Abstract
Treatment of chronic hepatitis C virus (HCV) infection with direct acting antivirals (DAAs) results in a sustained virologic response (SVR) in most patients. While highly efficacious, ~3–5% of patients do not achieve SVR despite having virus that appears susceptible. It is unclear whether host factors contribute to treatment failures, although innate and adaptive immunity may play a role. Previous studies showed that after DAA treatment, the composition of intrahepatic immune cells does not normalize relative to healthy volunteers, even in cases where SVR is achieved. We used paired pre- and post-treatment liver biopsies from 13 patients treated with sofosbuvir and ribavirin, 4 of whom relapsed, to analyze intracellular immune changes during DAA treatment and explore correlations with inflammation and treatment outcome. We performed single marker immunohistochemistry followed by electronic image capture, manual annotation of parenchymal and non-parenchymal regions, and quantitative image analysis. The predominant cellular change during treatment was a decrease in CD8+ cellular density in both parenchymal and non-parenchymal regions. CD68+ Kupffer cell density correlated with hepatic inflammation (AST, ALT) pre-treatment, but did not change during treatment. CD4+ cellular density decreased in non-parenchymal regions and, intriguingly, was lower pre-treatment in subjects who eventually relapsed. Other cellular markers (CD56, CD20), as well as markers of apoptosis (TIA-1) and activated stellate cells, did not change significantly during treatment or differ by treatment outcome.
Conclusion:
The predominant intrahepatic cellular change during DAA treatment of chronic HCV infection is a reduction in CD8+ cellular density, but this did not correlate with treatment outcome.
Keywords: hepatitis C virus, direct acting antiviral, intrahepatic immunity, SVR, relapse
Introduction
Approximately 71 million persons worldwide are chronically infected with hepatitis C virus (HCV) (1). In the United States, HCV is the leading cause of chronic liver disease, cirrhosis, hepatocellular cancer, and liver transplantation, and for over a decade deaths due to HCV infection have exceeded those from human immunodeficiency virus (HIV) (2). Treatment with direct-acting antivirals (DAAs) results in a sustained virologic response (SVR), synonymous with cure, in most patients, including HIV co-infected and cirrhotic populations who have historically been harder to cure (3).
For reasons that are poorly understood, relapse of HCV infection after DAA therapy occurs in a minority of patients, reinfection can occur, and no protective vaccine is available (4–6). Relapse can occur in patients with excellent adherence and who demonstrate anticipated viral decline during treatment, suggesting factors other than viral susceptibility and medication adherence play a role (4–6). As such, exploring whether host correlates of functional and dysfunctional immune responses to HCV may influence the risk of treatment relapse remains of practical clinical relevance.
Innate and adaptive immune function have previously been shown to change during DAA treatment, as multiple studies have now correlated achieving SVR with functional restoration of innate and adaptive immune function in both blood and liver (7–10). Patients with SVR have higher expression of an endogenous hepatic interferon gene signature at the end of treatment as well as greater restoration of a peripheral HCV-specific, polyfunctional CD8+ T-cell response compared to patients who experience virologic relapse (7–10). In addition, natural killer cell (NK) phenotype and function and mucosal-associated invariant T-cells (MAIT) normalize in liver in parallel with reduced inflammation associated with HCV viral decline during DAA therapy (11–14). Other studies revealed persistently elevated frequencies of regulatory T-cells 4 years after HCV clearance, indicating a sustained impact of HCV on hepatic immune function long after viral eradication (15). Most prior studies focused on detailed phenotype and functional analyses of specific cellular types, but bulk enumeration of intrahepatic immune cellular densities has not been previously reported.
In this study, we used a unique collection of paired liver biopsies from patients treated with DAA therapy to ask how a broad array of intrahepatic immune cellular populations change during DAA therapy, and explored whether there were associations with treatment outcome. We consider these findings in the context of prior work showing that multiple peripheral cellular populations change rapidly after DAA therapy, which we hypothesized was due to efflux of intrahepatic lymphocytes as well as reduced de novo migration (16).
Materials and Methods
Liver Immunohistochemistry
Liver biopsies were obtained from 13 treatment naïve HCV subjects enrolled in the SPARE clinical trial (NCT01441180) which was conducted at the National Institute of Allergy and Infectious Diseases (NIAID) and has been previously described in detail (9, 17). Subjects received sofosbuvir (an HCV NS5B inhibitor) combined with low dose or weight based ribavirin for 24 weeks (17). Paired liver biopsies obtained pre-treatment and at the end of treatment (prior to virologic relapse) were available from 9 patients who achieved SVR and 4 who relapsed. Single analyte immunohistochemistry (IHC) was performed using formalin-fixed paraffin embedded liver sections with antibodies specific for CD4 (1F6, Novocastra/Leica), CD8 (CD8/144B, Dako), an NK marker CD56 (1B6, Novocastra/Leica), a Kupffer cell marker CD68 (KP-1, Dako), a B-cell marker CD20 (L26, Dako), an apoptosis marker TIA-1 (2G9A10F5, Immunotech/Beckman Coulter), and alpha-smooth muscle actin (ASMA, 1A4, Dako), a marker of stellate cell activation. Subsequent staining was performed using the Ultra View DAB detection kits (Ventana Medical Systems). Insufficient clinical material was available to perform all stains on all samples, with the reported “n” for each stain as follows: CD4=13, CD8=13, CD68=13, TIA-1=12, ASMA=11, CD56=8, CD20=7.
The study received approval from the Institutional Review Boards at the National Institute of Allergy and Infectious Diseases and the Medical University of South Carolina and was conducted in concordance with the 1975 Declaration of Helsinki. All patients provided written informed consent, as previously reported with initial publication of the clinical trial result (17).
IHC Digital Image Analysis
Virtual whole slide scans of liver biopsies were performed using a Hamamatsu Nanozoomer HT 2.0 slide scanner from Hamamatsu Photonics (Hamamatsu, Japan). Images were then extracted as NDPI Files as a series of 20x Tiff images using the NDPI Tools plugin for ImageJ (v.1.46r, National institutes of Health, USA). Sample images were imported into Visiopharm Analysis software v5.3.1 (Hoersholm, Denmark) and manually annotated to distinguish parenchymal (hepatocyte predominant) and non-parenchymal (portal triad predominant) regions. Sections of tissue that had fibrous tissue with no clear cellular component or areas of artifact due to tissue folds, edge effect, or irregular staining were manually identified and excluded from subsequent analysis. Quantification of DAB positive regions and total tissue area was performed using 20x Tiff files and data are reported as cell count or pixel count per unit-less area. Customized evaluation protocols were optimized and trained for Visiopharm’s automated detection against randomly selected images for each individual biomarker and subsequently batch processed to minimize technical variation or potential bias. Biomarkers were quantitated using either cellular count, where appropriate based on staining characteristics and non-overlapping signal, or total pixel count when cell count could not be reliably determined due to signal irregularity or cellular overlap. The operator was blinded to sample identification during data collection.
Statistical Analysis
Data were analyzed with parametric assumptions using either paired or unpaired t-tests using Prism (v7.01). P-values <0.05 were considered to be statistically significant.
Results
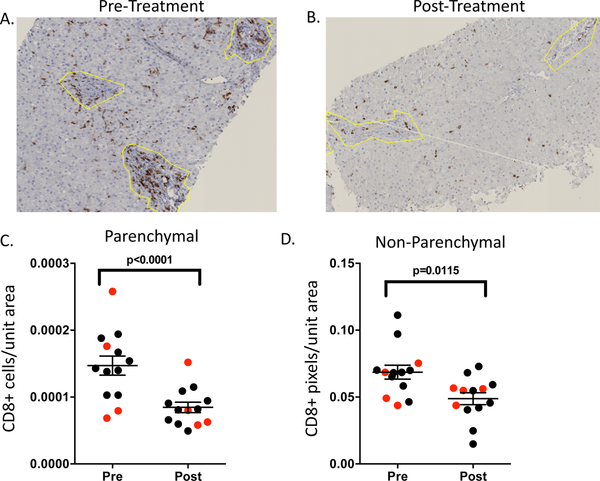
We performed single analyte IHC to understand cellular changes in liver during DAA treatment of HCV and explore associations with inflammation and outcome. After image analysis and un-blinding of samples, the most obvious visual change over the course of treatment was a reduction in CD8 signal (Figure 1A–B). To more rigorously assess this observation, we performed quantitative image analysis and identified a significant decrease in CD8 staining over the course of treatment in both parenchymal and non-parenchymal regions in an analysis considering all samples (Figure 1C–D). A separate analysis comparing CD8 signal pre- and post-treatment in patients achieving SVR vs. relapse identified no significant differences (data not shown), suggesting this decline in CD8+ T-cell density represents an intrahepatic cellular response to reduction in viral burden, but not necessarily viral eradication.
Figure 1:
Representative pre- (A) and post- (B) treatment FFPE liver sections stained with an anti-CD8 antibody. Quantitative changes in CD8 signal intensity in regions demarcated as parenchymal (C) or non-parenchymal (D) for 13 patients with available biopsies. Data from subjects who achieved SVR are in black and data from subjects who relapsed are in red. Cell count or pixel count per surface area were determined as described in the methods section. Non-parenchymal regions are denoted by the yellow line. Statistical analysis was by paired t-test considering all subjects, with mean and standard error shown.
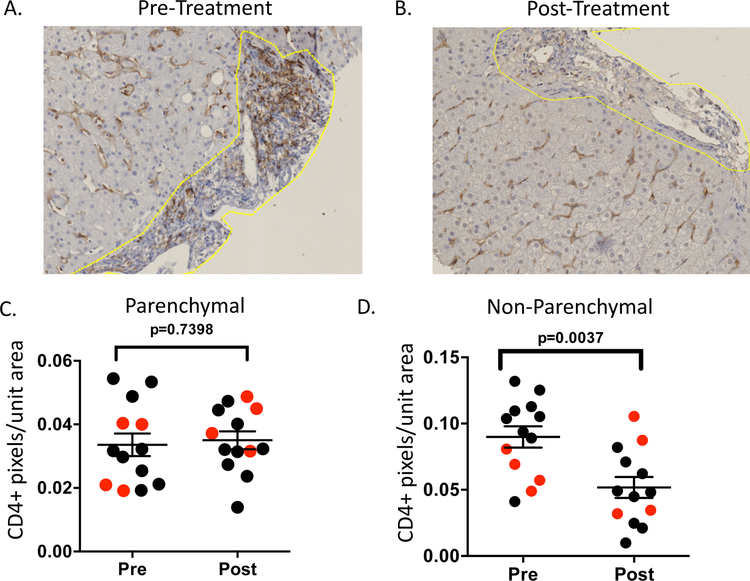
We next analyzed CD4+ cells, as previous work showed that even after clearance of HCV with DAA treatment, there is a sustained increase in regulatory CD4+ T-cells in the liver relative to healthy volunteers (15). We used a CD4-antibody to assess changes in bulk populations of cells over time, and found this antibody stained cells with a cellular morphology consistent with lymphocytes in non-parenchymal regions but a morphology consistent with tissue-resident macrophages in parenchymal regions (Figure 2A–B). We identified no change in CD4 expression in parenchymal regions during treatment, but a significant decrease in CD4 expression in non-parenchymal regions, where cells more closely resembled lymphocytes (Figure 2C–D). Interestingly, patients who achieved SVR had higher pre-treatment CD4 signal in non-parenchymal regions relative to patients who relapsed, while no significant differences by treatment outcome were seen post-treatment (Supplemental Figure 1), and no differences in CD4 by treatment outcome were observed at either time point in parenchymal regions (data not shown).
Figure 2:
Representative pre- (A) and post- (B) treatment FFPE liver sections stained with an anti-CD4 antibody. Quantitative parenchymal and non-parenchymal CD4 signals (C-D) were quantitated by pixel count to infer cellular density from 13 patients (SVR =9 shown in black, relapse =4 shown in red). Non-parenchymal regions are denoted by the yellow line. Statistical analysis is by paired t-test considering all patients.
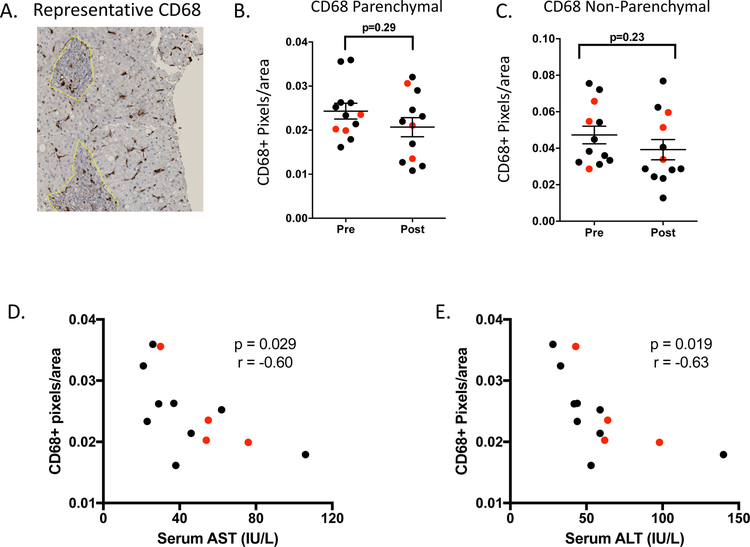
Chronic HCV infection has also been associated with B-cell and NK cell functional perturbations, as well as activation of tissue resident Kupffer cells and hepatic stellate cells (18–21), and functional perturbations have been shown to improve with DAA-mediated HCV suppression (7, 11, 13). To address changes in these cellular populations, we performed staining with CD20 (B-cell marker), CD56 (NK-cell marker), and CD68 (Kupffer cell marker), as well as with markers of stellate cell activation (ASMA) and apoptosis (TIA-1) (representative stains are shown in Figure 3A for CD68 and Supplemental Figure 2). None of these cellular populations or markers changed significantly over the course of treatment or differed by treatment outcome (Figure 3B–C for CD68 and Supplemental Figure 2), although the number of patients with available samples for these stains was limited, as described in the methods section.
Figure 3:
CD68+ cellular density correlates with hepatic inflammation. (A) Representative CD68 stain. (B-C) No change in CD68+ cellular density during treatment in parenchymal and non-parenchymal regions (D-E) Correlation of CD68+ cellular density pre-treatment in parenchymal regions with AST and ALT. (SVR =9 shown in black, relapse =4 shown in red). Non-parenchymal regions are denoted by the yellow line. Statistical analysis is by paired t-test (B-C) or Pearson correlation (D-E) considering all patients.
The mechanisms that drive the magnitude of inflammation in chronic HCV infection are complex and not entirely understood. There can be marked clinical variability in the extent to which hepatic transaminases are elevated, and many patients with prolonged high level viremia have liver enzymes within the normal range of the assay. Cells including hepatocytes, hepatic stellate cells, and Kupffer cells are known to be activated and respond to hepatic inflammation (13, 19–21). To gain insight into variables that may impact the extent of hepatic inflammation, we sought correlations between cellular density and AST/ALT pre-treatment. While no correlation was observed between CD68 cell density in non-parenchymal regions and ALT, AST or viral load (data not shown), we did identify a significant negative correlation between parenchymal Kupffer cell density and ALT and AST enzyme levels pre-treatment (Fig 3 D–E). In contrast, no correlation was found between hepatic inflammation levels and other cell densities including CD4 and CD8 (data not shown).
Discussion
In this study we characterized bulk changes in immune cell frequencies in liver as a result of DAA treatment and report significant findings related to hepatic inflammation and treatment outcome. First, with respect to changes related to hepatic inflammation, we show that CD8+ cells are the predominant cellular population that changes significantly over the course of treatment (Figure 1), CD4+ cells decrease in non-parenchymal regions only (Figure 2), while most other cellular populations did not change. In addition, we found that CD68 density pre-treatment correlated with hepatic inflammation rather than CD8 or CD4 density (Figure 3). Second, we identified an intriguing difference in CD4 density in non-parenchymal regions before treatment between patients who subsequently achieved SVR vs. relapsed, with relapsers having lower pre-treatment CD4 density (Figure 2 and Supplemental Figure 1).
Markers of hepatic inflammation decline rapidly during HCV suppression by DAAs (17), and we now add to this understanding by showing that CD8+ and CD4+ cells decrease markedly during treatment and that Kupffer density pre-treatment correlates with AST/ALT, even though Kupffer cell density did not change during treatment. These findings are consistent with prior observation correlating the frequency of pro-inflammatory monocytes in the liver during HCV infection with MAIT cell activation (13), suggesting innate immune cell density and functionality in inflamed liver impacts adaptive immune cell density and function, and thus the magnitude of inflammation. Whether individual differences in the nature of interaction between Kupffer cells and CD8+ cells can explain variability in the magnitude of hepatic transaminase elevation observed in patients with HCV infection requires further study. While intriguing, changes that correlated with reduced hepatic inflammation were not found to differ by treatment outcome, consistent with the notion that gross changes in hepatic inflammation may associate with a reduction in HCV burden by DAA suppression rather than with events associated with viral elimination (17, 22, 23). We were unable to assess changes in HCV-specific immune cells in the liver in the context of this study due to lack of sample availability amenable to cellular extraction and functional analysis, and thus cannot correlate our findings with those showing restoration of peripheral anti-HCV CD8+ T-cell function during therapy (7, 10).
Efforts to understand treatment outcome and why some patients relapse yielded one significant finding in this study, in that density of CD4 signal in non-parenchymal regions pre-treatment was lower in eventual relapsers relative to patients who achieved SVR. As noted above, the nature of this stain, which appeared to stain both lymphocytes and Kupffer cells, complicates interpretation of this finding. This caveat aside, the data raise the intriguing prospect that a less robust CD4 response at baseline could impact the nature of the subsequent immune response during DAA-induced HCV suppression in a way that impairs viral eradication. It is possible that higher CD4+ T cell infiltration in the extra parenchymal hepatic compartment may be vital in the development of a coordinated antiviral response during DAA therapy and contribute to elimination of HCV in those with SVR (24). This would be consistent with other data correlating the strength of “recovery” of innate and adaptive immune function in both liver and peripheral blood during DAA therapy with treatment outcome (7–10).
Several limitations to the approach pursued for this study merit discussion. As noted above, the lack of cellular specificity for the CD4 stain temper the strength of the hypothesis derived from this analysis. Unfortunately, there was not significant remaining tissue to perform more detailed staining to delineate CD4+ T-cell subsets, such as regulatory T-cells or CD4+ MAIT cells, or to verify with a CD3 co-stain that CD8 and CD4 staining populations were T-lymphocytes. As stated above, while we did not identify clear changes in NK and B-cell density, or any changes in apoptosis or stellate cell activation, the sample size for these analyses was limited based on tissue availability. Prior studies have shown phenotypic and functional changes in intrahepatic NK-cells as a result of DAA therapy (11), but we were unable to address this possibility here. Finally, although our study design was blinded and robust with respect to image analysis, differences in both sample staining and parenchymal vs. non-parenchymal composition of individual biopsies may have precluded the power to detect subtle, but meaningful, biologic differences.
In conclusion, utilizing a unique collection of paired liver biopsies from patients who achieved SVR or relapsed with DAA treatment, we report that a decrease in CD8+ cells was the predominant cellular change in liver immune cell density, a decrease that correlated with the rapid decrease in hepatic inflammation associated with viral suppression and that occurred irrespective of eventual treatment outcome. CD4+ cells also decreased in non-parenchymal regions, while most other cellular populations did not change. Lower CD4 density in the non-parenchymal regions of eventual relapsers before treatment merits further study to determine whether this baseline host response to HCV infection subsequently impairs odds of SVR with DAA treatment.
Supplementary Material
Supplemental Figure 1: Intrahepatic cellular density of CD4+ staining in non-parenchymal hepatic regions of liver differs pre-treatment in patients who relapse (n=4, shown in red) vs. achieve SVR (n=9, shown in black). Statistical analysis is by unpaired t-test.
Supplemental Figure 2: Additional cellular markers do not change significantly with treatment. (A). Representative stains for the indicated cellular markers. Non-parenchymal regions were demarcated by manual encircling as shown in yellow. (B-C) No significant quantitative changes were found in cellular frequency for CD56+ NK cells (n=8) (B) or CD20+ B-cells (n=7) (C) over the course of treatment or based on treatment outcome. Pixel count was used to enumerate signal in non-parenchymal areas, and cell count was used for parenchymal. Data from patients achieving SVR are shown in black and from relapsers in red. Statistical analysis is by paired t-test.
Acknowledgements:
This work was supported by the National Institute of Allergy and Infectious Diseases (K08AI121348 to E.G.M.), the National Institutes of Health Critical Care Medicine Department, and the National Institute of Health Division of Allergy and Infectious Diseases Intramural Research Program. This research was also supported in part by the L Core, Center for Oral Health Research, Medical University of South Carolina funded by the National Institute of General Medical Sciences of the National Institutes of Health (P30GM103331). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Conflict of Interest: None declared.
References
- 1.Hepatitis C Fact Sheet, Updated October 2017. http://wwwwhoint/mediacentre/factsheets/fs164/en/.
- 2.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012;156(4):271–8. [DOI] [PubMed] [Google Scholar]
- 3.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA : the journal of the American Medical Association. 2014;312(6):631–40. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B HCV in 2015: Advances in hepatitis C research and treatment. Nat Rev Gastroenterol Hepatol 2016;13(2):70–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Midgard H, Bjoro B, Maeland A, Konopski Z, Kileng H, Damas JK, et al. Hepatitis C reinfection after sustained virological response. J Hepatol 2016;64(5):1020–6. [DOI] [PubMed] [Google Scholar]
- 6.Sacks-Davis R, Grebely J, Dore GJ, Osburn W, Cox AL, Rice TM, et al. Hepatitis C Virus Reinfection and Spontaneous Clearance of Reinfection--the InC3 Study. J Infect Dis 2015;212(9):1407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, et al. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis 2016;213(2):216–23. [DOI] [PubMed] [Google Scholar]
- 8.Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat 2015;22(12):983–91. [DOI] [PubMed] [Google Scholar]
- 9.Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest 2014;124(8):3352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol 2014;61(3):538–43. [DOI] [PubMed] [Google Scholar]
- 11.Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149(1):190–200 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, et al. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNalpha. Gut. 2017;66(4):724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolte FJ, O’Keefe AC, Webb LM, Serti E, Rivera E, Liang TJ, et al. Intra-Hepatic Depletion of Mucosal-Associated Invariant T Cells in Hepatitis C Virus-Induced Liver Inflammation. Gastroenterology. 2017;153(5):1392–403 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaan M, van Oord GW, Janssen HL, de Knegt RJ, Boonstra A. Longitudinal analysis of peripheral and intrahepatic NK cells in chronic HCV patients during antiviral therapy. Antiviral Res 2015;123:86–92. [DOI] [PubMed] [Google Scholar]
- 15.Spaan M, Claassen MA, Hou J, Janssen HL, de Knegt RJ, Boonstra A. The Intrahepatic T Cell Compartment Does Not Normalize Years After Therapy-Induced Hepatitis C Virus Eradication. J Infect Dis 2015;212(3):386–90. [DOI] [PubMed] [Google Scholar]
- 16.Meissner EG, Kohli A, Higgins J, Lee YJ, Prokunina O, Wu D, et al. Rapid changes in peripheral lymphocyte concentrations during interferon-free treatment of chronic hepatitis C virus infection. Hepatol Commun 2017;1(7):586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;310(8):804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehermann B Natural Killer Cells in Viral Hepatitis. Cell Mol Gastroenterol Hepatol 2015;1(6):578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int 2016;10(3):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol 2014;61(3):660–71. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Li J, Wang X, Sang M, Ho W. Hepatic stellate cells, liver innate immunity, and hepatitis C virus. Journal of gastroenterology and hepatology. 2013;28 Suppl 1:112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alao H, Cam M, Keembiyehetty C, Zhang F, Serti E, Suarez D, et al. Baseline intrahepatic and peripheral innate immunity are associated with hepatitis C virus clearance during direct-acting antiviral therapy. Hepatology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meissner EG, Kohli A, Virtaneva K, Sturdevant D, Martens C, Porcella SF, et al. Achieving sustained virologic response after interferon-free hepatitis C virus treatment correlates with hepatic interferon gene expression changes independent of cirrhosis. J Viral Hepat 2016;23(7):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrivastava S, Wilson E, Poonia B, Tang L, Osinusi A, Kohli A, et al. Augmentation of HCV specific immunity and Sustained virological response (SVR). J Viral Hepat 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Intrahepatic cellular density of CD4+ staining in non-parenchymal hepatic regions of liver differs pre-treatment in patients who relapse (n=4, shown in red) vs. achieve SVR (n=9, shown in black). Statistical analysis is by unpaired t-test.
Supplemental Figure 2: Additional cellular markers do not change significantly with treatment. (A). Representative stains for the indicated cellular markers. Non-parenchymal regions were demarcated by manual encircling as shown in yellow. (B-C) No significant quantitative changes were found in cellular frequency for CD56+ NK cells (n=8) (B) or CD20+ B-cells (n=7) (C) over the course of treatment or based on treatment outcome. Pixel count was used to enumerate signal in non-parenchymal areas, and cell count was used for parenchymal. Data from patients achieving SVR are shown in black and from relapsers in red. Statistical analysis is by paired t-test.