Abstract
Structural brain deficits are linked to risk for suicidal behavior. However, there is disagreement about the nature of these deficits, probably due to the heterogeneity of suicidal behavior in terms of the suicidal act’s lethality. We hypothesized that individuals with major depressive disorder (MDD) and history of more lethal suicide attempts would have lower gray matter volume (GMV) of the prefrontal regions and insula compared with MDD lower-lethality attempters and MDD non-attempters. We collected structural MRI scans on 91 individuals with MDD; 11 with history of higher-lethality suicide attempts, 14 with lower-lethality attempts, and 66 were non-attempters. Differences in GMV between these three groups were examined using both regions-of-interest (ROI) and brain-wide voxel-based morphometry (VBM) analyses. Both ROI and VBM analyses showed that higher-lethality suicide attempters have greater GMV of the prefrontal cortical regions and insula, compared with the other two groups. Although this contrasts with our hypothesis, the observed larger prefrontal cortex GMV in higher-lethality suicide attempters may underlie the set of attributes observed previously in this suicidal subgroup, including enhanced suicide attempt planning, greater response inhibition, and delayed reward capabilities. Future studies should further examine the role of these brain regions in relation to suicidal intent and planning.
Keywords: voxel-based morphometry, gray matter, suicidal behavior, major depressive disorder
1. Introduction:
Suicide is a major public health problem (World Health Organization, 2016). There is a high attributable risk for suicidal acts associated with major depressive disorder (MDD) (Bernal et al., 2007). In USA, approximately 75% of suicide decedents are identified to have a history of depressive disorder (Stone et al., 2018). Among adults aged 18 years and older, for each suicide there are about 30 adults who reported making a suicide attempt (Center for Disease Control and Prevention (CDC), 2015; Substance Abuse and Mental Health Services Administration (SAMHSA), 2013), but thus far, clinicians must rely on the individual’s willingness to report their intentions or past history of attempts to identify those at elevated risk. Identifying biomarkers of suicidal behavior in depression may aid in predicting and preventing future suicide.
Structural brain deficits have been linked to increased risk for suicidal behavior (van Heeringen et al., 2014; van Heeringen and Mann, 2014) although there is disagreement over the exact nature and location of these deficits. Whereas some postmortem studies show that depressed suicide decedents have lower neuron density in dorsal and ventral prefrontal cortex (Underwood et al., 2012), and smaller hippocampi (Altshuler et al., 1990), others report larger fibrous astrocytes in anterior cingulate (Torres-Platas et al., 2011) compared with sudden-death controls. In vivo structural neuroimaging studies in MDD with suicidal behavior also report mixed results. The majority (Colle et al., 2015; Cyprien et al., 2011; Ding et al., 2015; Dombrovski et al., 2012; Hwang et al., 2010; Lee et al., 2016a; Monkul et al., 2007; Pan et al., 2015; Wagner et al., 2011), but not all (Gifuni et al., 2016), of these studies show that MDD suicide attempters, compared with MDD non-attempters (or healthy controls), have lower gray matter volume (GMV) in a wide variety of regions, mostly involving prefrontal cortex (PFC). Interestingly, Gifuni et al. (2016) reported that lethality of suicidal behavior correlates negatively with the volume of the nucleus accumbens in depressed patients, suggesting a possible association of regional brain volume deficits with specific aspects of suicidal behavior.
Discrepant findings also exist in studies of suicide attempters with borderline personality disorder (Soloff et al., 2012), bipolar disorder (Benedetti et al., 2011; Duarte et al., 2017; Johnston et al., 2017; Lijffijt et al., 2014; Matsuo et al., 2010) and psychotic disorders (Aguilar et al., 2008; Giakoumatos et al., 2013; Rüsch et al., 2008; Spoletini et al., 2011). Again, some of these studies observed that the GMV changes are confined to high-lethality suicide attempters (Duarte et al., 2017; Soloff et al., 2012) with evidence of positive correlation between suicidal act lethality and the orbitofrontal cortex (OFC) and insula GMV (Duarte et al., 2017).
One reason for the disagreement in the literature may be the heterogeneity of suicidal behavior, particularly in terms of lethality and intent. Suicidal behavior varies from very lethal to low-lethality attempts, and from impulsive attempts to carefully planned and determined attempts (Chaudhury et al., 2016; Keilp et al., 2014). Research samples where one type of attempt is overrepresented can potentially yield different results compared with other studies. High-lethality suicide attempts are characterized by more planning and a lower chance of rescue (Beck et al., 1975; Stengel, 1964). Individuals who make such attempts may resemble suicide decedents demographically and clinically (Chaudhury et al., 2016), as well as in terms of brain biology (Mann and Malone, 1997).
To reduce the impact of the heterogeneity of suicidal behavior on findings, and to focus on nonfatal suicidal behavior that is potentially more closely related to suicide, this study sought to examine potential neuroanatomical abnormalities in medication-free individuals with MDD and history of at least one higher-lethality suicide attempt defined on the basis of having caused significant medical damage. We compared the GMV of 5 a priori regions-of-interest (ROIs), involving the prefrontal areas and insula, in this group to MDD participants with a history of lower-lethality attempts and MDD without a history of prior suicide attempts. We hypothesized that depressed higher-lethality suicide attempters would show lower GMV in the 5 a priori ROIs. We also conducted exploratory whole-brain analysis using voxel-based morphometry (VBM) to confirm our findings. Additionally, we examined the possible effects of current depression and suicidal ideation severity, lifetime impulsiveness and aggression, cognitive control capability, and time since the most recent suicide attempt on any observed group differences in GMV.
2. Methods
2.1. Participants:
Participants were recruited through multiple study protocols in the Molecular Imaging and Neuropathology Division (MIND) Clinic at The New York State Psychiatric Institute (NYSPI) and Columbia University Medical Center (New York, NY). These protocols were approved by the NYSPI Institutional Review Board and all participants gave written informed consent. Participants included in the current study were selected based on the availability of structural MRI scan and relevant clinical and neuropsychological data. Ninety-one participants who met DSM-IV criteria for major depressive disorder (MDD) were included. A medical lethality scale (Beck et al., 1975) was used to classify participants into higher-lethality suicide attempters with a lethality score of at least 3 or above (physical injury sufficient to require medical intervention) (N = 11), lower-lethality suicide attempters with a lethality score of 2 or less (N = 14), and non-attempters (N = 66) (see Section 2.2 below for details). Inclusion criteria were assessed through clinical interview, chart review, review of systems, physical examination, routine blood tests, pregnancy test, urine toxicology, and ECG. Inclusion criteria included: 1) 18–65 years of age; 2) DSM-IV diagnosis of MDD as assessed using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995); 3) Hamilton Depression Rating Scale-17 item score ≥ 16 (HDRS) (Hamilton, 1960); and 4) capacity to provide informed consent. Exclusion criteria included: 1) unstable medical conditions (i.e., any active illness that may affect the brain such as blood dyscrasias, lymphomas, endocrinopathies, renal failure, chronic obstructive lung disease, systemic autoimmune disorders or malignancy); 2) current alcohol or substance use disorder (past diagnosis allowed if in remission for ≥ 6 months); 3) other current or past major psychiatric disorders such as bipolar disorder or schizophrenia (comorbid anxiety disorders were not excluded); 4) pregnancy, currently lactating, planning to conceive during the course of study participation or abortion in the past two months; 5) dementia; 6) any other neurological disease or prior head trauma with evidence of consequent cognitive impairment; 7) first-degree family history of schizophrenia if the participant is less than 33 years old (mean age of onset of schizophrenia plus two standard deviations) (Sham et al., 1994) to exclude possible prodromal phase of schizophrenia; 8) currently taking fluoxetine (due to long half-life); 9) metal implants or paramagnetic objects contained within the body (including heart pacemaker, shrapnel, or surgical prostheses) which may present a risk to the subject or interfere with the MR scan; and 10) claustrophobia significant enough to interfere with MRI scanning. Patients on antidepressant treatment at the time of enrollment underwent a medication washout and were drug-free for at least two weeks prior to neuroimaging except for the as-needed use of benzodiazepines for anxiety.
2.2. Clinical and Neuropsychological Measures:
Diagnosis of MDD was based on the Structured Clinical Interview for DSM-IV (SCID I) (First et al., 1995). The Beck Depression Inventory (BDI) (Beck et al., 1961) and the Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) assessed self- and clinician-rated depression severity, respectively. Lifetime history of impulsiveness and aggression were assessed using the Barratt Impulsiveness Scale (BIS) (Barratt, 1985) and the Brown-Goodwin Aggression Inventory (B-G) (Brown et al., 1979), respectively.
Suicide history was assessed with the Columbia Suicide History Form (Oquendo et al., 2003a), which carefully distinguishes between different forms of suicidal behavior and non-suicidal self-injurious behavior. To be considered suicide attempter, the participant must have history of at least one actual suicide attempt defined as: “A potentially self-injurious act committed with at least some wish to die, as a result of act. Behavior was in part thought of as method to kill oneself. Intent does not have to be 100%. If there is any intent/desire to die associated with the act, then it can be considered an actual suicide attempt. There does not have to be any injury or harm, just the potential for injury or harm (e.g., If person pulls trigger while gun is in mouth but gun is broken so no injury results, this is considered an attempt”. Participants who had other forms of suicidal behavior such as aborted or interrupted suicide attempt, but no actual suicide attempts, were not considered suicide attempters.
Suicidal ideation was evaluated using the Beck Scale for Suicidal Ideation (SSI) (Beck et al., 1979). Suicide intent was assessed using the Beck Suicide Intent Scale (Beck et al., 1974) for the most recent as well as the maximum-lethality suicide attempt. Lethality of these suicide attempts was assessed using a medical damage rating scale that scored physical injury resulting from an attempt (Beck et al., 1975). This scale measures the medical lethality of any suicide attempt for one of eight possible methods (sedative drugs, non-sedative drugs and other substances, shooting, immolation, drowning, cutting, jumping, hanging) on an ordinal scale from 0 (minimal or no damage) to 8 (death). Since the primary aim of this study is to identify structural brain abnormalities related to higher-lethality suicide attempters, we classified the suicide attempters into two groups by performing a median split for the lethality score of the maximum-lethality attempt. Higher lethality suicide attempters included participants with suicide attempts that were scored a lethality scoring of three or more (at minimum, mild physical injury requiring medical intervention). Given that the cut-off lethality score between these two groups (i.e., score of ≥3) is lower than the score used to distinguish between high vs. low lethality attempts in the literature (Keilp et al., 2014; Oquendo et al., 2009), we used the terms “higher” and “lower” lethality attempters.
The computerized Stroop task, described elsewhere (Keilp et al., 2008; Rizk et al., 2017), was used to assess cognitive control as part of our exploratory analyses to provide context for our initial findings. This task was adapted from standard color/word versions of the task (Macleod, 1991), using a single item presentation and a button press response. Briefly, three blocks of conditions were presented, in a manner similar to the standard paper-and-pencil task: a Word condition (identify color names in black letters), a Color condition (identify the color of a string of four X’s displayed in one of the three colors), and a Color/Word condition (identify display color of a stimulus containing an incongruous color name, ignoring the text). Auditory feedback was provided for correct and incorrect responses. Word and Color blocks had 45 stimulus trials; Color/Word block had 90 trials. Percent Stroop Interference (percent change in median reaction time to color/word vs. color responses) was used to summarize performance. This has been used as an indicator of cognitive inhibition by others (Snyder, 2013), and our group (Keilp et al., 2008; Keilp et al., 2013; Keilp et al., 2001; Kikuchi et al., 2012; Rizk et al., 2017).
2.3. Image Acquisition:
All MRI scans were acquired using a 3T SignaHDx scanner (General Electric Medical Systems, Milwaukee, WI) at the New York State Psychiatric Institute ((NYSPI). Seventy-two scans were performed using an 8-channel head coil (4 higher-lethality suicide attempters, 11 lower-lethality suicide attempters and 57 non-attempters) and 19 scans were performed using a 32-channel head coil (7 higher-lethality suicide attempters, 3 lower-lethality suicide attempters and 9 non-attempters).
T1-weighted MRI scans were acquired using the following parameters: TR=~6 ms, TE=minimum 2400 ms, flip angle = 9, FOV= 25.6 cm × 25.6 cm, slice thickness=1 mm, number of slices= 164, matrix size= 256 × 256 pixels. All 32-channel scans were performed with TI=450 and flip angle = 12, while the 8-channel coil scans were scanned with flip angle=9, with some subjects (N = 36) having TI=500 and the remainder (N = 36) having TI=900. In a preliminary analysis, we found that head coil type, but not the sequence parameters (p > 0.05 for all ROIs), had an effect on ROI GMV values so we included the type of head coil as a covariate (in addition to age, sex and total intracranial volume (TIV)) in all analyses. Detailed quality control using visual inspection was performed to rule out any motion artifacts and gross neuropathology.
2.4. Image Processing:
2.4.1. Preprocessing:
The T1-weighted images were processed with Statistical Parametric Mapping 8 (SPM8) software package (www.fil.ion.ucl.ac.uk/spm; Wellcome Department of Imaging Neuroscience) using Voxel-based Morphometry 8 (VBM8) toolbox (http://dbm.neuro.uni-jena.de/vbm/). Images were bias corrected, segmented, and spatially normalized to standard Montreal Neurological Institute (MNI) space at a voxel size of 1.5 × 1.5 × 1.5 mm3 using 12-parameter affine linear transformation and diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) (Ashburner, 2007). To preserve the actual gray matter values locally, segmented gray matter images were multiplied by the measure of warped and unwarped structures derived from the nonlinear step of the spatial normalization. The modulated gray matter volume (referred to as GMV) images were smoothed with an isotropic Gaussian kernel of 8 mm full width half maximum (FWHM).
2.4.2. ROI determination:
A standard space ROI atlas was used to label regions on VBM images. The Desikan-Killiany atlas was used (Desikan et al., 2006) with ROI GMV calculated as the average VBM value within each region.
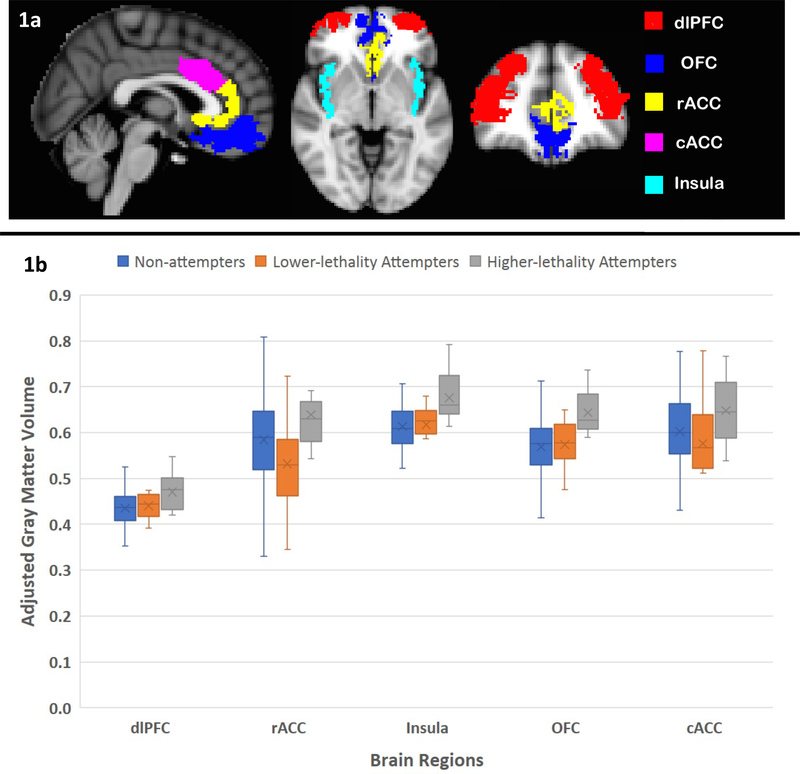
ROIs (Figure 1a) were selected based on results from previous structural and functional neuroimaging studies on suicidal behavior. ROIs included dorsolateral prefrontal cortex (dlPFC) (Giakoumatos et al., 2013; Jollant et al., 2008; Oquendo et al., 2003b), orbitofrontal cortex (OFC) (Jollant et al., 2008; Monkul et al., 2007; Oquendo et al., 2003b; Soloff et al., 2012), insula (Giakoumatos et al., 2013; Soloff et al., 2012), rostral and caudal anterior cingulate cortex (rACC and cACC) (Dombrovski et al., 2013; Oquendo et al., 2003b; Pan et al., 2011). ROIs were averaged bilaterally to minimize the number of comparisons.
Figure 1.
a) Sagittal, axial and coronal brain views showing the extension of the 5 a priori regions-of-interest. b) Box and Whisker plot showing the means and standard deviations of the gray matter volume (adjusted for age, sex, total intracranial volume and head coil type) in the 5 a priori regions-of-interest across higher-lethality suicide attempters, lower-lethality attempters and non-attempters. Abbreviations: cACC= caudal anterior cingulate cortex, dlPFC= dorsolateral prefrontal cortex, OFC= orbitofrontal cortex, rACC= rostral anterior cingulate cortex.
2.5. Statistical Analysis:
In preliminary analyses, data were statistically evaluated using IBM SPSS Statistics (SPSS Inc., Chicago, Illinois, USA, Version 24.0). All quantitative variables, by diagnostic group, were checked for normal distribution and outliers. Comparisons of demographic and clinical variables between the higher-lethality suicide attempters, lower-lethality suicide attempters and non-attempters were performed using one-way analysis of variance (ANOVA) for continuous variables and Chi-square analyses for categorical variables. The relationship between lethality and intent of the highest lethality suicide attempt was tested using Spearman’s correlation analysis. Given that research shows that GMV may be affected by individual differences in handedness (Sun and Walsh, 2006), educational level (Arenaza-Urquijo et al., 2013), history of substance use disorder (Thayer et al., 2017) or comorbid borderline personality disorder (Soloff et al., 2012), we performed preliminary analyses to check if these variables have any effect on GMV in the current study. These analyses yielded no significant association between these factors and GMV.
For the ROI analysis, we fitted a linear mixed-effects model with the GMV values as outcome, region, group (categorical variable with three levels), age, sex, TIV and head coil type as fixed effects, and subject as the random effect, in order to properly account for the covariance structure of the data. There was strong evidence for different variances across regions and so our linear mixed model allowed a different variance for each region. This ROI analysis was performed using R version 3.3.3 (http://cran.r-project.org). Power calculation showed that, with our sample sizes, based on summary statistics taken from the data, it is possible to perform determine hypothetical differences that could be detected with 80% power. We can do this separately for each region and for each comparison (i.e., higher-lethality vs. lower-lethality attempters and higher-lethality attempters vs. non-attempters), giving 10 minimally detectable effect sizes in all. This calculation varies considerably from region to region and from comparison to comparison based on volumes and sizes of comparison groups (66 non-attempters, but only 14 lower-lethality attempters). Overall, we are 80% powered to detect differences from as small as 8% to as large as 20.4%.
Possible confounding effect of clinical variables (current depression severity, suicidal ideation, life-time impulsiveness, aggression history, Stroop task interference, and time passed since most recent attempt [> 5 years vs. < 5 years]) were examined by refitting the linear mixed-effects model as above also including each of these variables in turn as an additional fixed effect.
For the voxel-wise analysis, a one-way ANOVA was performed in SPM8 to compare the smoothed gray matter maps of higher-lethality suicide attempters, lower-lethality attempters and non-attempters’ scans with age, sex, TIV and head coil type as covariates of no interest. The statistical parametric maps were thresholded at a voxel-level p-value of < 0.001 and a cluster-level FWE corrected p-value of < 0.05.
3. Results
3.1. Demographic, Clinical, and Neuropsychological Measures:
Demographic and clinical characteristics (N = 91) are shown in Table 1. The higher-lethality suicide attempters, lower-lethality suicide attempters and non-attempters did not differ in terms of age, sex, race, ethnicity and handedness. Current suicidal ideation scores were higher in higher-lethality attempters compared with the other two groups. No statistically significant difference was found between the three groups regarding depression severity, impulsiveness, lifetime aggression or Stroop interference scores.
Table 1.
Demographic and Clinical Characteristics of the Study Sample:
| Variable | MDD Higher-lethality Suicide Attempters (N= 11) | MDD Lower-lethality Suicide Attempters (N= 14) | MDD Suicide non-attempters (N= 66) | Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics | ||||||||
| N | % | N | % | N% | % | χ2 | pa | |
| Sex; Female | 5 | 45 | 9 | 64.3 | 46 | 70 | 2.487 | 0.305 |
| Race; White | 5 | 45 | 7 | 50 | 44 | 67.7 | 13.499 | 0.118 |
| Handedness; Right | 7 | 63.6 | 10 | 71.4 | 52 | 78.8 | 2.692 | 0.315 |
| Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | F | pb | |
| Age (years) | 18–56 | 33.4 (14.3) | 19–60 | 35.9 (12.8) | 18–64 | 34.5 (11) | 0.151 | 0.860 |
| Education (years) | 11–18 | 14.3 (2.9) | 11–22 | 15.7 (3.7) | 11–22 | 16.1 (2.6) | 1.630 | 0.202 |
| Clinical & Neuropsychological Measures | ||||||||
| Beck Depression Inventory | 13–38 | 23.6 (10.0) | 13–38 | 26.1 (9.1) | 13–47 | 24.3 (9.0) | 0.270 | 0.764 |
| Hamilton Depression Rating Scale (17-item) | 16–25 | 19.9 (3.7) | 16–25 | 17.5 (4.7) | 16–31 | 17.9 (5.0) | 0.890 | 0.415 |
| Barratt Impulsivity Scale | 23–87 | 52.1 (19.6) | 36–65 | 49.3 (10.1) | 21–95 | 52.0 (14.9) | 0.142 | 0.868 |
| Brown Goodwin Aggression Scale | 10–26 | 17.0 (5.5) | 10–24 | 17.1 (4.1) | 10–32 | 16.4 (3.7) | 0.236 | 0.791 |
| Stroop Interference$ | 0.050–0.820 | 0.348 (0.267) | 0.010–0.730 | 0.280 (0.190) | −0.0201.040 | 0.317 (0.221) | 0.296 | 0.744 |
| N | % | N | % | N | % | χ2 | pc | |
| Comorbid Anxiety Disorder | 4 | 36.4 | 7 | 50.0 | 30 | 45.5 | 0.236 | 0.887 |
| Past Substance Use Disorder | 1 | 9.1 | 2 | 14.3 | 8 | 12.1 | 0.289 | 0.951 |
| Borderline Personality Disorder | 6 | 54.5 | 3 | 21.4 | 4 | 6.1 | 15.147 | <0.001 |
| Suicide History | ||||||||
| Scale for Suicidal Ideation | 0–33 | 16.7 (13.1) | 0–24 | 6.3 (7.6) | 0–21 | 3.9 (5.7) | 15.137 | <0.001 |
| Number of actual suicide attempts | 1–6 | 2.3 (1.7) | 1–7 | 2.1 (1.7) | 0.086 | 0.773 | ||
| Suicide intent score of maximum-lethality attempt | 6–25 | 16.2 (5.7) | 11–24 | 16.7 (4.1) | 0.067 | 0.798 | ||
| Lethality score of maximum- | 3–7 | 3.6 | 0–2 | 0.7 | 51.693 | <0.001 | ||
| lethality attempt | (1.2) | (0.8) | ||||||
| N | % | N | % | χ2 | pa | |||
| Recency of last attempt; < 5 years | 7 | 63.6 | 6 | 42.9 | 1.066 | 0.428 | ||
Chi-square analysis.
One-way ANOVA.
Fisher’s Exact test.
Score negatively scaled. Higher scores indicate poorer performance.
Abbreviations: MDD = major depressive disorder
The two groups of attempters did not differ in the number of lifetime suicide attempts, suicide intent for the most lethal attempt or time passed since the most recent attempt. Within the suicide attempter group, the lethality of the most lethal suicide attempt did not correlate with intent (Spearman’s rho = 0.233, p = 0.297).
3.2. Image analysis:
3.2.1. ROI analysis:
Considering all ROIs simultaneously, GMV differed between higher-lethality attempters, lower-lethality attempters, and non-attempters (F(2, 85) = 5.261, p = 0.007), with evidence of heterogeneity of this effect across regions (region × group interaction: F(8, 352) = 2.110, p = 0.034). Exploratory post hoc individual ROI comparisons within the model (Figure 1b) showed that, compared with the MDD lower-lethality attempters, the MDD higher-lethality suicide attempters had greater GMV in OFC (p = 0.002), rACC (p = 0.006), cACC (p = 0.034), and insula (p = 0.009) but not in dlPFC (p = 0.126). Also, compared with the MDD non-attempters, the MDD higher-lethality suicide attempters had greater GMV in dlPFC (p = 0.042), OFC (p < 0.001), insula (p = 0.001), but not in rACC (p = 0.089) or cACC (p = 0.106).
Re-running the model with each of the clinical variables as an additional covariate, the main effect of group on GMV remained significant. That was true when including BDI score (F(2, 80) = 5.887, p = 0.004), HDRS score (F(2, 82) = 5.367, p = 0.006), SSI score (F(2, 81) = 7.311, p = 0.001), BIS score (F(2, 65) = 6.633, p = 0.002), B-G score (F(2, 76) = 4.438, p = 0.015), Stroop task interference (F(2, 84) = 5.290, p = 0.007) and time since most recent attempt (F(1, 19) = 5.496, p = 0.030).
3.2.2. Voxel-wise analysis:
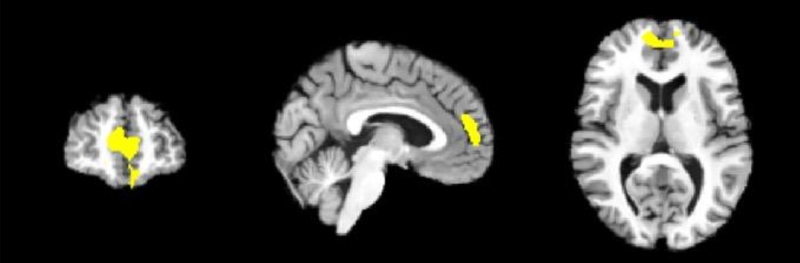
Compared with lower-lethality suicide attempters and non-attempters, higher-lethality attempters had greater GMV in a large cluster (size = 1,500 voxels, cluster-level p(FWE) < 0.001, maximum T-score = 4.02 at MNI coordinates 4,50,12) mainly encompassing the right prefrontal lobe (Figure 2). No clusters were identified where lower-lethality attempters or non-attempters had greater GMV than other groups.
Figure 2.
Results from an optimized voxel-based morphometry analysis, showing coronal, sagittal and axial views of the cluster (size = 1,500 voxels, maximum T-score = 4.02 at MNI coordinates x,y,z = 4,50,12) with greater gray matter volume in higher-lethality suicide attempters compared to lower-lethality attempters and non-attempters at a voxel-level p-value of < 0.001 and a cluster-level FWE corrected p-value of < 0.05.
4. Discussion:
The current study examined the neuroanatomical correlates of higher-lethality suicidal behavior in medication-free adults with major depression. Contrary to our hypothesis, individuals with MDD and a history of at least one higher-lethality suicide attempt had greater gray matter volume of prefrontal regions and insula compared to MDD participants with a history of lower-lethality suicide attempters and MDD participants with no history of suicide attempts. This association was not explained by current depression or suicidal ideation severity, lifetime severity of impulsiveness or aggression, cognitive control ability or time passed since the most recent suicide attempt, as the association remained significant after controlling for these variables.
The regions found to be larger in higher-lethality suicide attempters largely contribute to cognitive control of thoughts and emotions. Specifically, the OFC (particularly its lateral part), dlPFC and ACC play significant roles in cognitive reappraisal of negative emotions (Buhle et al., 2014; Ochsner et al., 2012). Further, the dlPFC and ACC subserve the attention control network (Leung et al., 2009; MacDonald et al., 2000; Shenhav et al., 2016), and the dlPFC has been particularly linked to planning of action sequences (Goel, 2002; Otto et al., 2013; Owen, 2005; Tanji et al., 2007). Besides its well-known function in negative emotional processing (Adolphs, 2003; Ochsner et al., 2002), some studies showed the anterior insula play an essential role in voluntary control and intentional actions (Brass and Haggard, 2010; Nelson et al., 2010).
It may seem counterintuitive that higher-lethality suicide attempters had larger volumes of brain regions sub-serving planning, intentional actions, executive functions, and emotion regulation. However, previous studies showed that a subset of higher-lethality suicide attempters has a high degree of planning prior to the suicidal act (Chaudhury et al., 2016), and lower delayed discounting (Dombrovski et al., 2011) and thus can suppress their desire for immediate satisfaction in favor of delayed reward. Higher-lethality depressed suicide attempters also perform better on an object alternation task, indicating greater executive control, and response organization (Keilp et al., 2014), compared with lower-lethality suicidal attempters. Interestingly, Vang et al. (2010) looked at the differences in brain subcortical volumes between a sample of suicide attempters with a high intent to die and healthy controls. They found this subgroup of attempters had smaller globus pallidus volumes that in turn correlated negatively with solidity, a measure of non-impulsive temperament. Further, Dombrovski et al. (2012) found larger putamen and pallidum in individuals with late-life depression and history of higher-lethality suicide attempts compared with lower-lethality suicide attempters. Collectively, these findings suggest that suicide attempters with higher degree of suicidal act lethality may represent a non-impulsive subgroup. In other words, the larger prefrontal regional volumes in this group may relate to their better planning and response organization abilities. Partially supporting this hypothesis is the established positive moderate correlation between lethality and intent of suicide attempt (van Heeringen and Mann, 2014), which was not found in our current study, probably due to small sample size. Although we have not found differences between higher-lethality suicide attempters, lower-lethality attempters and non-attempters in terms of the impulsivity, aggression, or cognitive control measures utilized in this study, these groups may show differences in other domains of executive function not captured by these measures.
An alternative explanation for our finding is that increased GMV in the higher-lethality suicide attempters may represent a compensatory process in response to chronic and elevated stress prior to the suicidal act. Consistent with this hypothesis, Steiner et al. (2008) found elevated microglial density in suicidal individuals suggesting that microglial activation might be a consequence of pre-suicidal stress. This microglial activation results in an increase in soma size and development of thicker, branched processes (LaVoie et al., 2004), a change that may be reflected in the observed greater volume of the brain regions in the current study. However, this interpretation should be considered with caution as the physiological mechanisms of increased regional brain volume detected by VBM remain incompletely elucidated.
Of note, the direction of gray matter abnormalities in our study is consistent with a recent study showing that high-lethality suicide attempters with bipolar disorder have greater GMV in the insula and ACC compared to low lethality attempters and non-attempters (Duarte et al., 2017). Interestingly, in their meta-analyses of VBM studies in different psychiatric diagnoses, Goodkind et al. (2015) argued that deficits in dorsal ACC and anterior insula may represent a transdiagnostic pathological process across major psychiatric disorders. Future studies should test if volumetric changes in these regions is linked to high lethal suicidal behavior across different psychiatric diagnoses.
In contrast to our finding, many previous studies reported that depressed suicide attempters have lower GMV in different brain regions compared with depressed non-attempters and healthy controls. These include findings in the anterior cingulate cortex (Wagner et al., 2011), lateral PFC (Ding et al., 2015), dorsomedial PFC (Hwang et al., 2010), OFC (Ding et al., 2015; Monkul et al., 2007), temporal gyrus (Pan et al., 2015), hippocampus (Colle et al., 2015), angular gyrus, cerebellum (Lee et al., 2016a) and posterior third of corpus callosum (Cyprien et al., 2011). The differences between these studies and the current one may possibly be explained by the heterogeneity of suicidal behavior in terms of degree of lethality and intent of suicidal act. We have assessed the history and characteristics of suicide attempts more thoroughly compared with previous studies with contrasting brain structural findings. For example, most previous studies focused on the differences between suicide attempters and non-attempters in general and did not assess different forms of suicidal behavior (Colle et al., 2015; Cyprien et al., 2011; Hwang et al., 2010; Lee et al., 2016b; Pan et al., 2015; Wagner et al., 2011). Although (Ding et al., 2015) examined the relationship between lethality of suicide attempt and brain regional volumes, they used a different measure for assessment of lethality of suicidal behavior, namely the Risk Rescue Rating Scale (Weisman and Worden, 1972). Another reason why our finding is different from some previous studies may be the differences in methodological approaches used by to calculate and analyze the GMV data.
4.1. Limitations:
Median-split of the suicide attempters based on the degree of medical lethality in this study resulted in a cut-off lethality score of 3, which is lower than what has been previously used in literature to distinguish the high vs. low lethality attempters. Although the modest sample size of the high lethality suicide attempters may limit the generalizability of the findings, this study provides support for considering the heterogeneity of suicidal behavior, particularly the lethality, in analyses of underlying neurobiology. The mixed-effects model for ROI analysis showed an overall effect of the group on GMV across regions. However, post hoc individual ROI analysis showed that in some regions the difference between higher-lethality suicide attempters and one of the other two groups did not reach statistical significance (p=0.1 for dlPFC in higher-lethality vs. lower-lethality attempters, and for rACC and cACC in higher-lethality attempters vs. non-attempters). It is not uncommon to find a robust difference in the overall model combining all brain regions and not in individual regions where the statistical power is usually lower. Future studies should seek replication of the findings in larger sample size that can more adequately do a post hoc test of individual brain region. Without comparisons to a healthy control group, it is impossible to assess the degree of structural abnormality of these findings or to conclude which group, attempters or non-attempters, if either, show differences as compared to healthy controls.
4.2. Future Directions:
The current study provides evidence for structural brain changes associated with higher-lethality suicidal behavior. We examined the effect of specific neurocognitive and behavioral functions, including attentions control, impulsiveness and aggression, on the observed brain structural findings of higher-lethality suicidal behavior. These assessments map onto the cognitive domain of the National Institute of Mental Health Research Domain Criteria (RDoC). Future work could seek to more comprehensively relate relevant RDoC domains to suicide risk and brain structural abnormalities. Additional studies are also required to determine whether the neural correlates of suicide risk are shared across these psychiatric conditions or are diagnosis-specific.
Highlights.
Risk for suicidal behavior has been linked to structural brain deficits.
Suicidal behavior is heterogeneous in terms of suicidal act’s lethality.
Higher-lethality suicidal behavior may be associated with larger prefrontal cortex.
Acknowledgments
We thank the study participants and entire staff of the Division of Molecular Imaging and Neuropathology for their time and effort.
Role of funding source
This research was supported by grants from the National Institute of Mental Health: P50 MH090964 (PI: J. Mann), P50 MH062185 (PI: J. Mann), R01 MH040695 (PI: J. Mann), R01 MH093637 (PI: M. Milak), K08 MH079033 (PI: M. Sublette) and K08 MH085061 (PI: J. Miller). Mina M. Rizk is supported by a scholarship from the Egyptian Cultural and Educational Bureau, Embassy of Egypt, Washington, D. C.
Footnotes
Conflict of interest
Maria A. Oquendo and J. John Mann receive royalties for commercial use of the Columbia Suicide Severity Rating Scale from the Research Foundation for Mental Hygiene. Dr. Oquendo’s family owns stock in Bristol Myers Squibb. Other authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- Adolphs R, 2003. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience 4, 165–178. [DOI] [PubMed] [Google Scholar]
- Aguilar EJ, Garcia-Marti G, Marti-Bonmati L, Lull J, Moratal D, Escarti M, et al. , 2008. Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 32, 1673–1676. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE, 1990. The Hippocampus and Parahippocampus in Schizophrenic, Suicide, and Control Brains. Arch Gen Psychiat 47, 1029–1034. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 1994. DSM-IV: Diagnostic and statistic manual of mental disorders, American Psychiatric Association, Washington DC. [Google Scholar]
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mezenge F, Perrotin A, et al. , 2013. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 83, 450–457. [DOI] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. NeuroImage 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Barratt ES, 1985. Impulsiveness defined within a system model of personality, in: Spielberger CD, Butcher JN (Eds.), Advances in Personality Assessment. Erlbaum, New York, pp. 113–132. [Google Scholar]
- Beck AT, Beck R, Kovacs M, 1975. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry 132, 285–287. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 47, 343–352. [DOI] [PubMed] [Google Scholar]
- Beck AT, Schuyler D, Herman I, 1974. Development of suicidal intent scales, in: Beck AT, Resnick HLP, Lettieri DJ (Ed.), The Prediction of Suicide. Charles Press Publishers, Bowie, MD, pp. 45–58. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Arch Gen Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Poletti S, Locatelli C, Falini A, Colombo C, et al. , 2011. Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affect Disord 135, 139–147. [DOI] [PubMed] [Google Scholar]
- Bernal M, Haro JM, Bernert S, Brugha T, de Graaf R, Bruffaerts R, et al. , 2007. Risk factors for suicidality in Europe: results from the ESEMED study. Journal of affective disorders 101, 27–34. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P, 2010. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct Funct 214, 603–610. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF, 1979. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1, 131–139. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. , 2014. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC), 2015. National center for injury prevention and control. Web‐based Injury Statistics Query and Reporting System (WISQARS). Retrieved January 21, 2015, from www.cdc.gov/injury/wisqars/index.html.
- Chaudhury SR, Singh T, Burke A, Stanley B, Mann JJ, Grunebaum M, et al. , 2016. Clinical correlates of planned and unplanned suicide attempts. J Nerv Ment Dis 204, 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle R, Chupin M, Cury C, Vandendrie C, Gressier F, Hardy P, et al. , 2015. Depressed suicide attempters have smaller hippocampus than depressed patients without suicide attempts. J Psychiatr Res 61, 13–18. [DOI] [PubMed] [Google Scholar]
- Cyprien F, Courtet P, Malafosse A, Maller J, Meslin C, Bonafe A, et al. , 2011. Suicidal behavior is associated with reduced corpus callosum area. Biol Psychiatry 70, 320–326. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. , 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Ding Y, Lawrence N, Olie E, Cyprien F, le Bars E, Bonafe A, et al. , 2015. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry 5, e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H, 2012. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med 42, 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Clark L, Reynolds CF, Siegle GJ, 2013. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA psychiatry 70, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Siegle GJ, Wallace ML, Forman SD, Sahakian B, et al. , 2011. Lethal forethought: delayed reward discounting differentiates high- and low-lethality suicide attempts in old age. Biol Psychiatry 70, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte DGG, Neves MCL, Albuquerque MR, Turecki G, Ding Y, de Souza-Duran FL, et al. , 2017. Structural brain abnormalities in patients with type I bipolar disorder and suicidal behavior. Psychiatry Res 265, 9–17. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0). Biometrics Research Dept., New York State Psychiatric Institute, New York. [Google Scholar]
- Giakoumatos CI, Tandon N, Shah J, Mathew IT, Brady RO, Clementz BA, et al. , 2013. Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? J Psychiatr Res 47, 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifuni AJ, Ding Y, Olie E, Lawrence N, Cyprien F, Le Bars E, et al. , 2016. Subcortical nuclei volumes in suicidal behavior: nucleus accumbens may modulate the lethality of acts. Brain Imaging and Behavior 10, 96–104. [DOI] [PubMed] [Google Scholar]
- Goel V, 2002. Planning: neural and psychological. Encyclopedia of cognitive science.
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. , 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, et al. , 2010. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol 23, 171–184. [DOI] [PubMed] [Google Scholar]
- Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. , 2017. Multimodal Neuroimaging of Frontolimbic Structure and Function Associated With Suicide Attempts in Adolescents and Young Adults With Bipolar Disorder. Am J Psychiatry 174, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, et al. , 2008. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry 165, 740–748. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ, 2008. Attention deficit in depressed suicide attempters. Psychiatry Res 159, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, et al. , 2013. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med 43, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ, 2001. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry 158, 735–741. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Wyatt G, Gorlyn M, Oquendo MA, Burke AK, John Mann J, 2014. Intact alternation performance in high lethality suicide attempters. Psychiatry Res 219, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Miller JM, Schneck N, Oquendo MA, Mann JJ, Parsey RV, et al. , 2012. Neural responses to incongruency in a blocked-trial Stroop fMRI task in major depressive disorder. J Affect Disord 143, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG, 2004. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Experimental Neurology 187, 47–57. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim S, Gwak AR, Kim SJ, Kang S-G, Na K-S, et al. , 2016a. Decreased regional gray matter volume in suicide attempters compared to suicide non-attempters with major depressive disorders. Comprehensive psychiatry 67, 59–65. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim S, Gwak AR, Kim SJ, Kang SG, Na KS, et al. , 2016b. Decreased regional gray matter volume in suicide attempters compared to suicide non-attempters with major depressive disorders. Compr Psychiatry 67, 59–65. [DOI] [PubMed] [Google Scholar]
- Leung KK, Lee TM, Wong MM, Li LS, Yip PS, Khong PL, 2009. Neural correlates of attention biases of people with major depressive disorder: a voxel-based morphometric study. Psychol Med 39, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Rourke ED, Swann AC, Zunta-Soares GB, Soares JC, 2014. Illness-course modulates suicidality-related prefrontal gray matter reduction in women with bipolar disorder. Acta Psychiatr Scand 130, 374–387. [DOI] [PubMed] [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS, 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Macleod CM, 1991. Half a century of research on Stroop an integrative view. Psychological Bulletin 109, 163–203. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, 1997. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiat 41, 162–171. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nielsen N, Nicoletti MA, Hatch JP, Monkul ES, Watanabe Y, et al. , 2010. Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neuroscience letters 469, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. , 2007. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry 12, 360–366. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE, 2010. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct 214, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD, 2002. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14, 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT, 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Carballo JJ, Rajouria N, Currier D, Tin A, Merville J, et al. , 2009. Are high-lethality suicide attempters with bipolar disorder a distinct phenotype? Arch Suicide Res 13, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ, 2003a. Risk factors for suicidal behavior: utility and limitations of research instruments, in: First MB (Ed.), Standardized Evaluation in Clinical Practice, 1st ed. American Psychiatric Publishing, Washington DC, pp. 103–130. [Google Scholar]
- Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, et al. , 2003b. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry 60, 14–22. [DOI] [PubMed] [Google Scholar]
- Otto AR, Gershman SJ, Markman AB, Daw ND, 2013. The curse of planning: dissecting multiple reinforcement-learning systems by taxing the central executive. Psychol Sci 24, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, 2005. Cognitive planning in humans: new insights from the Tower of London (TOL) task, in: Morris R, Ward G (Eds.), The Cognitive Psychology of Planning. Psychology Press, Hove-New York, pp. 135–151. [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida JR, Segreti A, Akkal D, Hassel S, et al. , 2011. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. J Am Acad Child Adolesc Psychiatry 50, 602–611 e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LA, Ramos L, Segreti A, Brent DA, Phillips ML, 2015. Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br J Psychiatry 206, 339–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk MM, Rubin-Falcone H, Keilp J, Miller JM, Sublette ME, Burke A, et al. , 2017. White matter correlates of impaired attention control in major depressive disorder and healthy volunteers. J Affect Disord 222, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch N, Spoletini I, Wilke M, Martinotti G, Bria P, Trequattrini A, et al. , 2008. Inferior frontal white matter volume and suicidality in schizophrenia. Psych. Res. Neuroimaging 164, 206–214. [DOI] [PubMed] [Google Scholar]
- Sham PC, MacLean CJ, Kendler KS, 1994. A typological model of schizophrenia based on age at onset, sex and familial morbidity. Acta Psychiatrica Scandinavica 89, 135–141. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM, 2016. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci 19, 1286–1291. [DOI] [PubMed] [Google Scholar]
- Snyder HR, 2013. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA, 2012. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res 46, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoletini I, Piras F, Fagioli S, Rubino IA, Martinotti G, Siracusano A, et al. , 2011. Suicidal attempts and increased right amygdala volume in schizophrenia. Schizophrenia research 125, 30–40. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. , 2008. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. Journal of Psychiatric Research 42, 151–157. [DOI] [PubMed] [Google Scholar]
- Stengel E, 1964. Suicide and Attempted Suicide. Penguin Books, Oxford, England. [Google Scholar]
- Stone DM, Simon TR, Fowler KA, Kegler SR, Yuan K, Holland KM, et al. , 2018. Vital Signs: Trends in State Suicide Rates—United States, 1999–2016 and Circumstances Contributing to Suicide—27 States, 2015, Morbidity and Mortality Weekly Report, pp. 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2013. Results from 2012 National survey on drug use and health: Mental health findings. NSDUH Series H‐47, HHS Publication No. (SMA) 13‐4805. Retrieved January 2015, 20, from: http://www.samhsa.gov/data/sites/default/files/2k12MH_Findings/2k12MH_Findings/NSDUHmhfr2012.htm.
- Sun T, Walsh CA, 2006. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci 7, 655–662. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K, Mushiake H, 2007. Concept-based behavioral planning and the lateral prefrontal cortex. Trends in cognitive sciences 11, 528–534. [DOI] [PubMed] [Google Scholar]
- Thayer RE, YorkWilliams S, Karoly HC, Sabbineni A, Ewing SF, Bryan AD, et al. , 2017. Structural neuroimaging correlates of alcohol and cannabis use in adolescents and adults. Addiction 112, 2144–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, et al. , 2011. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 36, 2650–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V, 2012. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol 15, 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C, 2014. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Frontiers in human neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ, 2014. The neurobiology of suicide. Lancet Psychiatry 1, 63–72. [DOI] [PubMed] [Google Scholar]
- Vang FJ, Ryding E, Traskman-Bendz L, van Westen D, Lindstrom MB, 2010. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res 183, 177–179. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG, 2011. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage 54, 1607–1614. [DOI] [PubMed] [Google Scholar]
- Weisman AD, Worden JW, 1972. Risk-rescue rating in suicide assessment. Arch Gen Psychiatry 26, 553–560. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2016. World health statistics 2016: monitoring health for the SDGs sustainable development goals World Health Organization, Geneva, Switzerland. [Google Scholar]