Abstract
Tizanidine, a widely used muscle relaxant that can lower blood pressure, is metabolized by the cytochrome P450 1A2 (CYP1A2). We studied 1,626 patients prescribed tizanidine and 5,012 prescribed cyclobenzaprine concurrently with a strong CYP1A2 inhibitor. The primary outcome was severe hypotension, defined as systolic blood pressure (SBP)≤70 mm Hg during periods of drug co-exposure. Severe hypotension occurred more often in the tizanidine group [2.03% (n=33)] than the cyclobenzaprine group [1.28% (n=64)]; OR= 1.60, p=0.029. This difference remained statistically significant after adjustment for a log-transformed propensity score that included age, sex, race, Charlson’s comorbidity index, and concurrent use of antihypertensive medications (OR=1.57, p=0.049). A sensitivity analysis that defined hypotension as SBP<90 mm Hg also yielded higher rates of hypotension among patients prescribed tizanidine. In conclusion, CYP1A2 inhibition increases the risk of hypotensive episodes associated with the use of tizanidine in routine clinical practice.
Keywords: CYP1A2, drug interaction, tizanidine, cyclobenzaprine, hypotension
INTRODUCTION
Tizanidine, a muscle relaxant widely used to treat musculoskeletal pain and spasticity, is a centrally-acting alpha2A adrenergic receptor (α2A-AR) agonist and is metabolized by cytochrome P450 1A2 (CYP1A2). Drugs such as ciprofloxacin and fluvoxamine inhibit this enzyme and increase plasma concentrations of tizanidine profoundly. Previous research using healthy volunteers found that co-administration of ciprofloxacin or fluvoxamine increased tizanidine area-under-the-concentration time curve (AUC) levels 10-fold1 and 33-fold2, respectively. A drug-drug interaction of this magnitude is unusual and is likely to increase the risk for side effects.
As a centrally-acting α2A-AR agonist, tizanidine lowers central sympathetic outflow and thus decreases resting muscle tone. Tizanidine possesses the same mechanism of action as clonidine (another α2A-AR agonist and a prototypical antihypertensive drug) and may decrease blood pressure as a side effect. Based on studies in healthy volunteers, the tizanidine package insert warns against using tizanidine concomitantly with strong inhibitors of CYP1A23 including ciprofloxacin and fluvoxamine. Indeed, case reports have noted instances of severe hypotension when tizanidine was administered concomitantly with ciprofloxacin,4 as well as dizziness when it was administered with fluvoxamine.5 Furthermore, in double-blind randomized cross-over studies in healthy volunteers, the maximum decrease in systolic blood pressure was 35 mm Hg with concurrent use of tizanidine and ciprofloxacin,1 and concurrent use of tizanidine and fluvoxamine dropped the minimum systolic blood pressure from 115 to 79 mm Hg.2
Despite these concerns, tizanidine is commonly co-prescribed with CYP1A2 inhibitors; moreover, there is limited information about the clinical consequences of the interaction in routine clinical practice. Thus, we compared the frequency of severe hypotensive episodes in patients receiving tizanidine and cyclobenzaprine when co-prescribed ciprofloxacin or fluvoxamine. We chose cyclobenzaprine as an active control comparator drug because it is a widely-used muscle relaxant with similar indications to those of tizanidine, but with a different mechanism of action. Also, its metabolism is not primarily dependent on CYP1A2.6,7,8 We tested the hypothesis that, when used in combination with the CYP1A2 inhibitors ciprofloxacin or fluvoxamine, tizanidine is associated with higher rates of severe hypotension than cyclobenzaprine.
RESULTS
Baseline cohort characteristics
The cohort included 1,626 patients prescribed tizanidine and 5,012 prescribed cyclobenzaprine concurrently with a strong CYP1A2 inhibitor (either ciprofloxacin or fluvoxamine) (Table 1). Patients taking cyclobenzaprine were older than those prescribed tizanidine [55 (42–64) and 52 (42–62) years of age, respectively (p<0.001)]. There were more Caucasians in the tizanidine group than the cyclobenzaprine group (83.7% versus 81.9%, p=0.006), but there were no significant differences between the groups in the distribution of sex (p=0.21). Systolic blood pressure at baseline was similar in both groups [125 (112–140) and 126 (115–139), p=0.23].
Table 1:
Characteristics of the tizanidine and cyclobenzaprine cohorts
| Tizanidine (n=1626) | Cyclobenzaprine (n=5012) | p-value | |
|---|---|---|---|
| Age (years), median [IQR]a | 52 [42,62] | 55 [42,64] | <0.001 |
| Sex | |||
| Female, n (%) | 1063 (65.38%) | 3315 (66.14%) | 0.21 |
| Male | 519 (31.92%) | 1598 (31.88%) | |
| Unknown | 44 (2.71%) | 99 (1.98%) | |
| Race | |||
| White | 1361 (83.70%) | 4104 (81.88%) | 0.006 |
| Black | 168 (10.33%) | 656 (13.09%) | |
| Other (and unknown) | 97 (5.97%) | 252 (5.03%) | |
| Co-exposure Drug | |||
| Ciprofloxacin | 1595 (98.09%) | 4936 (98.48%) | 0.31 |
| Fluvoxamine | 31 (1.91%) | 76 (1.52%) | |
| Systolic blood pressure at baseline (mm Hg) | 125 (112–140) | 126 (115–139) | 0.23 |
| Lowest Blood Pressure during study period | |||
| Systolic blood pressure (mm Hg) | 115 [100,130] | 117 [103,130] | 0.003 |
| Diastolic blood pressure (mm Hg) | 68.5 [59,79] | 70 [60,80] | 0.001 |
| Charlson comorbidities, by ICD9 codes | |||
| Myocardial Infarction | 65 (4.00%) | 205 (4.09%) | 0.94 |
| Congestive Heart Failure | 146 (8.98%) | 418 (8.34%) | 0.41 |
| Peripheral Vascular Disease | 72 (4.43%) | 176 (3.51%) | 0.10 |
| Cerebrovascular Disease | 137 (8.43%) | 324 (6.46%) | 0.008 |
| Dementia | 4 (0.25%) | 17 (0.34%) | 0.80 |
| Pulmonary Disease | 210 (12.92%) | 667 (13.31%) | 0.71 |
| Connective Tissue Disease | 13 (0.80%) | 59 (1.18%) | 0.22 |
| Peptic Ulcer Disease | 29 (1.78%) | 43 (0.86%) | 0.003 |
| Liver Disease | 61 (3.75%) | 177 (3.53%) | 0.70 |
| Diabetes with or without complication | 310 (19.07%) | 976 (19.47%) | 0.37 |
| Paralysis/Hemiplegia | 55 (3.38%) | 79 (1.58%) | <0.001 |
| Renal Disease | 150 (9.23%) | 460 (9.18%) | 0.96 |
| Malignancy with or without metastasis | 119 (7.32%) | 395 (7.88%) | 0.48 |
| HIV/AIDS | 14 (0.86%) | 45 (0.90%) | 1.000 |
| Modified Charlson Score | 0 [0,2] | 0 [0,2] | 0.09 |
| Concurrent anti-hypertensive drug | |||
| Angiotensin converting enzyme inhibitors | 439 (27.0%) | 1,340 (26.74%) | 0.85 |
| Angiotensin receptor blockers | 190 (12.1%) | 680 (13.57%) | 0.05 |
| Calcium channel blockers | 347 (21.34%) | 1,071 (21.37%) | 1.0 |
| Beta blockers | 539 (33.15%) | 1,656 (33.04%) | 0.95 |
| Diuretics | 592 (36.41%) | 1,831 (36.53%) | 0.95 |
| Alpha-agonists | 148 (9.1%) | 325 (6.48%) | 0.001 |
| Others | 113 (7.0%) | 285 (5.69%) | 0.07 |
| Propensity score | 0.24 (0.22–0.27) | 0.24 (0.22–0.26) | <0.001 |
Data are presented as median [interquartile range] for continuous variables and number (%) for categorical variables.
Missing age for 216. Baseline systolic blood pressure available in 4,353 patients. HIV: Human immunodeficiency virus, AIDS: Acquired Immunodeficiency syndrome.
The co-prescription of ciprofloxacin accounted for the majority of co-exposure windows in both groups. The median modified Charlson score and the distribution of the individual co-morbidity components were similar in both cohorts, with the exception of cerebrovascular disease, hemiplegia/paraplegia, and peptic ulcer disease, which were more common in the tizanidine cohort. Concurrent use of antihypertensive medications was frequent, and there were small, but statistically significant differences in antihypertensive medication use. Patients in the cyclobenzaprine group received more concurrent ARBs, but fewer alpha-agonists, than patients in the tizanidine group.
Severe hypotension and concurrent use of tizanidine with a CYP1A2 inhibitor
The event rate of severe hypotension was higher in the tizanidine group [2.03% (n=33)] than in the cyclobenzaprine group [1.28% (n=64)](OR=1.60, 95% CI=1.05–2.45, P=0.029, univariate analysis). The results remained statistically significant after adjustment for the log-transformed propensity score, which included age, sex, race, Charlson’s score, and concurrent use of antihypertensive medication classes (OR=1.57, 95% CI= 1.00–2.45, p=0.049) (Table 2). A sensitivity analysis, excluding 2,017 episodes during which there was concurrent use of other CYP1A2 inhibitors, yielded similar results [OR=1.77 (1.03–3.03), p=0.038].
Table 2:
Association between concurrent prescription of tizanidine and a CYP1A2 inhibitor and severe hypotension (SBP≤70 mm Hg)
| Tizanidine (n=1,626) | Cyclobenzaprine (n=5,012) | p-value | |
|---|---|---|---|
| Windows with event (SBP≤70 mm Hg) | 33 | 64 | |
| Rate of Event (%) | 2.03 | 1.28 | 0.032 |
| Odds Ratio (95% CI) | 1.60 (1.05, 2.45) | Reference | 0.029 |
| Adjusted Odds Ratio – adjusted for age, race, and sex | 1.69 (1.08, 2.64) | Reference | 0.021 |
| Adjusted for logarithmically transformed PS* | 1.57 (1.0, 2.45) | Reference | 0.049 |
PS included the following variables: age, use of ciprofloxacin vs fluvoxamine, race, sex, Charlson score, current use of ARBs, ACE inhibitors, beta-blockers, calcium channel blockers, diuretics, alpha-agonist agents, and other antihypertensive agents.
Table S3 presents another sensitivity analysis among the 4,353 patients for whom there was baseline systolic blood pressure data. The baseline systolic blood pressure was added to the above variables to calculate an additional propensity score. The adjusted result was similar to the one presented for the primary analysis (OR=1.97, 95% C.I. 1.08–3.59, p=0.027).
Secondary outcomes: hypotension and lowest systolic blood pressure:
Patients in the tizanidine group had a 33% increased odds of hypotension (SBP<90 mmHg) compared to the cyclobenzaprine group, and this finding remained statistically significant after propensity score adjustment (OR=1.26, p=0.025) (Table 3).
Table 3:
Association between concurrent prescription of tizanidine and a CYP1A2 inhibitor and hypotension (SBP<90 mm Hg)
| Tizanidine (n=1,626) | Cyclobenzaprine (n=5,012) | p-value | |
|---|---|---|---|
| Windows with event (SBP<90 mm Hg) | 159 | 377 | |
| Rate of Event (%) | 9.78 | 7.52 | 0.005 |
| Odds Ratio (95% CI) | 1.33 (1.10, 1.62) | Reference | 0.004 |
| Odds Ratio – adjusted for age, race, and sex | 1.33 (1.09, 1.63) | Reference | 0.005 |
| Adjusted for logarithmically transformed PS* | 1.26 (1.03, 1.54) | Reference | 0.025 |
PS included the following variables: age, use of ciprofloxacin vs fluvoxamine, race, sex, Charlson score, current use of ARBs, ACE inhibitors, beta-blockers, calcium channel blockers, diuretics, alpha-agonist agents, and other antihypertensive agents.
The association between the lowest systolic blood pressure and inclusion in the tizanidine group was significant (Beta coefficient=−1.61, p=0.007), but the results were attenuated after propensity score adjustment (p=0.127) (Table 4).
Table 4:
Association between concurrent prescription of tizanidine and a CYP1A2 inhibitor and lowest systolic blood pressure
| Beta coefficient | 95% CI | p-value | |
|---|---|---|---|
| Beta coefficient (95% CI), unadjusted | −1.61 | −2.77, −0.44 | 0.007 |
| Beta coefficient – adjusted for age, race, and sex | −1.28 | −2.46, −0.11 | 0.032 |
| Beta coefficient-adjusted for logarithmically transformed PS* | −0.92 | −2.10, 0.26 | 0.127 |
PS included the following variables: age, use of ciprofloxacin vs fluvoxamine, race, sex, Charlson score, current use of ARBs, ACE inhibitors, beta-blockers, calcium channel blockers, diuretics, alpha-agonist agents, and other antihypertensive agents.
Role of comorbidities and concurrent use of antihypertensive agents
We performed an exploratory analysis to identify groups of patients at the highest risk for severe hypotension. After stratification by Charlson score, the highest risk for severe hypotension was among those individuals receiving tizanidine who had a Charlson score of 3 or more as compared with patients who were in the cyclobenzaprine group and had a Charlson score of zero (OR=10.60, 95% C.I. 4.19–26.81, p<0.001) (Figure 1).
Figure 1:
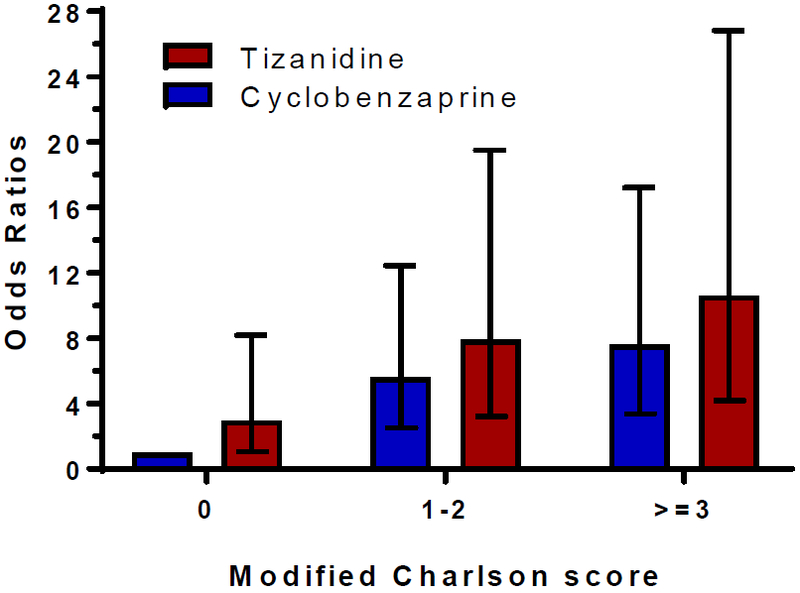
Co-prescription of strong CYP1A2 inhibitors and the risk of tizanidine-associated severe hypotension, results stratified by modified Charlson scores
Results adjusted for age, sex, and race. Error bars represent odds ratios and 95% confidence intervals as compared to reference group (cyclobenzaprine and Charlson score = 0).
Similarly, Figure 2 presents results stratified by the number of concurrent prescribed antihypertensive classes. Our results indicate that the risk for severe hypotension increases as the number of concurrent antihypertensive classes increase. The highest risk for severe hypotension was observed in patients in the tizanidine group who had concurrent use of three or more antihypertensive drug classes compared to the risk in patients in the cyclobenzaprine group who were not taking any concurrent antihypertensive (OR=5.64, 95% C.I. 2.30–13.81, p<0.001).
Figure 2:
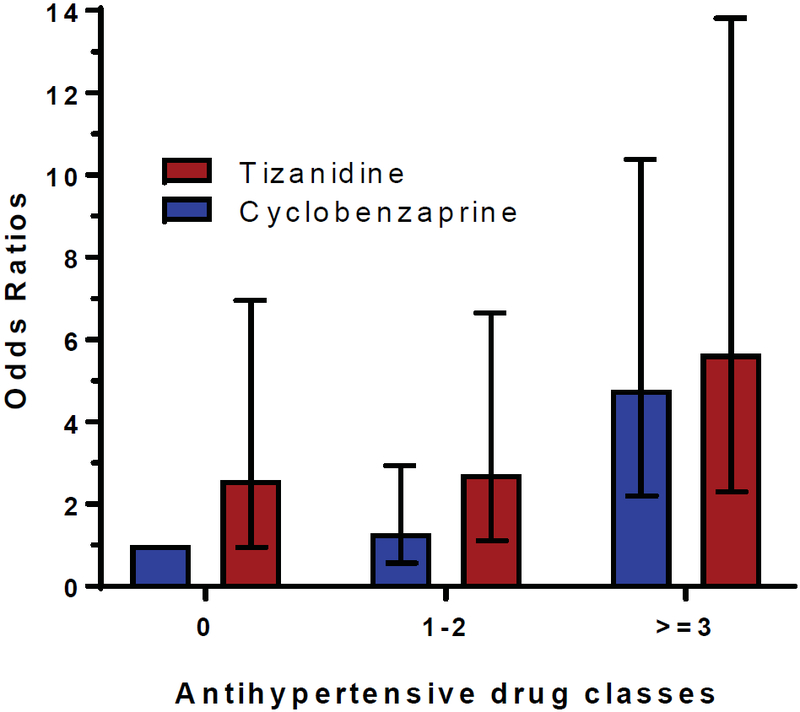
Co-prescription of strong CYP1A2 inhibitors and the risk of tizanidine-associated severe hypotension, results stratified by concurrent prescription of antihypertensive drug classes
Results adjusted for age, sex, and race. Error bars represent odds ratios and 95% confidence intervals as compared to reference group (cyclobenzaprine and no concurrent use of antihypertensive drugs).
DISCUSSION
To the best of our knowledge, this is the first study providing evidence of a drug-drug interaction between tizanidine and strong CYP1A2 inhibitors using electronic health records. This study shows that in a real-world clinical setting, the concurrent use of tizanidine and a strong CYP1A2 inhibitor is associated with higher rates of both severe (SBP≤70 mmHg) and less severe hypotensive events (SBP<90 mmHg) than those observed in the cyclobenzaprine group, independent of potential confounders. This is clinically important because co-prescription—particularly of tizanidine and ciprofloxacin—is common, despite package warnings.
Our results indicate that increased risk of hypotension and severe hypotension in patients prescribed concurrent tizanidine and a strong CYP1A2 inhibitor is not only plausible,1, 2 but it is clinically meaningful for several reasons. Because our data are from a real-world clinical population, the findings are more generalizable than any prior study that included small numbers of healthy volunteers. In particular, the inclusion criteria were broad, and thus this study encompassed patients typically excluded from randomized clinical studies (e.g., those with multiple comorbidities and multiple antihypertensive medication co-prescriptions). Moreover, the distribution of the Charlson’s score revealed a cohort with a wide range of co-morbidities.
Another strength of this study is the inclusion of an active comparator, cyclobenzaprine. Most notably, blood pressure effects are not associated with the use of cyclobenzaprine. Moreover, our data show the similarity between patients’ characteristics and the significant overlap in the propensity score distributions in both groups of patients, further supporting its appropriateness as an active comparator. Using such a system of comparison allows us to account for multiple potential confounders without overfitting the multivariate models. This breadth of patient characteristics and the use of an active comparator allows this study to provide information on the risks inherent in treating patients with concurrent co-morbidities and to examine risk stratification.
Our results have implications for the development of risk stratification. Starting with the implementation of the Framingham risk score in routine practice, there has been increased interest in how to optimize risk stratification for the prediction of cardiac outcomes.9 The present study suggests a trend towards higher risk for hypotension associated with concurrent use of tizanidine and a strong CYP1A2 inhibitor in patients with higher Charlson comorbidity scores as well as those with the highest number of prescribed anti-hypertensive drug classes. Prior to the present study, the information related to the drug-drug interaction between tizanidine and strong CYP1A2 inhibitors was limited to research reporting area-under-the-concentration time curve in healthy volunteers or to case reports; thus, neither the Charlson score nor the use of antihypertensive classes has been studied in the setting of co-prescription of CYP1A2 inhibitors with tizanidine. In the case reports, researchers have presented tizanidine-induced hypotension in patients with liver cirrhosis and suggest that the hypotensive episodes can be mediated by these patients’ impaired CYP1A2 activity.10 Similarly, some cases of severe hypotension are attributed to concurrent use of tizanidine and lisinopril,11–13 and one of the authors acknowledged that it is unclear whether this effect is drug or class specific.12 Our results suggest that the association between concurrent use of strong CYP1A2 inhibitors in combination with tizanidine and severe hypotension is present across all anti-hypertensive drugs and is directly related to the number of antihypertensive drugs prescribed.
In contrast to the observation that patients in the tizanidine group had a higher risk of hypotension and severe hypotension independently of potential confounders, the absolute difference in median systolic blood pressures between the two study groups was small. Although there was an inverse association between being in the tizanidine group and the lowest systolic blood pressure as a continuous variable, the results were no longer statistically significant after multivariate adjustment. This indicates that the main effect of tizanidine in combination with the strong CYP1A2 inhibitors is more evident among patients with the lowest systolic blood pressure measurements. Although no antihypertensive agents appear in the list of sensitive to moderate sensitive CYP1A2 substrates,14 it is possible that fluvoxamine or ciprofloxacin could have interacted with some antihypertensive agents. For example, triamterene, verapamil, betaxolol, carvedilol, and propranol have complex metabolism partially involving CYP1A2,15 and fluvoxamine increases the AUC of the B-blocker nebivolol from 12.1 ± 11.0 to 19.3 ± 19.5 ng*h/mL.16
The study has some limitations. First, it reflects hypotensive events experienced at the hospital. As we were not able to account for episodes that patients experienced in their homes, we underestimated the total number of hypotensive episodes. Second, medication exposure was ascertained by MedEx,17 which extracts and standardizes medication information in clinical text from the electronic health records; because we included information from outpatient visits, we do not have data on time of medication ingestion. Third, we based our definition of “exposure window” (i.e., drug coexposure) on prescribed medications, and we lack information on medication compliance. Nevertheless, as the use of d-EHR data for research continues to rise,18 we provide a proof of concept of the usefulness of this design to study drug-drug interactions. Fourth, there were only 107 windows of exposure in the fluvoxamine group, with no cases of severe hypotensive episodes and seven cases of moderate hypotensive episodes; therefore, we could not conduct a stratified analysis by CYP1A2 inhibitor.
METHODS
This was a retrospective cohort study in the de-identified electronic health record (d-EHR) database at Vanderbilt University Medical Center. The d-EHR is a copy of the hospital records with HIPAA identifiers removed by identification software and custom algorithms.19 The d-EHR database includes health records of more than 2.2 million patients, which are a date-shifted (within 1 calendar year) shadow of the EHR; temporal relationships within each patient’s course remain consistent.19 The Vanderbilt University Medical Center IRB approved the study and waived need for informed consent.
Cohort:
We used MedEx17 to identify patients 18 years of age or older who were prescribed the study muscle relaxants (tizanidine or cyclobenzaprine) along with ciprofloxacin or fluvoxamine in the same window of co-exposure (Figure S1). We defined window of co-exposure as the days when prescriptions of a muscle relaxant and an inhibitor overlapped. We set the maximum potential overlap to be six days (i.e., prescription date+5 days) because ciprofloxacin is usually prescribed for short periods and because adverse events due to a drug interaction are most likely to manifest early in the period of co-exposure. For further clarification of window of co-exposure scenarios, see Figure S2. We restricted our analysis to a patient’s unique, first window of co-exposure. Patients with overlapping windows of co-exposure for both study drugs (i.e., tizanidine and cyclobenzaprine) were excluded (N=95). We also excluded windows of co-exposure during which patients had no blood pressure readings recorded.
Outcomes:
We extracted all blood pressure measurements in the d-EHR during each patient’s window of co-exposure and identified the lowest systolic blood pressure (SBP) during this time. Of these, we reviewed the records of patients with SBP values >300 mmHg or <30 mmHg as well as any SBP lower than its associated diastolic blood pressure to identify likely errors in blood pressure recording. We corrected one flipped measure based on chart notes and replaced four measures of “0” with the second lowest SBP reading. We excluded six windows of co-exposure with SBP measures that were extreme outliers (e.g., SBP>1000) and that also had no additional measures.
The primary outcome was the occurrence of severe hypotension, defined as SBP ≤70 mm Hg, during the window of co-exposure (tizanidine with CYP1A2 inhibitor vs. cyclobenzaprine with CYP1A2 inhibitor). Secondary outcomes included hypotension (defined as SBP<90 mm Hg) and the lowest SBP (as a continuous variable) during the window of co-exposure.
Covariates
We extracted demographic variables, including age, race, and sex, as well as the specific CYP1A2 inhibitor drug from the d-EHRs. We then used ICD-9 codes to identify the presence of comorbid conditions during the prior year, and codes were collapsed according to the categories described in the Charlson/Deyo modified score.20 In brief, this score ranges from 0–25, and the list of comorbidities includes myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, pulmonary disease, connective tissue disease, peptic ulcer, liver disease, diabetes, hemiplegia, renal disease, malignancies, and HIV/AIDS, with the highest scores corresponding to the sickest patients.20 Table S1 provides a full list of diagnostic codes (ICD-9 codes) used in the analysis.
Drug exposures to antihypertensive medications were identified from Vanderbilt’s d-EHRs by electronic-prescribing tools and MedEx;17 at least one of the following identifiers—dose, route, frequency, or duration—was required to consider a drug exposure true. Table S2 includes the list of antihypertensive classes and medications within each class. We defined prescribed anti-hypertensive agents by class as “current” if the records indicated prescription of them in the 90 days prior to the start of the window of co-exposure. We defined baseline SBP as the closest outpatient reading before the start of the window of co-exposure (restricted to the prior year). We only included outpatient measurements.
Statistics
We present demographics and other covariates as mean and standard deviation (SD), median and interquartile range for continuous variables, and frequencies and percentages for categorical variables. We compared group characteristics using Wilcoxon rank sum tests and Fisher’s exact tests, as appropriate.
To examine the association between hypotension/severe hypotension and the concurrent use of tizanidine and a strong CYP1A2 inhibitor, we modeled multivariate logistic regressions with two pre-specified adjusted models: (1) adjusted for age, sex, and race; (2) model 1 plus comorbidities and concurrent use of antihypertensives. Given the large number of covariates for the second adjusted model, we estimated a propensity score,21 which we defined as the probability of assignment to tizanidine given demographics, Charlson score, and current use of antihypertensives (by class). After logarithmic transformation, we used the propensity score as a single covariate in the second model. A sensitivity analysis, including baseline SBP in the propensity score, was conducted among those patients for whom an outpatient blood pressure was recorded in the year prior to study entry. In an additional sensitivity analysis, we excluded 2,017 episodes during which there was concurrent use of other CYP1A2 inhibitors (norfloxacin, rofecoxib, enoxacin, zafirlukast, methoxsalen, acyclovir, allopurinol, cimetidine, peg-interferon, zileuton, oral contraceptives, and hormone replacement therapy). To examine the association between the lowest SBP and concurrent use of tizanidine and a strong CYP1A2 inhibitor, we built multiple linear regressions using the same adjustments, as explained above.
In a final step, we defined groups of patients at the highest risk for severe hypotensive episodes by examining the association of hypotension in subgroups according to Charlson scores or to the number of concurrent antihypertensive drugs and using multivariate logistic regression models. We considered p-values of ≤0.05 statistically significant. All analyses were performed using STATA (Version 15.1, College Station, TX).
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Concurrent use of tizanidine with strong CYP1A2 inhibitors is frequent. Prior to this study, information was limited to randomized clinical studies in healthy volunteers and case reports indicating risk of hypotension, but there was no data in routine clinical practice.
What question did this study address?
We tested the hypothesis that, when used in combination with strong CYP1A2 inhibitors, tizanidine is associated with higher rates of hypotension than cyclobenzaprine.
What does this study add to our knowledge?
This is the first study providing evidence of a drug-drug interaction between tizanidine and strong CYP1A2 inhibitors using electronic health records. We provide evidence that the risk of severe tizanidine-associated hypotension is higher in patients with more comorbidities and those who use three or more antihypertensive agents.
How might this change clinical pharmacology or translational science?
Clinicians should avoid co-prescribing these medications, particularly in patients with higher comorbidity indices and in those who receive three or more antihypertensive agents.
Acknowledgments
FUNDING:
Supported by grants: F31DK10844, GM109145, HL56693, GM120523, K23AR064768 (NIAMS), and the Rheumatology Research Foundation. The datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s Synthetic Derivative, which is supported by institutional funding and by the CTSA grant ULTR000445 from NCATS/NIH. The funding sources had no role in the collection, analysis, or interpretation of data, writing of the manuscript, or decision to submit for publication.
Footnotes
CONFLICT OF INTEREST: The authors declared no competing interests for this work.
References
- 1.Granfors MT, Backman JT, Neuvonen M and Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther 2004;76:598–606. [DOI] [PubMed] [Google Scholar]
- 2.Granfors MT, Backman JT, Neuvonen M, Ahonen J and Neuvonen PJ. Fluvoxamine drastically increases concentrations and effects of tizanidine: a potentially hazardous interaction. Clin Pharmacol Ther 2004;75:331–41. [DOI] [PubMed] [Google Scholar]
- 3.Momo K, Doki K, Hosono H, Homma M and Kohda Y. Drug interaction of tizanidine and fluvoxamine. Clin Pharmacol Ther 2004;76:509–10. [DOI] [PubMed] [Google Scholar]
- 4.Momo K, Homma M, Kohda Y, Ohkoshi N, Yoshizawa T and Tamaoka A. Drug interaction of tizanidine and ciprofloxacin: case report. Clin Pharmacol Ther 2006;80:717–9. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Matsuo N, Yamamoto A, Hazui T, Yagi H, Nakano M, Suzuki Y, Miki A, Ohtani H and Sawada Y. Piloerection induced by replacing fluvoxamine with milnacipran. Br J Clin Pharmacol 2007;63:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang RW, Liu L and Cheng H. Identification of human liver cytochrome P450 isoforms involved in the in vitro metabolism of cyclobenzaprine. Drug Metab Dispos 1996;24:786–91. [PubMed] [Google Scholar]
- 7.Peng WX, Wang LS, Li HD, Abd El-Aty AM, Chen GL and Zhou HH. Evidence for the involvement of human liver microsomes CYP1A2 in the mono-hydroxylation of daidzein. Clin Chim Acta 2003;334:77–85. [DOI] [PubMed] [Google Scholar]
- 8.Granfors MT, Backman JT, Laitila J and Neuvonen PJ. Tizanidine is mainly metabolized by cytochrome p450 1A2 in vitro. Br J Clin Pharmacol 2004;57:349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H and Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 10.Momo K, Homma M, Abei M, Hyodo I and Kohda Y. Tizanidine-induced hypotension in patients with liver cirrhosis. Eur J Clin Pharmacol 2008;64:647–8. [DOI] [PubMed] [Google Scholar]
- 11.Johnson TR and Tobias JD. Hypotension following the initiation of tizanidine in a patient treated with an angiotensin converting enzyme inhibitor for chronic hypertension. J Child Neurol 2000;15:818–9. [DOI] [PubMed] [Google Scholar]
- 12.Kao CD, Chang JB, Chen JT, Wu ZA, Shan DE and Liao KK. Hypotension due to interaction between lisinopril and tizanidine. Ann Pharmacother. 2004;38:1840–3. [DOI] [PubMed] [Google Scholar]
- 13.Publow SW and Branam DL. Hypotension and bradycardia associated with concomitant tizanidine and lisinopril therapy. Am J Health Syst Pharm 2010;67:1606–10. [DOI] [PubMed] [Google Scholar]
- 14.Administration USFD. Drug Development and Drug Interactions; Table of Substrates, Inhibitors and Inducers. 2017.
- 15.Zisaki A, Miskovic L and Hatzimanikatis V. Antihypertensive drugs metabolism: an update to pharmacokinetic profiles and computational approaches. Current pharmaceutical design. 2015;21:806–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheldiu AM, Vlase L, Popa A, Briciu C, Muntean D, Bocsan C, Buzoianu A, Achim M, Tomuta I, Todor I and Neag M. Investigation of a Potential Pharmacokinetic Interaction Between Nebivolol and Fluvoxamine in Healthy Volunteers. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2017;20:68–80. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Stenner SP, Doan S, Johnson KB, Waitman LR and Denny JC. MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assoc. 2010;17:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemingway H, Feder GS, Fitzpatrick NK, Denaxas S, Shah AD and Timmis AD. Using nationwide ‘big data’ from linked electronic health records to help improve outcomes in cardiovascular diseases: 33 studies using methods from epidemiology, informatics, economics and social science in the ClinicAl disease research using LInked Bespoke studies and Electronic health Records (CALIBER) programme Southampton (UK); 2017. [PubMed] [Google Scholar]
- 19.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR and Masys DR. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical Pharmacology and Therapeutics. 2008;84:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC and Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, Schneeweiss S and Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


