ST segment elevation myocardial infarction (STEMI) or non-ST segment elevation myocardial infarction (NSTEMI) at 765 clinical sites between January 1, 2007 and December 31, 2014, were included in this analysis. From a total population of 596,820, patients with missing age, sex, or angiographic data (n=61,761), recent cocaine use (n=3632), STEMI with thrombolytic therapy prior to catheterization (n=12,076), prior revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) (n=186,829), or cardiac arrest (n=9999) were excluded. Patients with missing data on season of presentation (n=635) were excluded in the season of MI presentation analysis. An additional 49,410 patients did not have data on time of presentation and were omitted from the time of day analysis.
Demographics, geographic region within the US, and characteristics of symptom onset including season and time of day were compared by CAD status (MI-CAD versus MINOCA) and sex. Geographic regions were divided into 4 categories: Northeast, South, Midwest, and West based on the US census divisions.10 There were no missing data for geographic region of MI cases. Symptom onset referred to the time when acute ischemic symptoms began at rest lasting ≥10 minutes and occurred up to 24 hours before admission or up to 72 hours for STEMI.9 Obstructive CAD was defined as ≥50% stenosis in any major coronary epicardial vessel and non-obstructive CAD was defined as <50% stenosis in all major epicardial vessels.
Seasons were defined as follows: Winter (December 1-February 28/29), Spring (March 1-May 31), Summer (June 1-August 31), and Fall (September 1-November 30). Time of day of symptom onset was divided into 4 six-hour windows: 2:01am-8:00am, 8:01am-2:00pm, 2:01pm-8:00pm, and 8:01pm-2:00am. Continuous variables are reported as median (25th, 75th percentile) and were compared using Wilcoxon rank sum tests. Categorical variables were compared using Pearson chi square tests.
Results:
A total of 322,523 patients with MI in the ACTION-GWTG registry met inclusion criteria for this analysis. MINOCA was present in 18,918 patients (5.9%) and was more common in women than men (10.5% vs 3.4%, p<0.0001, Tables 1 and 2).11 Patients with MINOCA were younger than those with MI-CAD, and men were younger than women in both MI-CAD and MINOCA groups (p<0.0001). Among patients with MINOCA, a majority of men (65.3%) were under the age of 60 years, while only 39.9% of women with MINOCA were less than 60 years old.11 Approximately half of patients across all MI subgroups were from the Southern United States, without differences by CAD status or sex.
Table 1: Presentation of myocardial infarction by CAD status.
Categorical variables are shown as % (N) and are compared using Pearson chi-square tests.
| All Patients | MI-CAD | MINOCA | ||
|---|---|---|---|---|
| N=322,523 | N=303,605 | N=18,918 | P-value | |
| Age Group | <0.0001 | |||
| <50 | 18% (57879) | 18% (53095) | 25% (4784) | |
| 50–59 | 27% (86744) | 27% (82151) | 24% (4593) | |
| 60–69 | 26% (84060) | 26% (79705) | 23% (4355) | |
| 70–79 | 18% (56588) | 18% (53316) | 17% (3272) | |
| 80–89 | 12% (37252) | 12% (35338) | 10% (1914) | |
| Geographic region | <0.0001 | |||
| Northeast | 8% (27053) | 8% (25569) | 8% (1484) | |
| South | 48% (155238) | 48% (145204) | 53% (10034) | |
| Midwest | 30% (97869) | 31% (92586) | 28% (5283) | |
| West | 13% (42363) | 13% (40246) | 11% (2117) | |
| Symptom Onset | ||||
| Season | <0.0001 | |||
| Winter | 25% (79131) | 25% (74658) | 24% (4473) | |
| Spring | 25% (80385) | 25% (75969) | 23% (4416) | |
| Summer | 25% (80758) | 25% (75814) | 26% (4944) | |
| Fall | 25% (81614) | 25% (76565) | 27% (5049) | |
| Unknown | 0.2% (635) | 0.2% (599) | 0.2% (36) | |
| Time of day | <0.0001 | |||
| 2am-8am | 24% (65467) | 24% (61865) | 23% (3602) | |
| 8am-2pm | 29% (78714) | 29% (74150) | 29% (4564) | |
| 2pm-8pm | 25% (67463) | 25% (63323) | 27% (4140) | |
| 8pm-2am | 22% (60834) | 22% (57606) | 21% (3228) | |
| Unknown | 16% (50045) | 15% (46661) | 18% (3384) | |
Table 2: Sex differences in demographics and presentation of myocardial infarction by CAD status.
Continuous variables are shown as median (25th, 75th percentiles) and compared using Wilcoxon rank sum tests between MI-CAD and MINOCA groups and sex (within MI-CAD and MINOCA groups). Categorical variables are shown as % (N) and are compared using Pearson chi-square tests.
|
MI-CAD |
MINOCA |
||||||
|---|---|---|---|---|---|---|---|
| All Patients | Men | Women | Men | Women | |||
| N=322,523 | N=202,821 | N=100,784 | P-Value | N=7,155 | N=11,763 | P-Value | |
| Age | 61 (52, 71) | 59 (51, 69) | 66 (56, 77) | <0.0001 | 54 (44, 64) | 63 (53, 74) | <0.0001 |
| <50 | 18% (57879) | 20% (40015) | 13% (13080) | 38% (2732) | 17% (2052) | ||
| 50–59 | 27% (86744) | 30% (61653) | 20% (20498) | 27% (1942) | 23% (2651) | ||
| 60–69 | 26% (84060) | 27% (54654) | 25% (25051) | 19% (1386) | 25% (2969) | ||
| 70–79 | 18% (56588) | 15% (30803) | 22% (22513) | 10% (746) | 22% (2526) | ||
| 80–89 | 12% (37252) | 8% (15696) | 20% (19642) | 5% (349) | 13% (1565) | ||
| Geographic region | <0.0001 | 0.9121 | |||||
| Northeast | 8% (27053) | 8% (17136) | 8% (8433) | 8% (564) | 8% (920) | ||
| South | 48% (155238) | 48% (96850) | 48% (48354) | 53% (3814) | 53% (6220) | ||
| Midwest | 30% (97869) | 30% (60923) | 31% (31663) | 28% (1988) | 28% (3295) | ||
| West | 13% (42363) | 14% (27912) | 12% (12334) | 11% (789) | 11% (1328) | ||
| Symptom Onset | |||||||
| Season | <0.0001 | 0.0315 | |||||
| Winter | 25% (79131) | 25% (49804) | 25% (24854) | 24% (1706) | 24% (2767) | ||
| Spring | 25% (80385) | 25% (51025) | 25% (24944) | 24% (1692) | 23% (2724) | ||
| Summer | 25% (80758) | 25% (51002) | 25% (24812) | 27% (1920) | 26% (3024) | ||
| Fall | 25% (81614) | 25% (50613) | 26% (25952) | 26% (1825) | 28% (3224) | ||
| Unknown | 0.2% (635) | 0.2% (377) | 0.2% (222) | 0.2% (12) | 0.2% (24) | ||
| Time of day | <0.0001 | <0.0001 | |||||
| 2am-8am | 24% (65467) | 24% (41480) | 24% (20385) | 26% (1521) | 22% (2081) | ||
| 8am-2pm | 29% (78714) | 30% (51109) | 28% (23041) | 28% (1631) | 30% (2933) | ||
| 2pm-8pm | 25% (67463) | 25% (42915) | 24% (20408) | 25% (1453) | 28% (2687) | ||
| 8pm-2am | 22% (60834) | 22% (37864) | 24% (19742) | 22% (1270) | 20% (1958) | ||
| Unknown | 16% (50,045) | 15% (29453) | 17% (17208) | 18% (1280) | 18% (2104) | ||
No seasonal pattern in MI presentation was observed overall and in patients with MI-CAD (Table 1, Figure 1). This finding was consistent in all geographic regions (Supplemental Table 1). However, MINOCA occurred more commonly in the summer and fall in both sexes (Table 2). The fall peak was driven more by women with MINOCA compared to men (28% vs. 26%, p=0.0315). Circadian variation in MI was present, with a peak of onset between 8am-2pm overall (29% of all patients). This finding remained present in both MINOCA and MI-CAD groups and in both sexes (Table 2, Figure 2). A higher proportion of women with MINOCA also presented between 2pm-8pm (28%).
Figure 1:
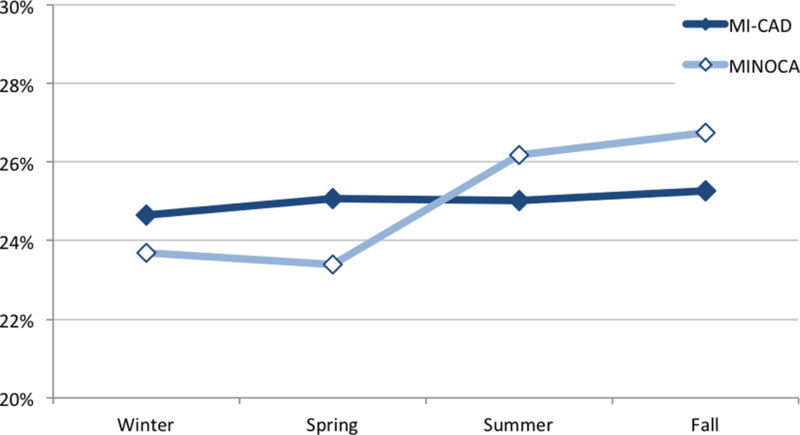
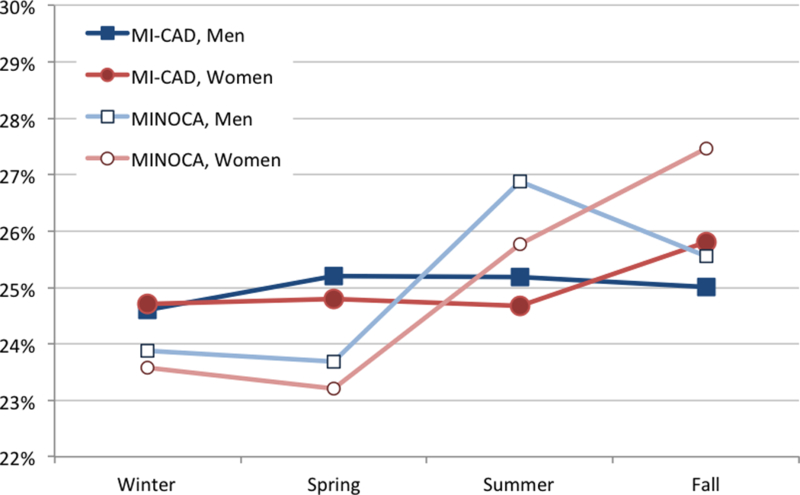
Percentage of patients presenting with MI each season. Panel A, MI-CAD vs. MINOCA; panel B, MI-CAD vs. MINOCA by sex.
Figure 2:


Percentage of patients presenting with MI over different time frames. Panel A, MI-CAD vs. MINOCA; panel B, MI-CAD vs. MINOCA by sex.
Discussion:
Seasonal and circadian variations associated with acute MI presentation have been described and became of great interest to help identify pathophysiologic triggers leading to acute coronary syndromes.1–7 Increases in sympathetic tone, blood pressure, platelet count and activity, and catecholamines associated with colder temperatures and morning hours have been hypothesized to explain these patterns.1, 2, 4–7 More recently, an association between infectious diseases that peak in winter (i.e. influenza, pneumococcal pneumonia, and other respiratory tract infections) and MI incidence and cardiovascular death has been described.12, 13
Interestingly, we did not observe a seasonal pattern of MI presentation in the ACTION Registry-GWTG overall in contrast to prior reports.1–7 We examined a similarly sized or larger patient population (n=322,523) than previously published studies.1–7 Given the association between influenza and MI, it is possible that increases in influenza vaccine coverage in the US from 1992 to 200914 may explain a decrease in winter MI presentations since prior studies were published.1–7 Pneumococcal vaccine administration may also explain the lower proportion of winter MI presentations in the contemporary era compared with prior analyses.15–17 The absence of a seasonal pattern of MI in the ACTION Registry-GWTG was observed in all geographic regions, including regions with milder climates, such as the South, as well as regions with more extreme weather, such as the Northeast. A winter peak has been previously reported even in milder climates (South Atlantic US, Greece, Australia).1–3
Interestingly, we observed a seasonal pattern in patients with MINOCA, with a peak in summer and fall that was consistent in all geographic regions of the United States. This finding may relate to mechanisms of MINOCA other than plaque rupture, such as coronary vasospasm, takotsubo syndrome, and unrecognized myocarditis.18 In fact, recent research on the presentation of takotsubo syndrome has demonstrated peaks in summer and early fall.7, 19 Interestingly, the fall peak in our data was more notable in women with MINOCA compared to men, which may have been driven by takotsubo syndrome as it predominantly affects women.19
When evaluating circadian trends, we found an increase in MI presentation during morning and early afternoon hours that was similar across subgroups defined by sex and CAD status. The morning peak in both MI-CAD and MINOCA suggests that despite different mechanisms associated with MINOCA, patients with non-obstructive CAD may be similarly susceptible to biological changes and stressors that occur during the morning hours. Coronary spasm may be responsible for MINOCA. Provocation of coronary spasm has been reported to occur predominantly in the morning hours, and this may contribute to the diurnal variation with the incidence of MINOCA.20 Small studies on circadian rhythm of takotsubo presentation also seem to indicate a morning predominance, perhaps related to circadian rhythm in catecholamine levels.7 We also observed a secondary peak of MINOCA presentations during late afternoon hours among women, but at present there is not a clear explanation for this observation. This report underscores the significant role chronobiology may play in MI incidence even in patients without obstructive CAD, and warrants further investigation.
There are several limitations to our study. We restricted analysis to patients undergoing coronary angiography and without prior revascularization because of our focus on differences between patients with and without obstructive CAD. Some patients were excluded from the analysis due to missing data. ACTION Registry-GWTG does not collect pre-admission medications, which may be relevant to seasonal and circadian rhythms.9 Furthermore, the underlying pathophysiology of infarction in patients with MINOCA in this registry was not systematically evaluated, and therefore, the mechanisms hypothesized are speculative. This represents an unavoidable limitation of observational analyses, and highlights the need for additional investigation on this important topic.
In conclusion, there was no seasonal variation in MI presentation overall or in MI-CAD in the ACTION Registry-GWTG from 2007 to 2014. However, MINOCA incidence was higher in the summer-fall in both sexes, with the fall peak driven mostly by women. Patients with MINOCA and MI-CAD in both sexes presented more frequently during morning and early afternoon hours. A secondary peak in women with MINOCA during late afternoon hours was also identified. These different patterns may relate to the pathophysiology of MINOCA.
Supplementary Material
What is already known about this subject?
Seasonal and circadian patterns associated with incidence of myocardial infarction (MI) have been reported to show that MI is more common during winter months and morning hours.
What does this study add?
We evaluated seasonal and circadian patterns of MI presentation by CAD status and sex in patients with MI. Men and women with MI and non-obstructive coronary arteries (MINOCA) presented more often in the summer and fall while MI with obstructive CAD presentations were equally distributed across seasons. The most common time of presentation was 8am-2pm regardless of CAD status or sex. A secondary peak in women with MINOCA during late afternoon hours was also identified.
How might this impact on clinical practice?
These findings underscore the significant role chronobiology plays in MI incidence, including in patients without obstructive CAD. Seasonal and circadian patterns may relate to underlying MI pathophysiology and warrant further study.
Acknowledgments
Grant Support: This study was funded by the American College of Cardiology National Cardiovascular Data Registry.
References:
- 1.Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second national registry of myocardial infarction. J Am Coll Cardiol. 1998;31:1226–1233 [DOI] [PubMed] [Google Scholar]
- 2.Moschos N, Christoforaki M, Antonatos P. Seasonal distribution of acute myocardial infarction and its relation to acute infections in a mild climate. Int J Cardiol. 2004;93:39–44 [DOI] [PubMed] [Google Scholar]
- 3.Loughnan ME, Nicholls N, Tapper NJ. Demographic, seasonal, and spatial differences in acute myocardial infarction admissions to hospital in melbourne australia. Int J Health Geogr. 2008;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiza JR, de Llano JM, Messa JB, Lopez CA, Fernandez JA, Group AS. New insights into the circadian rhythm of acute myocardial infarction in subgroups. Chronobiol Int. 2007;24:129–141 [DOI] [PubMed] [Google Scholar]
- 5.Sayer JW, Wilkinson P, Ranjadayalan K, Ray S, Marchant B, Timmis AD. Attenuation or absence of circadian and seasonal rhythms of acute myocardial infarction. Heart. 1997;77:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reavey M, Saner H, Paccaud F, Marques-Vidal P. Exploring the periodicity of cardiovascular events in switzerland: Variation in deaths and hospitalizations across seasons, day of the week and hour of the day. Int J Cardiol. 2013;168:2195–2200 [DOI] [PubMed] [Google Scholar]
- 7.Manfredini R, Manfredini F, Fabbian F, Salmi R, Gallerani M, Bossone E, Deshmukh AJ. Chronobiology of takotsubo syndrome and myocardial infarction: Analogies and differences. Heart Fail Clin. 2016;12:531–542 [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFftUDoMI, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035 [DOI] [PubMed] [Google Scholar]
- 9.Peterson ED, Roe MT, Rumsfeld JS, Shaw RE, Brindis RG, Fonarow GC, Cannon CP. A call to action (acute coronary treatment and intervention outcomes network): A national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–499 [DOI] [PubMed] [Google Scholar]
- 10.Census Regions and Divisions of the United States. United States Census Bureau. 2010. Accessed 5/2018 https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.
- 11.Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, Reynolds HR. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the action registry-gwtg (acute coronary treatment and intervention outcomes network registry-get with the guidelines). Circ Cardiovasc Qual Outcomes. 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: A meta-analysis of case-control studies. Heart. 2015;101:1738–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Influenza Vaccination Coverage Among Children and Adults: United States, 2008–09 Influenza Season. United States Centers for Disease Control. 2009. Accessed 5/2018 https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5839a1.htm
- 15.Shen AK, Warnock R, Chu S, Kelman JA. Receipt of other routinely recommended vaccines relative to receipt of seasonal influenza vaccines: Trends from medicare administrative data, 2013–2015. Vaccine. 2018;36:4399–4403 [DOI] [PubMed] [Google Scholar]
- 16.Black CL, Williams WW, Arbeloa I, Kordic N, Yang L, MaCurdy T, Worrall C, Kelman JA. Trends in influenza and pneumococcal vaccination among us nursing home residents, 2006–2014. J Am Med Dir Assoc. 2017;18:735 e731–735 e714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamontagne F, Garant MP, Carvalho JC, Lanthier L, Smieja M, Pilon D. Pneumococcal vaccination and risk of myocardial infarction. CMAJ. 2008;179:773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aryal MR, Pathak R, Karmacharya P, Donato AA. Seasonal and regional variation in takotsubo cardiomyopathy. Am J Cardiol. 2014;113:1592. [DOI] [PubMed] [Google Scholar]
- 20.Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


