Abstract
BACKGROUND
Autoantibodies against GPIHBP1 cause chylomicronemia by blocking the ability of GPIHBP1 to bind lipoprotein lipase (LPL) and transport the enzyme to its site of action in the capillary lumen.
OBJECTIVE
A patient with multiple sclerosis developed chylomicronemia during interferon (IFN) β1a therapy. The chylomicronemia resolved when the IFN β1a therapy was discontinued. Here, we sought to determine whether the drug-induced chylomicronemia was caused by GPIHBP1 autoantibodies.
METHODS
We tested plasma samples collected during and after IFN β1a therapy for GPIHBP1 autoantibodies (by western blotting and with enzyme-linked immunosorbent assays). We also tested whether the patient’s plasma blocked the binding of LPL to GPIHBP1 on GPIHBP1-expressing cells.
RESULTS
During IFN β1a therapy, the plasma contained GPIHBP1 autoantibodies, and those autoantibodies blocked GPIHBP1’s ability to bind LPL. Thus, the chylomicronemia was due to the GPIHBP1 autoantibody syndrome. Consistent with that diagnosis, the plasma levels of GPIHBP1 and LPL were very low. After IFN β1a therapy was stopped, the plasma triglyceride levels returned to normal, and GPIHBP1 autoantibodies were undetectable.
CONCLUSION
The appearance of GPIHBP1 autoantibodies during IFN β1a therapy caused chylomicronemia. The GPIHBP1 autoantibodies disappeared when the IFN β1a therapy was stopped, and the plasma triglyceride levels fell within the normal range.
Keywords: GPIHBP1, hypertriglyceridemia, interferon β1a, chylomicronemia, autoantibodies
Introduction
GPIHBP1, glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1, is a protein of capillary endothelial cells that plays a key role in intravascular lipolysis.1, 2 GPIHBP1 plays three crucial functions in plasma triglyceride metabolism. First, it binds lipoprotein lipase (LPL) in the subendothelial spaces and transports it to its site of action in the capillary lumen.1, 2 Second, the GPIHBP1-bound LPL is responsible for the margination of triglyceride-rich lipoproteins (TRLs) along capillaries, allowing lipolysis to proceed.3 Third, GPIHBP1 stabilizes LPL against both spontaneous and ANGPTL4-mediated unfolding and inactivation.4, 5 The importance of GPIHBP1 in intravascular triglyceride processing is demonstrated by the fact that GPIHBP1 mutations cause lifelong, severe hypertriglyceridemia (chylomicronemia) associated with bouts of pancreatitis.6-12
Recent studies have demonstrated that some acquired cases of chylomicronemia are caused by GPIHBP1 autoantibodies (“GPIHBP1 autoantibody syndrome”).13, 14 GPIHBP1 autoantibodies block the ability of GPIHBP1 to bind LPL, preventing transport of the enzyme to the capillary lumen.13, 14 The hypertriglyceridemia associated with GPIHBP1 autoantibodies is typically severe13, 14 and is often associated with bouts of acute pancreatitis. The GPIHBP1 autoantibody syndrome is often, but not always, associated with another autoimmune disease (e.g., systemic lupus erythematosus).13 The frequency of GPIHBP1 autoantibodies among patients with chylomicronemia is not known but does not appear to be rare.13, 14
We previously reported a 34-year-old female patient who was admitted to the Okayama University Hospital for progressive numbness on the right side of her face and in her right upper and lower extremities.15 The initial examination revealed normal plasma triglyceride levels and elevated levels of anti-cardiolipin, anti-thyroglobulin, and anti-thyroid peroxidase antibodies.15 A brain MRI revealed lesions consistent with multiple sclerosis (MS), and the patient was treated with interferon (IFN) β1a therapy. Over a period of six months, the IFN β1a dose was gradually increased from 15 μg to 30 μg per week. The patient had no further signs of MS, but her plasma triglyceride levels increased from 97 mg/dl (at the time of presentation) to 2305 mg/dl (after a year on IFN β1a therapy).15 At that time, the serum LPL levels were low (22 ng/ml).15 No LPL mutations were identified.15 The patient was treated with 400 mg of bezafibrate, and because of abnormal thyroid function levothyroxine therapy was initiated.15 The patient’s thyroid tests normalized within a month, but the hypertriglyceridemia persisted.15 IFN β1a was replaced by fingolimod, and the plasma triglyceride levels normalized within 5 months.15
Because the patient had “thyroid autoantibodies” at initial presentation and because IFN β1a can in some cases “fuel” autoimmune diseases,16-18 we hypothesized that the chylomicronemia during the IFN β1a therapy was due to GPIHBP1 autoantibodies. We further hypothesized that the GPIHBP1 autoantibodies disappeared after IFN β1a therapy was discontinued. Here, we tested those hypotheses.
Materials and Methods
Subject
The 34-year-old female subject has been followed at the Okayama University Hospital for the past 6 years. This study was approved by the ethics committee of the Okayama University Hospital, and a written informed consent was obtained from the subject before the initiation of the study. Plasma samples free of any patient identifiers were shared with A.P.B. and S.G.Y. at UCLA.
Genetic and Blood Sample Analyses
Genomic DNA was extracted from the subject’s whole blood, and the coding regions of LPL, APOC2, APOC3, APOA5, GPIHBP1, LMF1, GCKR, CREB3L3, and GPD1 were sequenced.19, 20 The subject’s blood sample was collected after an overnight fast. LPL mass, hepatic lipase (HL) mass, endothelial lipase (EL) mass, and GPIHBP1 mass were measured by solid-phase immunoassays (ELISAs).21-25
Measurements of LPL and HL Activity
Pre- and post-heparin plasma was collected before and 10 min after an intravenous injection of heparin (50 IU/kg). LPL and HL activity were determined as described previously.26
Production of Recombinant Human GPIHBP1
Secreted versions of human GPIHBP1, CD177, C4.4A, and CD59 with an amino-terminal uPAR epitope tag were expressed in Drosophila S2 cells and purified on immunoaffinity column with a monoclonal antibody against uPAR (mAb R24).13, 27
ELISAs to Detect GPIHBP1 and LPL Autoantibodies in Human Plasma
GPIHBP1 autoantibodies were examined with two ELISAs.13 In the first ELISA, 0.5 μg of the uPAR-tagged GPIHBP1 was added to wells that had been coated with 0.5 μg of mAb R24. After washing, serial 1:2 dilutions of plasma samples were added to the wells and incubated overnight at 4°C. Human IgGs that bound to GPIHBP1 were detected with a horseradish peroxidase (HRP)–labeled goat anti-human [IgG + IgM] (1:50,000 in blocking buffer). After washing, 50 μl of TMB substrate was added to the wells, incubated on ice for 5 min, and the reaction was stopped with 50 μl of 2M sulfuric acid. The optical density (OD) was read at 450 nm.
In the second ELISA, plasma samples (1:500 dilution) were added to wells that had been coated with 0.5 μg of human GPIHBP1, CD177, C4.4A, or CD59. Human IgGs were then detected with HRP-labeled goat anti human [IgG + IgM]. In separate wells, known amounts of human IgGs were applied directly onto wells, and the autoantibody titer of the samples was determined by comparing the OD of the sample wells with the OD of human IgG-coated wells.
The possibility of LPL autoantibodies was tested with an ELISA in which FLAG-tagged human LPL from transfected CHO cells was captured on a plate coated with 0.5 μg/well of anti-FLAG antibody (Sigma Millipore). Plasma samples (1:500) were then added to the wells, and any human IgGs that bound to the LPL were detected with an HRP-labeled goat anti-human [IgG + IgM], exactly as described for the GPIHBP1 autoantibody ELISA. The binding of FLAG-tagged LPL to the anti-FLAG antibody was verified in a parallel ELISA with an HRP-labeled monoclonal antibody against human LPL (mAb 88B8).
The immunoglobulin classes (isotypes) and subclasses were determined by ELISA using the Human Immunoglobulin Class Screening Kit from Cygnus Technologies (Southport, NC).
Western Blots
Proteins in the conditioned medium of Drosophila S2 cells expressing human GPIHBP1 were size-fractioned by SDS-PAGE under nonreducing conditions and then transferred to a sheet of nitrocellulose. The membranes were incubated with plasma samples (1:40 dilution), and the binding of antibodies was detected with an IRDye-680–labeled donkey anti-human IgGs (Rockland, 1:200). The membrane was subsequently incubated with an IRDye-800–labeled human GPIHBP1-specific antibody RF4.21 A western blot was also performed with a rabbit antibody against human apolipoprotein CIII (Abcam). Antibody binding was visualized with a Li-Cor infrared scanner.
Immunocytochemistry
CHO pgsA-745 cells (2 × 106) were electroporated with 2 μg of plasmid DNA encoding S-protein–tagged versions of either wild-type human GPIHBP1 or GPIHBP1-W109S (a mutant GPIHBP1 that cannot bind LPL). On the next day, the transfected cells were incubated with plasma samples for 1 h at 4°C (1:20 dilution). After washing, the cells were incubated with V5-tagged human LPL (200 ng/well) for 1 h at 4°C. After fixation in 100% methanol, immunocytochemistry studies were performed on non-permeabilized cells with an Alexa Fluor 488–conjugated goat anti-human (IgG + IgM] (50 ng/ml); an Alexa Fluor 568–conjugated mouse anti-V5 antibody (1:50); and a rabbit antibody against the S-protein tag (0.2 μg/ml) followed by an Alexa Fluor 647–conjugated donkey anti-rabbit IgG (ThermoFisher, 2.5 μg/ml). Confocal images were taken with an Axiovert 200M microscope and processed with the Zen 2010 software (Zeiss).
Results
A patient with multiple sclerosis developed chylomicronemia during IFN β1a therapy. The plasma triglycerides increased from 97 mg/dl at the initial presentation to 2265 mg/dl during the IFN β1a therapy (Table 1). No coding mutations were found in LPL, APOC2, APOC3, APOA5, GPIHBP1, LMF1, GCKR, CREB3L3, or GPD1.
Table1.
Lipid and lipoprotein profiles during IFNβ1a therapy (on) and after discontinuing that therapy (off).
| IFNβ1a therapy |
Normal range | ||
|---|---|---|---|
| on | off | ||
| Total Chol. (mmol/l) | 4.45 | 5.48 | 3.36–5.69 |
| Triglycerides (mg/dl) | 2265 | 122 | 90.0–150 |
| HDL-C (mmol/l) | 0.59 | 1.5 | 1.06–2.59 |
| LDL-C (mmol/l) | 0.96 | 3.54 | 1.81–3.59 |
| Apo-AI (mg/dl) | 94 | 115 | 126–165 |
| Apo-AII (mg/dl) | 19.3 | 25.7 | 24.6–33.3 |
| Apo-AV ( μ g/ml)) | 1.38 | 0.27 | 0.09–1.78 |
| Apo-B (mg/dl) | 70 | 110 | 66.0–101 |
| Apo-CII (mg/dl) | 8.6 | 3.7 | 1.50–3.80 |
| Apo-CIII (mg/dl) | 22.7 | 9.2 | 5.40–9.00 |
| Apo-E (mg/dl) | 3.4 | 5.1 | 2.80–4.60 |
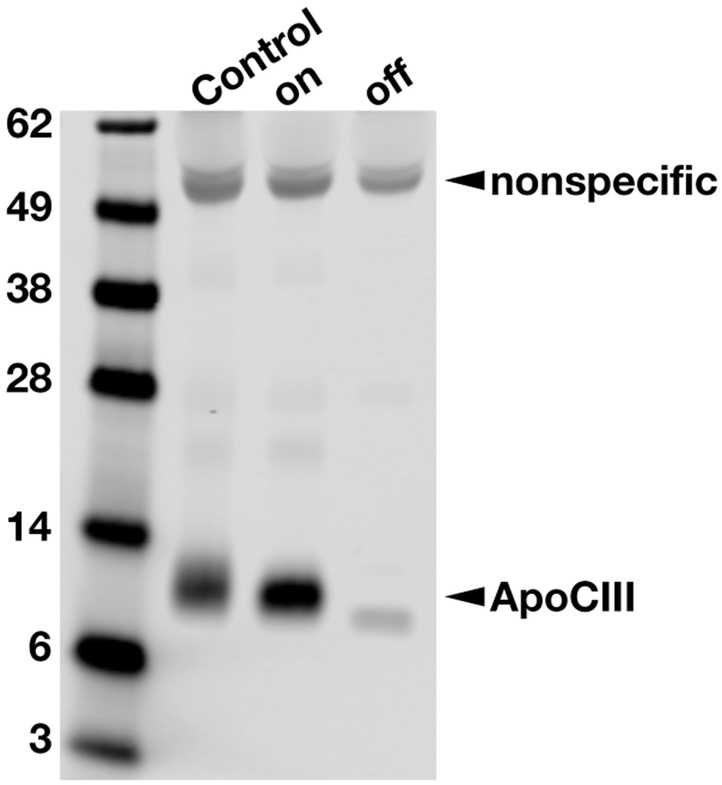
The chylomicronemia during IFN β1a treatment was associated with low plasma LPL mass and activity levels (Table 2). Endothelial lipase (EL) mass, and hepatic lipase (HL) mass and activity were within the normal range (Table 2). As expected, apo-CII and apo-CIII levels were slightly elevated during IFN β1a therapy but declined after the therapy was discontinued. (Table 1). Lower levels of plasma apo-CIII off IFN β1a therapy were also documented by western blotting (Fig. 1).
Table 2.
GPIHBP1 and lipase levels in the pre-and post-heparin plasma during IFNβ1a therapy (on) and after discontinuation of that therapy (off).
| On IFNβ1a |
Off IFNβ1a |
Normal range | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| GPIHBP1 mass (pg/ml) | 15.5 | 13.4 | 51.4 | 55.9 | 550-1528 |
| LPL mass (ng/ml) | 3.9 | 8.4 | 20.6 | 19.9 | 70.0-140 |
| HL mass (ng/ml) | 47.3 | 463 | 48.4 | 477 | 36.0-116 |
| EL mass (ng/ml) | 93 | 68.3 | 45.2 | 39.5 | 43.0-136 |
| LPL activity (U/L) | ND | 10.5 | ND | 27.5 | 40.0-209 |
| HL activity (U/L) | ND | 266 | ND | 351 | 198-859 |
Figure 1.
Assessing relative amounts of apo-CIII in the plasma by SDS-PAGE followed by western blotting with a goat polyclonal against human apo-CIII. The plasma levels of apo-CIII were higher on IFN β1a therapy (on) than after IFN β1a therapy was discontinued (off). A normolipidemic plasma sample (control) was also included as a control. A nonspecific band at ~50-kDa indicated that the loading of plasma samples was similar. IFN, interferon.
The chylomicronemia persisted for many months during IFN β1a therapy, but the triglyceride levels fell to normal after the IFN was discontinued.15 Most recently, the patient’s plasma triglyceride levels (off IFN β1a therapy) were 122 mg/dl (Table 1). After IFN β1a therapy was stopped, the preheparin plasma LPL levels increased from 3.90 ng/ml to 20.6 ng/ml; the post-heparin plasma LPL levels increased from 8.4 ng/ml to 14.2 ng/ml, and more recently, the post-heparin plasma LPL level was 19.9 ng/ml (Table 2).
Detection of GPIHBP1 autoantibodies in the patient’s plasma
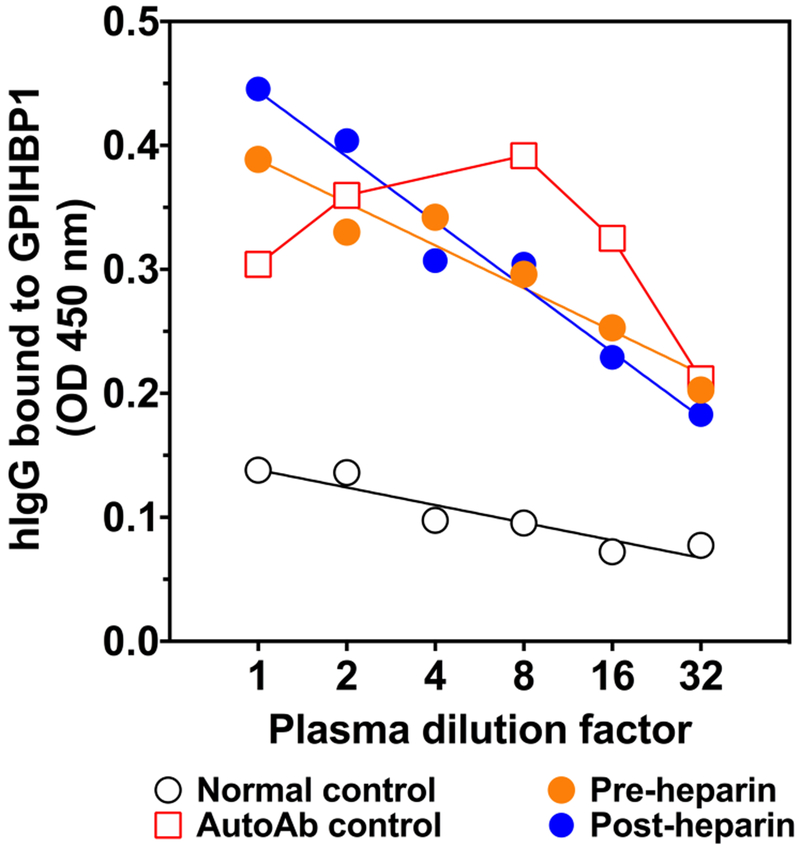
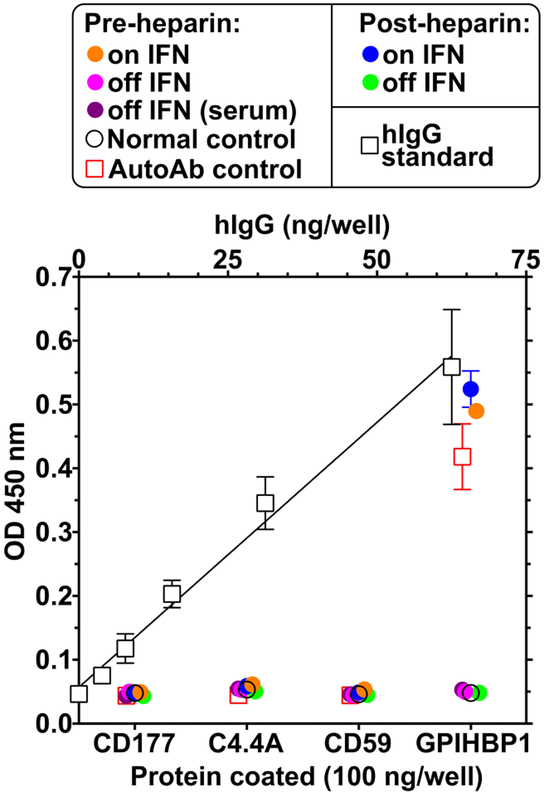
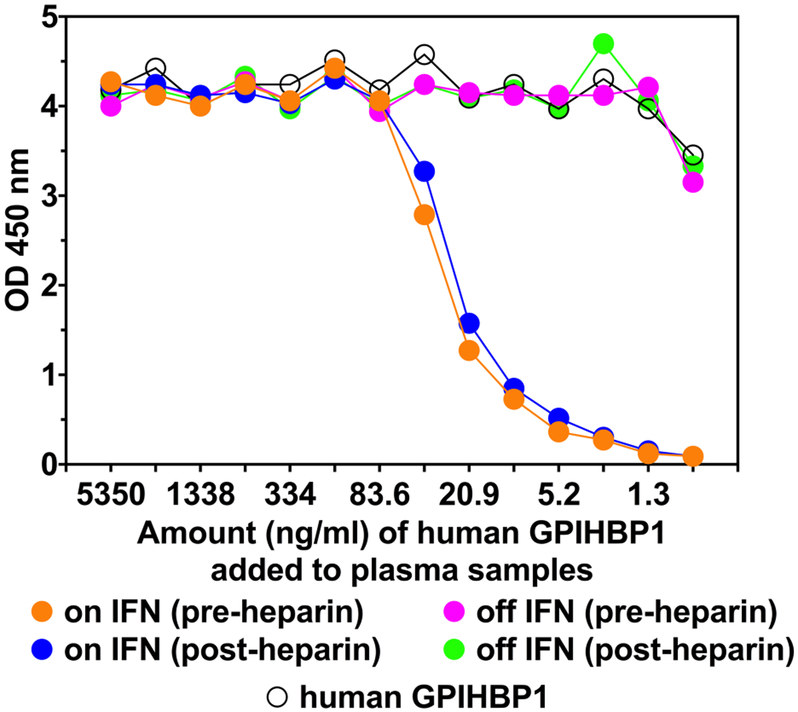
Because the patient had thyroid autoantibodies at the initial presentation and because the plasma levels of GPIHBP1 and LPL levels were low during IFN β1a therapy, we suspected that the chylomicronemia during IFN β1a therapy was due to the “GPIHBP1 autoantibody syndrome.” As judged by a solid-phase ELISA, both the pre- and post-heparin plasma samples collected during IFN β1a therapy contained GPIHBP1 autoantibodies at levels comparable to another patient with the GPIHBP1 autoantibody syndrome (Fig. 2). The autoantibodies during IFN β1a therapy bound specifically to GPIHBP1 and not to other LY6 proteins (Fig. 3). LPL autoantibodies could not be detected by ELISA. The GPIHBP1 autoantibody titer was 2160 U/ml in post-heparin plasma; a similar level (2476 U/ml) was detected in the preheparin plasma (Fig. 2). No GPIHBP1 autoantibodies could be detected in plasma or serum samples collected after the termination of the IFN β1a therapy (Fig. 3). The GPIHBP1 autoantibodies in the patient’s plasma interfered with the ability to detect recombinant GPIHBP1 after the GPIHBP1 was spiked into the patient’s plasma (Fig. 4).
Figure. 2.
The plasma of the patient treated with IFN β1a contains immunoglobulins that bind to GPIHBP1 in a dose-dependent manner. uPAR-tagged human GPIHBP1 produced in Drosophila S2 cells was captured on an ELISA plate with the uPAR-specific monoclonal antibody R24. Serial dilutions of the patient plasma samples (pre-heparin and post-heparin samples) were applied to the wells and incubated overnight. Immunoglobulins bound to GPIHBP1 were detected with an HRP-labeled goat anti-human IgG/IgM. Plasma samples from a normal control and a subject with GPIHBP1 antibodies (AutoAb control)13 were included as negative and positive controls, respectively. HRP, horseradish peroxidase; GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1.
Figure 3.
The IFN β1a treatment was associated with the presence of autoantibodies against GPIHBP1. The presence of autoantibodies in the patient’s blood, both during IFN therapy and after the therapy was discontinued, was assessed by ELISA. The samples consisted of plasma from a normal control, plasma from a documented GPIHBP1 autoantibody case (AutoAb control),13 and plasma or serum from the patient during (on IFN) and after discontinuing IFN β1a therapy (off IFN). ELISA plates were coated with 500 ng/well of purified human LY6 proteins (GPIHBP1, CD177, C4.4A, or CD59). After blocking, diluted (1:500) plasma or serum samples were added to the wells and incubated overnight. The binding of plasma IgGs to the purified proteins was quantified with an HRP-labeled goat anti-human IgG/IgM. The autoantibody titer was determined by comparing the optical density (OD) in the sample wells with the OD of wells that had been coated with known amounts of human IgG (open square). GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1; IFN, interferon.
Figure 4.
The presence of GPIHBP1 autoantibodies in the plasma compromises the ability of an ELISA to detect recombinant GPIHBP1. In this study, we tested the ability of a monoclonal antibody–based ELISA to detect GPIHBP1 in plasma after spiking the samples with different amounts of recombinant GPIHBP1 (from 5,350 ng/ml to 0.7 ng/ml). When the recombinant human GPIHBP1 was spiked into plasma samples obtained during IFN β1a therapy (i.e., when GPIHBP1 autoantibodies were present), the ability of the ELISA to detect GPIHBP1 was reduced (note the reduction in OD in plasma samples that had been spiked with less than 83.6 ng/ml of GPIHBP1). In contrast, the ability to detect recombinant GPIHBP1 was robust in plasma samples obtained after the termination of the IFN β1a therapy (when GPIHBP1 autoantibodies were undetectable). GPIHBP1, glycosylphosphatidyl-inositol-anchored high-density lipoprotein–binding protein 1; IFN, interferon; OD, optical density.
The GPIHBP1 autoantibodies during IFN β1a therapy were mainly subclass IgG4 and to a far lesser extent IgA. There were no IgG1, IgG2, or IgG3 autoantibodies. No IgG4 or IgA GPIHBP1 autoantibodies were detected in plasma after the discontinuation of IFN β1a therapy.
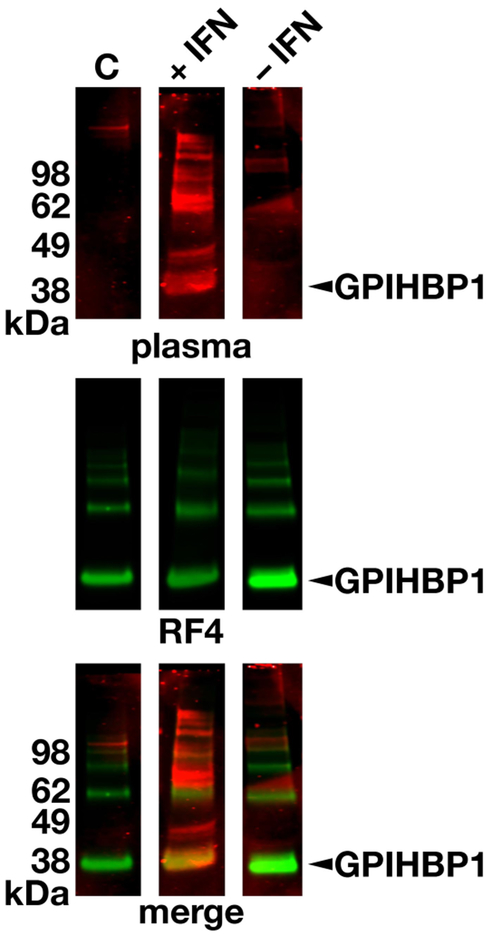
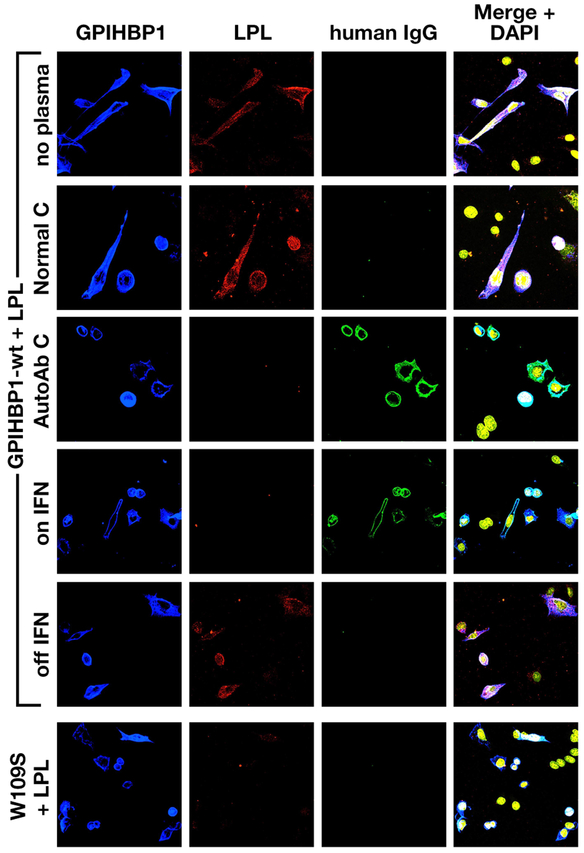
The presence of GPIHBP1 autoantibodies in the patient’s plasma during IFN β1a therapy was verified by western blotting (Fig. 5). The GPIHBP1 autoantibodies bound to the same band as a human GPIHBP1–specific monoclonal antibody (Fig. 5). We suspected that the GPIHBP1 autoantibodies in the patient’s plasma during the IFN β1a therapy would block the binding of LPL to GPIHBP1-expressing cells. Indeed, this was the case (Fig. 6). In contrast, the plasma collected after the termination of the IFN (–1a therapy (which was free of GPIHBP1 autoantibodies) had no capacity to block LPL binding to GPIHBP1-expressing cells (Fig. 6).
Figure 5.
Detecting GPIHBP1 autoantibodies by western blotting. Proteins in the conditioned medium of human GPIHBP1-expressing Drosophila S2 cells were size-fractionated under nonreducing conditions and then transferred to nitrocellulose membranes. The membranes were incubated with plasma from a normal control subject (C) or the plasma from the multiple sclerosis patient during IFN β1a therapy (+IFN) and after discontinuing IFN therapy (−IFN). Human immunoglobulins were detected with an IRDye680-labeled donkey anti-human IgGs (red, top row). The same membranes were subsequently incubated with an IRDye800-labeled human GPIHBP1-specific antibody RF421 (green, middle row). The bottom row shows the merged images. Arrowhead points to the GPIHBP1 band. GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1; IFN, interferon.
Figure 6.
Immunocytochemistry studies showing that the GPIHBP1 autoantibodies in the multiple sclerosis patient’s plasma block LPL binding to GPIHBP1. Cells expressing S-protein–tagged human GPIHBP1 were pre-incubated with plasma from: a normal subject (Normal C), a previously studied patient with GPIHBP1 autoantibodies (AutoAb C),13 or plasma collected from the multiple sclerosis patient during IFN β1a therapy (on IFN) and after discontinuing the therapy (off IFN). Next, V5-tagged human LPL was added to the wells. LPL binding to the GPIHBP1-transfected cells was visualized with an anti-V5 tag antibody (red). DNA was stained with DAPI (yellow). GPIHBP1 autoantibodies (green) in the patient’s plasma during IFN β1a therapy bound to the cells expressing S-protein–tagged human GPIHBP1 (blue); the binding of the GPIHBP1 autoantibodies to GPIHBP1-expressing cells abolished LPL binding. The plasma collected after discontinuing IFN treatment did not contain GPIHBP1 autoantibodies and had no capacity to block LPL binding. As expected, plasma from the previously studied patient with GPIHBP1 autoantibodies blocked LPL binding to GPIHBP1, while a normal plasma control did not. Cells expressing the mutant GPIHBP1-W109S6 did not bind LPL. GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1; IFN, interferon; LPL, lipoprotein lipase.
Discussion
We previously reported a patient with multiple sclerosis who developed chylomicronemia during treatment with IFN β1a.15 At the time of the initial report, the GPIHBP1 autoantibody syndrome had not yet been described. After the GPIHBP1 autoantibody syndrome was discovered, we began to suspect that the IFN β1a-treated patient with chylomicronemia may have had GPIHBP1 autoantibodies. First, like other patients with the GPIHBP1 autoantibody syndrome, the multiple sclerosis patient had pre-existing evidence of autoimmune disease (e.g., antibodies against cardiolipin, thyroglobulin, thyroid peroxidase).15 Second, IFN β1a therapy has been reported to exacerbate diseases caused by autoantibodies (e.g, SLE, rheumatoid arthritis, hyper- or hypothyroidism, thrombocytopenia).16-18 Third, the patient did not have gene mutations associated with lifelong chylomicronemia. Indeed, the patient’s triglyceride levels normalized after stopping the IFN β1a therapy.15 In the current study, we demonstrated that the patient had a high titer of GPIHBP1 autoantibodies while on IFN β1a therapy (comparable to the titers in other patients with the GPIHBP1 autoantibody syndrome). After the IFN β1a therapy was stopped, the GPIHBP1 autoantibodies disappeared and the plasma triglycerides normalized. As expected, the presence of the GPIHBP1 autoantibodies in the patient’s plasma limited the ability of an ELISA to detect GPIHBP1 (after spiking the plasma with recombinant GPIHBP1). Also, the GPIHBP1 autoantibodies inhibited the ability of GPIHBP1 to bind LPL. The latter finding—the ability of GPIHBP1 autoantibodies to block LPL binding to GPIHBP1—is the biochemical sine qua non of the GPIHBP1 autoantibody syndrome.
In our multiple sclerosis patient, the GPIHBP1 autoantibodies were predominantly subclass IgG4 and to a small extent IgA. In recent years, IgG4 autoantibodies have been shown to play a predominant role in many autoimmune diseases, including pemphigus foliaceus, pemphigus vulgaris, thrombotic thrombocytopenic purpura, and membranous nephropathy.28, 29 In general, IgG4 autoantibodies cause disease by disrupting the binding of essential protein–protein interactions rather than by causing structural damage to tissues.28, 29 In the GPIHBP1 autoantibody syndrome, the sole mechanism of disease, at least as far as we are aware, is disrupting the binding of LPL to GPIHBP1 on capillary endothelial cells.13, 14
Ours is the first case of the GPIHBP1 autoantibody syndrome associated with IFN β1a therapy. There are earlier reports of severe hypertriglyceridemia in the setting of IFNα therapy,30 but whether those cases were accompanied by GPIHBP1 autoantibodies is not known. Earlier publications have stated that IFN β1a therapy can “fuel” autoimmune disease syndromes, although hypertriglyceridemia is uncommon (less than 5%),31, 32 The package insert for IFN β1a contains a warning about the potential of autoimmune diseases during therapy (e.g., idiopathic thrombocytopenia, hyper- or hypothyroidism, and autoimmune hepatitis) and further states that the drug should be stopped if those complications occur. In the case of chylomicronemia due to the GPIHBP1 autoantibody syndrome, stopping the drug is imperative because of the high risk for acute pancreatitis.
A hallmark of functional GPIHBP1 deficiency (whether caused by autoantibodies or homozygous loss-of-function GPIHBP1 mutations) is low plasma levels of GPIHBP1 and LPL. In the setting of LPL deficiency, the plasma levels of LPL can be extremely low, but GPIHBP1 levels are normal.13 The low plasma levels of LPL in the GPIHBP1 autoantibody syndrome make sense, simply because the autoantibodies block transport of LPL to the capillary lumen. The low plasma levels of GPIHBP1 also make sense because the autoantibodies block detection of GPIHBP1 by ELISAs. Our IFN β1a-treated patient with chylomicronemia and GPIHBP1 autoantibodies had a presentation typical of the GPIHBP1 autoantibody syndrome—low plasma levels of both GPIHBP1 and LPL.
The plasma levels of GPIHBP1 and LPL increased after IFN β1a therapy was discontinued—when GPIHBP1 autoantibodies were absent and plasma triglyceride levels were normal. Even though the disappearance of GPIHBP1 autoantibodies resulted in higher GPIHBP1 and LPL levels, the levels of both GPIHBP1 and LPL levels remained rather low. We don’t know the reason for the persistently low levels of GPIHBP1 and LPL after discontinuation of IFN β1a therapy, but one possibility is that the GPIHBP1 autoantibodies in this patient, like some other endothelial cell–specific alloantibodies and autoantibodies,33, 34 damaged GPIHBP1-expressing endothelial cells and selected against their survival (i.e., GPIHBP1-expressing endothelial cells in capillaries were gradually replaced by endothelial cells of arterioles and venules, which lack GPIHBP1 expression). Against that possibility is the observation that IgG4 subclass autoantibodies, such as those present in our patient, generally do not cause tissue damage.28, 29 A second possibility is that the presence of GPIHBP1 autoantibodies re-set the expression of GPIHBP1 in capillary endothelial cells. In the renal transplantation literature, ABO-incompatible kidneys survive and function despite the presence of ABO alloantibodies in the circulation.35 In those cases, the endothelial cells of the recipient kidney appear to adapt by reducing the expression of ABO antigens on endothelial cells. In our case, the explanation for the persistently low GPIHBP1 and LPL levels after discontinuation of the IFN β1a therapy is unknown. However, the important lesson from this case is that the plasma triglyceride levels normalized completely after the GPIHBP1 autoantibodies disappeared from the circulation.
Acknowledgments
Supported by grants (HL090553, HL087228, and HL125335) from the National Heart, Lung, and Blood Institute and a Transatlantic Network Grant (12CVD04) from the Fondation Leducq.
Footnotes
Financial disclosures
The authors have no financial interests to disclose.
References
- 1.Beigneux AP, Davies B, Gin P, Weinstein MM, Farber E, Qiao X, Peale P, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG: Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies BS, Beigneux AP, Barnes RH 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyren R, Goldberg I, Olivecrona G, Bensadoun A, Young SG, Fong LG: GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulbourne C, Gin P, Tatar A, Nobumori C, Hoenger A, Jiang H, Grovenor C, Adeyo O, Esko J, Goldberg I, Reue K, Tontonoz P, Bensadoun A, Beigneux A, Young S, Fong L: The GPIHBP1–LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 2014;19:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mysling S, Kristensen KK, Larsson M, Beigneux AP, Gardsvoll H, Fong LG, Bensadouen A, Jorgensen TJ, Young SG, Ploug M: The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife. 2016;5:e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mysling S, Kristensen KK, Larsson M, Kovrov O, Bensadouen A, Jorgensen TJ, Olivecrona G, Young SG, Ploug M: The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. eLife. 2016;5:e20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigneux AP, Fong LG, Bensadoun A, Davies BS, Oberer M, Gardsvoll H, Ploug M, Young SG: GPIHBP1 missense mutations often cause multimerization of GPIHBP1 and thereby prevent lipoprotein lipase binding. Circ Res. 2014;116:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, Kastelein JJ, Fong LG, Dallinga-Thie GM, Young SG: Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009;29:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charrière S, Peretti N, Bernard S, Di Filippo M, Sassolas A, Merlin M, Delay M, Debard C, Lefai E, Lachaux A, Moulin P, Marçais C: GPIHBP1 C89F neomutation and hydrophobic C-Terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia. J Clin Endocrinol Metab. 2011;96:E1675–E1679. [DOI] [PubMed] [Google Scholar]
- 9.Coca-Prieto I, Kroupa O, Gonzalez-Santos P, Magne J, Olivecrona G, Ehrenborg E, Valdivielso P: Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass: identification of a novel GPIHBP1 mutation. J Intern Med. 2011;270:224–228. [DOI] [PubMed] [Google Scholar]
- 10.Franssen R, Young SG, Peelman F, Hertecant J, Sierts JA, Schimmel AWM, Bensadoun A, Kastelein JJP, Fong LG, Dallinga-Thie GM, Beigneux AP: Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ Cardiovasc Genet. 2010;3:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivecrona G, Ehrenborg E, Semb H, Makoveichuk E, Lindberg A, Hayden MR, Gin P, Davies BSJ, Weinstein MM, Fong LG, Beigneux AP, Young SG, Olivecrona T, Hernell O: Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J Lipid Res. 2010;51:1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plengpanich W, Young SG, Khovidhunkit W, Bensadoun A, Karnman H, Ploug M, Gardsvoll H, Leung CS, Adeyo O, Larsson M, Muanpetch S, Charoen S, Fong LG, Niramitmahapanya S, Beigneux AP: Multimerization of GPIHBP1 and familial chylomicronemia from a serine-to-cysteine substitution in GPIHBP1's Ly6 domain. J Biol Chem. 2014;289:19491–19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigneux AP, Miyashita K, Ploug M, Blom DJ, Ai M, Linton MF, Khovidhunkit W, Dufour R, Garg A, McMahon MA, Pullinger CR, Sandoval NP, Hu X, Allan CM, Larsson M, Machida T, Murakami M, Reue K, Tontonoz P, Goldberg IJ, Moulin P, Charriere S, Fong LG, Nakajima K, Young SG: Autoantibodies against GPIHBP1 as a Cause of Hypertriglyceridemia. N Engl J Med. 2017;376:1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Dallinga-Thie GM, Hovingh GK, Chang SY, Sandoval NP, Dang TLP, Fukamachi I, Miyashita K, Nakajima K, Murakami M, Fong LG, Ploug M, Young SG, Beigneux AP: GPIHBP1 autoantibodies in a patient with unexplained chylomicronemia. J Clin Lipidol. 2017;11:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawahara Y, Yamashita T, Ohta Y, Sato K, Tsunoda K, Takemoto M, Hishikawa N, Eguchi J, Abe K: Marked hypertriglyceridemia induced by interferon-beta1a therapy in a clinically isolated syndrome patient. J Neurol Sci. 2017;373:144–146. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J, Pascual V: Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. [DOI] [PubMed] [Google Scholar]
- 17.Silva MO: Risk of autoimmune complications associated with interferon therapy. Gastroenterol Hepatol. 2012;8:540–542. [PMC free article] [PubMed] [Google Scholar]
- 18.Di Domizio J, Cao W: Fueling autoimmunity: type I interferon in autoimmune diseases. Expert Rev Clin Immunol. 2013;9:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surendran RP, Visser ME, Heemelaar S, Wang J, Peter J, Defesche JC, Kuivenhoven JA, Hosseini M, Peterfy M, Kastelein JJ, Johansen CT, Hegele RA, Stroes ES, Dallinga-Thie GM: Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J Intern Med. 2012;272:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Labeur C, Xu CF, Ferrell R, Lins L, Brasseur R, Rosseneu M, Weiss KM, Humphries SE, Talmud PJ: Characterization of the lipid-binding properties and lipoprotein lipase inhibition of a novel apolipoprotein C-III variant Ala23Thr. J Lipid Res. 2000;41:1760–1771. [PubMed] [Google Scholar]
- 21.Hu X, Sleeman MW, Miyashita K, Linton MF, Allan CM, He C, Larsson M, Tu Y, Sandoval NP, Jung RS, Mapar A, Machida T, Murakami M, Nakajima K, Ploug M, Fong LG, Young SG, Beigneux AP: Monoclonal antibodies that bind to the Ly6 domain of GPIHBP1 abolish the binding of LPL. J Lipid Res. 2017;58:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyashita K, Fukamachi I, Nagao M, Ishida T, Kobayashi J, Machida T, Nakajima K, Murakami M, Ploug M, Beigneux AP, Young SG, Nakajima K: An enzyme-linked immunosorbent assay for measuring GPIHBP1 levels in human plasma or serum. J Clin Lipidol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machida T, Miyashita K, Sone T, Tanaka S, Nakajima K, Saito M, Stanhope K, Havel P, Sumino H, Murakami M: Determination of serum lipoprotein lipase using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2015;442:130–135. [DOI] [PubMed] [Google Scholar]
- 24.Miyashita K, Nakajima K, Fukamachi I, Muraba Y, Koga T, Shimomura Y, Machida T, Murakami M, Kobayashi J: A new enzyme-linked immunosorbent assay system for human serum hepatic triglyceride lipase. J Lipid Res. 2017;58:1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida T, Miyashita K, Shimizu M, Kinoshita N, Mori K, Sun L, Yasuda T, Imamura S, Nakajima K, Stanhope KL, Havel PJ, Hirata K: ELISA system for human endothelial lipase. Clin Chem. 2012;58:1656–1664. [DOI] [PubMed] [Google Scholar]
- 26.Imamura S, Kobayashi J, Nakajima K, Sakasegawa S, Nohara A, Noguchi T, Kawashiri MA, Inazu A, Deeb SS, Mabuchi H, Brunzell JD: A novel method for measuring human lipoprotein lipase and hepatic lipase activities in postheparin plasma. J Lipid Res. 2008;49:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gårdsvoll H, Hansen LV, Jorgensen TJ, Ploug M: A new tagging system for production of recombinant proteins in Drosophila S2 cells using the third domain of the urokinase receptor. Protein Expr Purif. 2007;52:384–394. [DOI] [PubMed] [Google Scholar]
- 28.Koneczny I. A New Classification System for IgG4 Autoantibodies. Front Immunol. 2018;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huijbers MG, Plomp JJ, van der Maarel SM, Verschuuren JJ. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Ann N Y Acad Sci. 2018;1413:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Miranda C, Castellano G, Guijarro C, Fernandez I, Schoebel N, Larumbe S, Gomez-Izquierdo T, del Palacio A: Lipoprotein changes in patients with chronic hepatitis C treated with interferon-alpha. Am J Gastroenterol. 1998;93:1901–1904. [DOI] [PubMed] [Google Scholar]
- 31.Byskosh PV, Reder AT: Interferon beta-1b effects on cytokine mRNA in peripheral mononuclear cells in multiple sclerosis. Mult Scler. 1996;1:262–269. [DOI] [PubMed] [Google Scholar]
- 32.Sena A, Pedrosa R, Ferret-Sena V, Almeida R, Andrade ML, Morais MG, Couderc R: Interferon beta1a therapy changes lipoprotein metabolism in patients with multiple sclerosis. Clin Chem Lab Med. 2000;38:209–213. [DOI] [PubMed] [Google Scholar]
- 33.Tanabe T, Ishida H, Horita S, Yamaguchi Y, Toma H, Tanabe K: Decrease of blood type antigenicity over the long-term after ABO-incompatible kidney transplantation. Transpl Immunol. 2011;25:1–6. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Zhang C, Wang J, Yang C, Xu M, Rong R, Zhu T, Zhu D: Endothelial Cells in Antibody-Mediated Rejection of Kidney Transplantation: Pathogenesis Mechanisms and Therapeutic Implications. J Immunol Res. 2017;2017:8746303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morath C, Zeier M, Dohler B, Opelz G, Susal C: ABO-Incompatible Kidney Transplantation. Front Immunol. 2017;8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]