Abstract
Background:
Mesenchymal stromal cell (MSC)–based therapy has great potential to modulate chronic inflammation and enhance tissue regeneration. Crosstalk between MSC-lineage cells and polarized macrophages is critical for bone formation and remodeling in inflammatory bone diseases. However, the translational application of this interaction is limited by the short-term viability of MSCs after cell transplantation.
Methods:
Three types of genetically modified (GM) MSCs were created: (1) luciferase-expressing reporter MSCs; (2) MSCs that secrete interleukin (IL)-4 either constitutively; and (3) MSCs that secrete IL-4 as a response to nuclear factor kappa-light-chain-enhancer of activated B cell (NFκB) activation. Cells were injected into the murine distal femoral bone marrow cavity. MSC viability and bone formation were examined in vivo. Cytokine secretion was determined in a femoral explant organ culture model.
Results:
The reporter MSCs survived up to 4 weeks post-implantation. No difference in the number of viable cells was found between high (2.5 × 106) and low (0.5 × 106) cell-injected groups. Injection of 2.5 × 106 reporter MSCs increased local bone mineral density at 4 weeks post-implantation. Injection of 0.5 × 106 constitutive IL-4 or NFκB-sensing IL-4–secreting MSCs increased bone mineral density at 2 weeks post-implantation. In the femoral explant organ culture model, LPS treatment induced IL-4 secretion in the NFκB-sensing IL-4–secreting MSC group and IL-10 secretion in all the femur samples. No significant differences in tumor necrosis factor (TNF)α and IL-1β secretion were observed between the MSC-transplanted and control groups in the explant culture.
Discussion:
Transplanted GM MSCs demonstrated prolonged cell viability when transplanted to a compatible niche within the bone marrow cavity. GM IL-4–secreting MSCs may have great potential to enhance bone regeneration in disorders associated with chronic inflammation.
Keywords: anti-inflammation, bone regeneration, interleukin-4, mesenchymal stromal cells
Introduction
Mesenchymal stromal cells (MSCs) are characterized by their capacity for immunomodulation and multi-lineage differentiation, and can be isolated from several sources including bone marrow and adipose tissue. MSC-based therapy has great potential to modulate chronic inflammation and enhance tissue regeneration, including bone, and has been applied in more than 400 clinical trials, such as graft-versus-host disease, diabetes and osteoarthritis [1]. In the bone and joint diseases, MSC transplantation showed improved bone structure and reduced fracture incidence in the patients with osteogenesis imperfecta [2,3]. Local administration of MSCs was shown to enhance cartilage quality and decrease progressive destruction of the joint [4]. Encouraged by the promising results in early-phase clinical trials, strategies have been developed to further enhance the therapeutic effects of MSCs, including cell preconditioning [5] and genetic modification [6]. However, the translational application of MSCs in chronic diseases is limited by the short cell survival period of only 3–7 days after implantation [7–9].
Bone marrow is one of the most abundant sources for MSC isolation. Endogenous MSCs are mainly located in a unique bone marrow niche that includes hematopoietic stem cells, endothelial cells, macrophages and osteoblasts [10,11]. Although the regulatory mechanisms of hematopoietic stem cell homeostasis in the quiescent endosteal and the active perivascular niche have been identified [12], the most crucial environmental factors to maintain MSC viability in vivo remain largely unknown.
Bone formation and remodeling are closely associated with crosstalk between MSC-lineage cells and polarized macrophages [13]. At the beginning of the inflammatory process, macrophages infiltrate the local area, and are polarized into pro-inflammatory macrophages (M1) that initiate the clearance of pathogens or debris from damaged tissues. Resolution of inflammation is then mediated by anti-inflammatory cytokines, such as interleukin (IL)-4, which polarize macrophages into an anti-inflammatory, pro-neovascularization, pro–tissue-repairing phenotype (M2). Recent studies suggest that the status of macrophage polarization determines changes in local bone mineral density [14–16]. To enhance bone regeneration in the presence of inflammatory bone diseases, we previously generated two types of genetically modified (GM) IL-4–secreting MSCs. In the first construct, IL-4 was over-expressed constitutively (Cytomegalovirus (promoter)[CMV]-IL-4); in the second one, IL-4 expression was induced by the activation of the inflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B cell (NFκB) (NFκB-IL-4), allowing targeted IL-4 delivery that is active only when the cells are placed in an inflammatory microenvironment. We subsequently demonstrated improved capabilities for immunomodulation of macrophage phenotype by these GM IL-4–secreting MSCs in vitro [6]. However, the application of these novel GM MSCs has not been examined in an in vivo model.
We hypothesized that the bone marrow environment could support implanted MSCs and prolong cell viability and function. In the current study, the viability of MSCs and their effects on bone formation were examined in a murine model in which luciferase reporter MSCs were transplanted into the marrow space. The therapeutic potential of GM IL-4–secreting MSCs [6] was also examined in vivo.
Materials and methods
Isolation of murine MSCs
The methods of isolating murine bone marrow–derived MSCs have been described previously [17]. Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) approved this isolation protocol (APLAC 17566) and institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project. In brief, bone marrow was collected from the femurs and tibias of 8- to 10-week-old Balb/c male mice. For MSC isolation, the cells were carefully suspended and passed through a 70-μm strainer, spun down and resuspended in alpha-minimal essential medium (α-MEM) supplied with 10% MSC certified fetal bovine serum (FBS; Invitrogen) and antibiotic anti-mycotic solution (100 U penicillin, 100 μg of streptomycin and 0.25 μg of Amphotericin B/mL; Hyclone, Thermo Scientific). Media was replaced the next day to remove the unattached cells (passage one). The immunophenotype of isolated MSCs (Sca1+/CD105+/CD44+/CD45-/CD34-/CD11b-; Supplementary Figure 1) was characterized using LSR II flow cytometer (BD Bioscience) at passage four.
Generation of IL-4–secreting MSCs
The luciferase reporter and IL-4–secreting lentiviral vector preparations were performed as previously described using human embryonic kidney 293T cells (ATCC) [18]. In brief, the luciferase reporter or IL-4–expressing (CMV-IL-4 or NFκB-IL-4) lentivirus vector, psPAX2 packaging vector and pMD2G VSV-G envelope vector were co-transfected into 293T cells using a calcium phosphate transfection kit (Clontech) with 25 μmol/L chloroquine. The culture supernatant was collected 48 h and 72 h post-transfection and the cellular debris was removed by centrifugation. The virus titer was determined by using 293T cells; the titer of the multiplicity of infection (MOI) on 293T cells was used to calculate the amount of virus used in MSC infection (40:1). The supernatant was mixed with MSC culture medium at 1:1 ratio and supplemented with 6 μg/mL of polybrene (Sigma Aldrich) and infected to murine MSCs. The infection efficiency (number of Green fluorescence protein (GFP)+ cells) was confirmed using LSRII flow cytometer (BD) 4 days post-infection. Flow cytometry analysis was done on instruments in the Stanford Shared FACS Facility.
Cell implantation
The model described by Takada et al. in which cells are implanted into the bone marrow [19] was used in the present study. The cell number and the injection site were modified as described below. Balb/c male mice (12 weeks old) were used as the recipients of MSC implantation. The mice were placed under isoflurane inhalation anesthesia and the left and right distal femurs were exposed under aseptic conditions via a lateral parapatellar arthrotomy. A 25-gauge needle was used to drill an axial hole to the middle of the intercondylar region to gain access to the medullary cavity. The luciferase reporter MSCs (500,000 or 2,500,000 cells/10 μL phosphate-buffered saline [PBS]; five mice per group) were injected into bone marrow cavity of the right distal femur using a Hamilton micro-syringe. The mice were euthanized 4 weeks post-operation. For the IL-4–secreting MSCs, [1] vehicle control (n = 3), [2] MSC control (n = 3), (3) CMV-IL-4 (n = 4) and [4] NFκB-IL-4 (n = 4) MSCs were injected (500,000 cells/10 μL PBS) into the distal end of both left and right femurs. The mice were euthanized 2 weeks post-operation. The study design is summarized in Figure 1a.
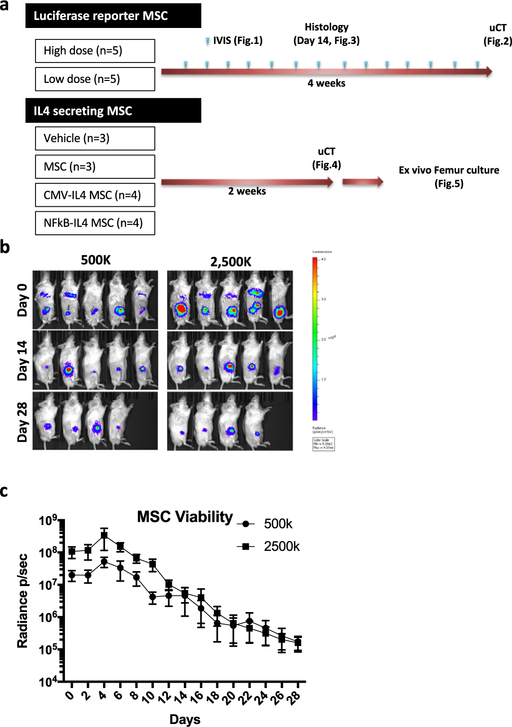
Figure 1.
Quantification of injected MSC viability using the IVIS system. The luciferase reporter MSCs (500,000 or 2,500,000 cells/10 μL PBS; five mice per group) were injected into bone marrow at femurs. Cell viability was evaluated using IVIS every 2 days for 28 days. (a) Summary of experiment design. (b) The IVIS images at day 0, 14 and 28. (c) Quantification results of MSC viability during 4 weeks of experiments (p/s/cm2/sr). p=photon, sr=surface radiance.
Quantification of implanted MSCs in vivo
The viability of the injected luciferase reporter MSCs was evaluated by the In Vivo Imaging System (IVIS; Perkin Elmer) immediately after the cell injection and then every 2 days (Caliper) for 28 days. Luciferase substrate D-luciferin was administered by intraperitoneal injection (150 mg/kg) 5 mins before imaging. One mouse from each group was euthanized at day 14 to examine the localization of implanted MSCs in femurs histologically. The data were analyzed using Living Image Software (Perkin Elmer) and presented as the bioluminescence (recorded as photon [p]/s/cm2/surface radiance [sr]) in the regions of interest (ROI) drawn over the injected femurs.
Micro-computed tomography scanning
The mice underwent micro-computed tomography (μCT) scans at day 14 (IL-4–secreting MSCs) or 28 (luciferase MSCs) post-injection using a Trifoil CT120 scanner with 50 μm resolution. Two three-dimensional (3D) ROI (ROI = 4 × 4 × 3 mm3) in the femurs were created at the epiphysis (beginning from distal end) and diaphysis (beginning 6 mm from the distal end of the femur). Quantification of bone mineral density (BMD; represents bone mineralization status or bone quality) and bone volume fraction (BVF; the mineralized bone per unit volume) and generation of 3D-reconstructed images were performed using GEMS MicroView. BMD was the primary outcome variable based on the cell transplantation model by Takada et al. [19].
Immunohistochemistry staining
Femurs were fixed in 4% paraformaldehyde overnight and decalcified in 0.5 mol/L ethylenediaminetetra acetic acid (EDTA; pH 7.4) for 2 weeks. The specimens were embedded in optimal cutting temperature compounds and cut into 10-μm transverse sections using Leica CM3050S Cryostat for subsequent histological staining. Implanted MSCs were identified by anti-luciferase antibody (Abcam) with the use of Avidin-biotin complex (Vector Laboratories) immunohistochemistry. Corresponding rabbit immunoglobulin (Ig)G antibodies were used to control the specificity of the staining.
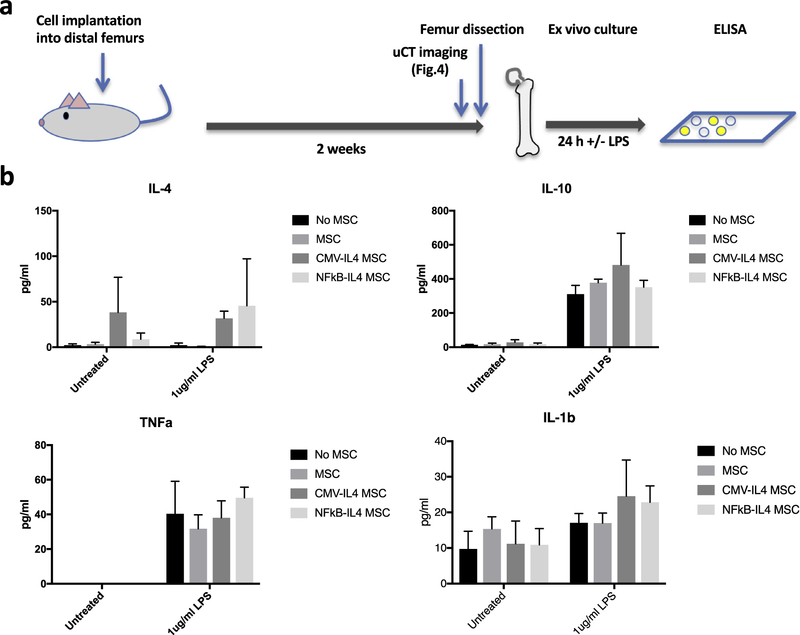
Femur organ culture model
Murine femurs implanted with IL-4–secreting MSCs or corresponding controls were dissected using sterile technique at 14 days post-operation. The femurs were placed in 12-well tissue culture plates supplemented with 1 mL of MSC growth medium with (left femurs) or without (right femurs) 1 μg/mL lipopolysaccharide (LPS). The supernatants were collected 24 h later, and the concentration of IL-4, tumor necrosis factor (TNF)α, IL-10 and IL-1β was measured using the DuoSet Enzyme-Linked ImmunoSorbent Assay (ELISA) kits (R&D system). The manufacturer’s protocol was followed carefully.
Statistical analysis
Non-paired t tests were performed for data with two groups, and a one-way analysis of variance (ANOVA) with Tukey post hoc test was performed for data with three or more groups. Two-way ANOVA was performed for data affected by two factors (LPS treatment and MSC injection). The statistical analysis was conducted using Prism 7 (GraphPad Software). Data are reported as mean ± standard deviations. P < 0.05 was chosen as the threshold of statistical significance.
Results
Prolonged cell viability of the luciferase reporter MSCs implanted into bone marrow
The luciferase signal was detected in both high (2,500,000) and low (500,000) MSC-injected mice throughout the 28-day experimental period (Figure 1b; five mice per group). The signal first increased, peaking at day 4, and gradually decreased thereafter (Figure 1c). The ratio in the signal strength between the high and low cell number groups remained around 5:1 until day 10, whereas no significant difference was found after day 12. The IVIS images showed that injected MSCs were distributed throughout the bone marrow cavity at earlier time points (Figure 1b), whereas at later time points the localization was restricted to the distal end of femurs (Figure 1b and Figure 2a).
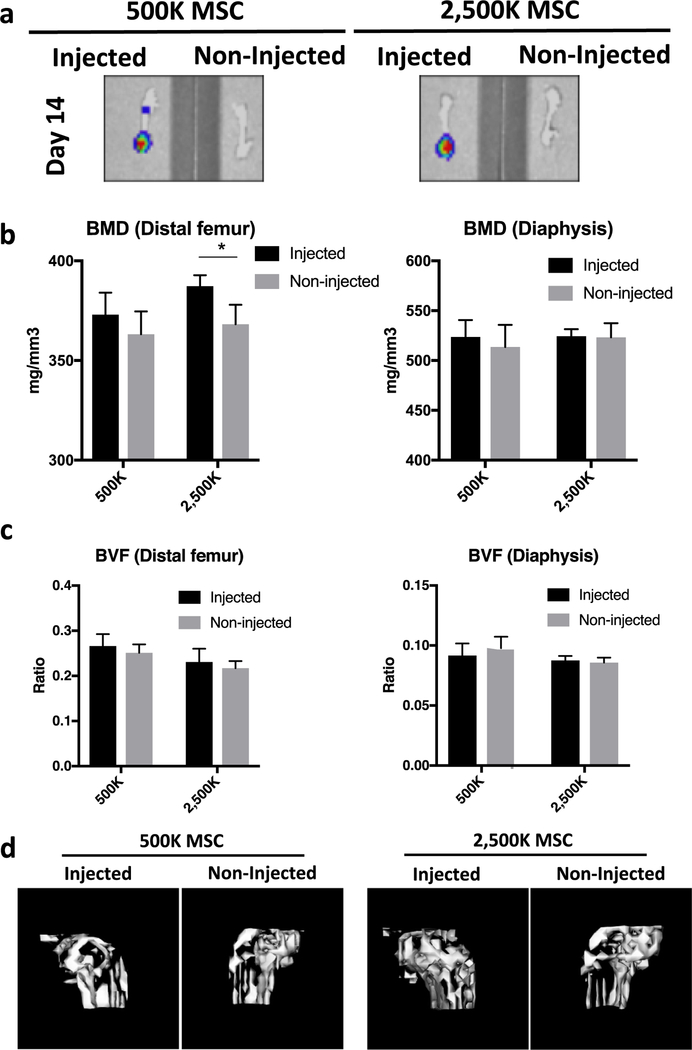
Figure 2.
Evaluation of the effects of injected MSCs on bone homeostasis using μCT. (a) Localization of injected luciferase reporter MSCs (500,000 or 2,500,000 cells per injection) examined using IVIS at day 14. (b and c) Quantification results of BMD (b) and BVF (c) in the femurs at the epiphysis (distal end) and diaphysis (700 HU) at day 28. (c) 3D reconstructed images at the distal end of femurs at day 28 (ROI = 2 × 4 × 3 mm3/1000 HU). *P < 0.05. HU= Hounsfield Unit (scale).
Increased BMD at the distal femurs implanted with higher dose of luciferase reporter MSCs
μCT analysis was performed at 4 weeks post-operation. The BMD at the distal femur was increased in the high-dose MSC-injected group (387.3 ± 5.52 mg/mm3; P=0.014) but not in the low-dose MSC-injected group (373.0 ± 11.01 mg/mm3) compared with the non-injected sites (368.2 ± 9.75 mg/mm3 and 363.2 ± 11.46 mg/mm3 in high- and low-dose MSC-injected groups, respectively). No significant difference of BMD between injected and non-injected sites was observed at the femoral diaphysis (Figure 2b and 2d). There was no significant difference of BVF between high- or low-dose MSC-injected versus non-injected sites in the femurs (Figure 2c).
Localization of implanted MSCs in bone marrow environments
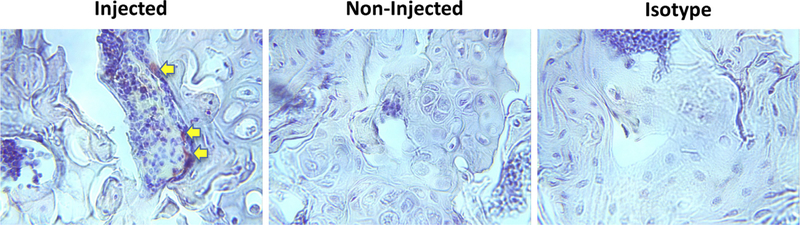
The femurs with or without MSC implantation at week 2 (Figure 2a) were processed for histological analysis. The immunohistochemistry staining showed that the luciferase-positive MSCs were lining the surface of the endosteum at the distal femur (Figure 3).
Figure 3.
Examination of the localization of injected MSCs in the bone marrow environment. The epiphyseal bone sections (40X) from the injected (left and right) or non-injected (middle) were stained with anti-luciferase antibody. Luciferase-positive cells indicated with yellow arrows.
IL-4–secreting MSCs increased BMD at the distal femur
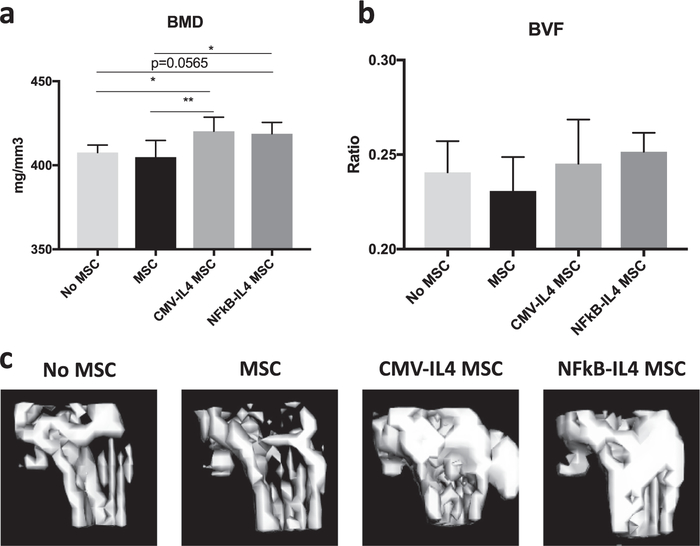
The GM MSCs with constitutive activity or NFκB-sensing IL-4 secretion [6] were injected into murine femurs, and μCT analysis was performed at 2 weeks post-operation. The BMD at the distal femur was significantly increased in CMV-IL-4 MSC– (420.3 ± 8.34 mg/mm3; four mice) and NFκB-IL-4 MSC– (418.8 ± 6.76 mg/mm3; four mice) injected groups (P = 0.005 and 0.013, respectively) compared with the unmodified MSC (407.7 ± 4.34 mg/mm3; three mice) or no MSC injected (404.9 ± 9.90 mg/mm3; three mice) control groups (Figure 4a and 4c). No significant difference in BVF among the groups was observed at the distal femurs (Figure 4b).
Figure 4.
Evaluation of the effects of injected IL-4–secreting MSCs on bone homeostasis by μCT. BMD (a) and BVF (b) at the femurs injected with vehicle control, MSC control, CMV-IL-4 MSCs and NFκB-IL-4 MSCs (500,000 cells) were analyzed (700 HU) 14 days post-operation. (c) 3D reconstructed images at the distal end of femurs at day 14 (ROI = 2 × 4 × 3 mm3/1000 HU). ★P < 0.05.
Secretion of IL-4 was detected in femurs injected with CMV-IL-4 and NFκB-IL-4 MSCs in the organ culture model
The MSC-injected femurs were dissected after μCT scanning and placed in culture medium for 24 h as illustrated in Figure 5a. IL-4 production was higher in the femurs injected with CMV-IL-4 and NFκB-IL-4 MSCs compared with the controls (column factor P < 0.05). Exposure to LPS showed a trend for increased IL-4 production in the NFκB-IL-4 group (P = 0.094). LPS exposure also significantly increased IL-10, TNFα and IL-1β secretion (row factor P < 0.005) in all four groups, but no differences were observed between the MSC-injected groups and controls (Figure 5b).
Figure 5.
Secretion of IL-4 by implanted MSCs in the ex vivo femur culture model. (a) Illustration of the ex vivo femur culture model. (b) IL-4, IL-10, TNFα and IL-1β secretions in the femur culture system for 24 h were analyzed using ELISA.
Discussion
Our findings indicate that transplanted exogenous luciferase reporter MSCs could survive up to 4 weeks, and the GM IL-4–secreting MSCs could survive for at least 2 weeks, when injected into the bone marrow cavity. Injection of a higher number of MSCs increased BMD at the injection site, and IL-4–secreting MSCs accelerated the process even when lower cell numbers were injected.
The amount of viable MSCs was quickly reduced to comparable amounts at later time points, irrespective of the initial number of implanted cells; the numbers reduced more slowly after day 18 (Figure 1c). These results suggest that the bone marrow environment could accommodate only a limited number of transplanted MSCs. It is likely that the excess amount of implanted MSCs were not able to survive due to limited space and nutrients, and a lack of necessary survival signals from paracrine factors and direct cell to cell contact. In the current study, it was not possible to study the localization of endogenous versus exogenous implanted MSCs in detail. Therefore, it is unclear whether implanted MSCs will compete with the endogenous cells for the limited space and resources for survival. This question could be clarified in future studies by using the MSC cell-tracking reporter murine models using nestin [10] or leptin receptor [20] promoters.
The majority of the MSCs that demonstrated long-term viability was located in the distal femur, rather than in the diaphyseal region (Figure 2a). This phenomenon could be explained by the unique osteoblast/endothelial cell niche within the trabecular bone region and the particular environmental conditions in this region. Several paracrine regulators have been reported to enhance MSC proliferation and survival in experimental models [21]. The potential paracrine regulation from osteoblast/endothelial cells on MSC viability remains to be clarified. Local oxygen level could also contribute to the determination of implanted MSC viability. Direct measurement of absolute local oxygen concentration showed that bone marrow has a hypoxic environment (range, 1.3–4.2%), despite the high vascular density [22]. The lowest oxygen concentration (1.3%) was found in the deeper peri-sinusoidal regions. Previous studies showed that cell proliferation and biological functions of ex vivo–expanded MSCs were improved under a hypoxic environment [23], and hypoxic preconditioning of MSCs enhanced the cell survival and angiogenesis in a heart ischemic rat model [24]. These findings indicate that a hypoxic environment is favorable for MSCs in vitro. Further investigation is required to clarify the optimal oxygen level for MSC survival in vivo.
Increased BMD was only observed in the high-dose MSC-injected femurs, suggesting that initial MSC number is crucial for long-term facilitation of the bone mineralization process. The mechanism of MSC-mediated tissue generation by direct cell differentiation or indirect paracrine regulation is still debated [25]. In our model, it is likely that the implanted MSCs enhanced bone mineralization through paracrine factors because the viable cell numbers at the study endpoints were similar.
IL-4 secretion can modulate the bone-remodeling process by different mechanisms. The sequential polarization of macrophages with IL-4 enhanced osteogenesis via crosstalk with MC3T3 osteoprogenitors [14] or murine MSCs (unpublished data) using in vitro co-culture models. A mechanistic study suggested that paracrine factors including CCL2, CCL5 and insulin growth factor 1 (IGF1) participate in the induction of MC3T3 cell differentiation [26]. Notably, the reduced osteogenesis in vitro using IL-4–secreting MSCs does not reflect the observation in the current in vivo model, indicating that the initial suppressive effect of IL-4 on osteogenesis could be a result of the in vitro culture conditions [6]. IL-4 can also modulate osteoclast activity through direct inhibition of osteoclast precursors [27,28] or indirect regulation on the osteoclast-activating signals mediated by immune cells [29]. Although the amounts of IL-4 at the injected femurs were not quantified in vivo, it is expected that IL-4 secretion by implanted NFκB-IL-4 MSCs was induced by transient inflammation caused by the trauma of the cell injection procedure, and diminished to basal levels a few days later [6]. The transient induction of IL-4 secretion was sufficient to enhance bone mineralization after implantation in vivo (Figure 4), suggesting that the “on-demand”, inflammation-provoked NFκB-sensing IL-4–secreting MSCs may have great potential to mitigate inflammation-associated bone loss with minimal adverse effects in vivo (30–34).
The constitutive secretion and “on-demand” secretion of IL-4 by the implanted MSCs were demonstrated in the femur explant organ culture model. However, the protective effects on the modulation of proinflammatory cytokines were not observed (Figure 5b) due to the significant induction of IL-10 by LPS. MSC-mediated IL-10 induction in macrophages is a crucial protective mechanism against sepsis-associated death [35]. No significant difference in TNFα and IL-1β levels was observed between the MSC injected femurs versus controls, suggesting that endogenous cells were sufficient to mediate the protective mechanism.
There are other limitations in this study. The cell viability of IL-4–secreting MSCs was not quantified due to their lacking the luciferase reporter expression. The potential effects of IL-4 on MSC viability in vivo were not addressed. In addition, the inflammatory response could be limited in the current model to induce a higher amount of IL-4 secretion in the NFκB-IL-4 MSC in vivo. The therapeutic effects of the IL-4–secreting MSCs will be further examined using inflammatory bone disease models.
In conclusion, injection of transplanted bone marrow MSCs into bone marrow cavity have extended cell survival and increased bone mineralization. Identification of critical factors supporting MSC cell survival in vivo could help to advance the design of bio-scaffolds for MSC-based cell therapy and tissue engineering.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko Foundation.
Footnotes
Disclosure of interests: The authors have no conflicts of interest to declare.
Supplementary materials
Supplementary data to this article can be found online at doi:10.1016/j.jcyt.2018.06.009.
References
- [1].Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplantation 2016;25(5):829–48. [DOI] [PubMed] [Google Scholar]
- [2].Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001;97(5):1227–31. [DOI] [PubMed] [Google Scholar]
- [3].Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A 2002;99 (13):8932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentis J, Sanchez A, Garcia-Sancho J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation 2013;95(12):1535–41. [DOI] [PubMed] [Google Scholar]
- [5].Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Engineering. Part A 2017;23 (21–22):1212–20. [DOI] [PubMed] [Google Scholar]
- [6].Lin T, Pajarinen J, Nabeshima A, Lu L, Nathan K, Yao Z, Goodman SB. Establishment of NF-kappaB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy 2017;19(9):1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Matsumoto K, Futamura K, Saito T, Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage 2016;24(6):1061–70. [DOI] [PubMed] [Google Scholar]
- [8].Deveza L, Choi J, Lee J, Huang N, Cooke J, Yang F. Polymer-DNA Nanoparticle-Induced CXCR4 Overexpression Improves Stem Cell Engraftment and Tissue Regeneration in a Mouse Hindlimb Ischemia Model. Theranostics 2016;6 (8):1176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li L, Chen X, Wang WE, Zeng C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int 2016;2016:9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466(7308):829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. Journal of Experimental Medicine 2011;208(3):421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nature Reviews. Immunology 2017;17 (9):573–90. [DOI] [PubMed] [Google Scholar]
- [13].Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone 2016;86:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loi F, Cordova LA, Zhang R, Pajarinen J, Lin TH, Goodman SB, Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Research & Therapy 2016;7(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomaterialia 2012;8(7):2815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ma QL, Zhao LZ, Liu RR, Jin BQ, Song W, Wang Y, Zhang YS, Chen LH, Zhang YM. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014;35(37):9853–67. [DOI] [PubMed] [Google Scholar]
- [17].Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, Pajarinen J, Egashira K, Yao Z, Goodman SB. NF-kappaB Decoy Oligodeoxynucleotide Enhanced Osteogenesis in Mesenchymal Stem Cells Exposed to Polyethylene Particle. Tissue engineering. Part A 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pajarinen J, Lin TH, Sato T, Loi F, Yao Z, Konttinen YT, Goodman SB. Establishment of Green Fluorescent Protein and Firefly Luciferase Expressing Mouse Primary Macrophages for In Vivo Bioluminescence Imaging. PloS one 2015;10(11):e0142736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takada K, Inaba M, Ichioka N, Ueda Y, Taira M, Baba S, Mizokami T, Wang X, Hisha H, Iida H, Ikehara S. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells 2006;24(2):399–405. [DOI] [PubMed] [Google Scholar]
- [20].Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15(2):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Research & Therapy 2010;1(4):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Cote D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014;508(7495):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. Journal of Cellular Physiology 2010;223(1):27–35. [DOI] [PubMed] [Google Scholar]
- [24].Liu XB, Wang JA, Ji XY, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Research & Therapy 2014;5(5):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 2011;9(1):11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cordova LA, Loi F, Lin TH, Gibon E, Pajarinen J, Nabeshima A, Lu L, Yao Z, Goodman SB. CCL2, CCL5, and IGF-1 participate in the immunomodulation of osteogenesis during M1/M2 transition in vitro. Journal of Biomedical Materials Research. Part A 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abu-Amer Y IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-kappaB. The Journal of Clinical Investigation 2001;107(11):1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wei S, Wang MW, Teitelbaum SL, Ross FP. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. The Journal of Biological Chemistry 2002;277(8):6622–30. [DOI] [PubMed] [Google Scholar]
- [29].Goodman SB, Gibon E, Pajarinen J, Lin TH, Keeney M, Ren PG, Nich C, Yao Z, Egashira K, Yang F, Konttinen YT. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. Journal of the Royal Society, Interface 2014;11(93):20130962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen C, Akiyama K, Wang D, Xu X, Li B, Moshaverinia A, Brombacher F, Sun L, Shi S. mTOR inhibition rescues osteopenia in mice with systemic sclerosis. Journal of Experimental Medicine 2015;212(1):73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fischer JE, Johnson JE, Kuli-Zade RK, Johnson TR, Aung S, Parker RA, Graham BS. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. Journal of Virology 1997;71(11):8672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol 2014;134(2):247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Relic B, Guicheux J, Mezin F, Lubberts E, Togninalli D, Garcia I, van den Berg WB, Guerne PA. Il-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. Journal of Immunology 2001;166(4):2775–82. [DOI] [PubMed] [Google Scholar]
- [34].Schmidt-Weber CB. Anti-IL-4 as a new strategy in allergy. Chem Immunol Allergy 2012;96:120–5. [DOI] [PubMed] [Google Scholar]
- [35].Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine 2009;15(1): 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.