Abstract
Objective:
An association between central venous pressure and AKI has been observed following cardiac surgery, but it is unknown whether this reflects intravascular volume status or impaired right ventricular myocardial performance. This study was performed to test the hypothesis that decreased right ventricular peak longitudinal strain (PLSS,) as measured by 2-D speckle tracking echocardiography, is associated with AKI following cardiac surgery.
Design:
Retrospective observational cohort study.
Setting:
Cardiovascular intensive care unit in a 576-bed referral hospital.
Participants:
Adult patients having undergone cardiac surgery in whom a transthoracic echocardiogram was performed within 48 hours following chest closure.
Interventions:
This was a retrospective study. Urine output and serum creatinine values were recorded at baseline and for 48h after surgery. Statistical analysis was performed to identify differences in baseline demographic and echo-derived values between patients with and without postoperative AKI criteria.
Measurements and main results:
199 subjects had post-processing of TTE performed. AKI was observed in 87% of patients (173/199.) Age, BMI, and preoperative serum creatinine were higher in the AKI group. The mean PLSS was −17.2% ± 4.3% vs. −17.1% ± 3.7% in patients with AKI versus those without (p = 0.95). The calculated right ventricular systolic pressure was elevated in the AKI group compared to the non-AKI group (38.9±9.9 vs 34.6±7.9 mmHg; p=0.02.)
Conclusions:
In this cohort of cardiac surgery patients, speckle-tracking analysis of RV myocardial performance was feasible. Elevated right ventricular systolic pressure associated with AKI, while speckle-tracking derived echo measurements did not.
Keywords: Acute Kidney Injury; Cardiac Surgical Procedures; Cardiorenal Syndrome; Echocardiography; Postoperative Care; Ventricular Function, Right
Background
Acute kidney injury (AKI,) a common complication following cardiothoracic surgery (CTS,) increases postoperative mortality and risk of chronic kidney disease.1–4 Perioperative management may alter these outcomes; thus, this is a growing area of investigation.5 Most previously reported risk factors of postoperative AKI after CTS are patient-specific and non-modifiable, with the exceptions of the use of cardiopulmonary bypass (CPB) and duration of CPB and aortic cross-clamping.6, 7
However, postoperative elevation of the central venous pressure (CVP,) an indicator of venous congestion, has recently demonstrated association with subsequent AKI.8, 9 This is a potentially modifiable contributor to acute postoperative cardiorenal syndrome; however, whether the rise in CVP is attributed to excess preload with normal right ventricular (RV) systolic function or due to impaired right ventricular systolic performance with elevated right-sided pressures as a secondary effect is unknown. As these disparate etiologies have distinct treatments, it is essential to evaluate the contributions of AKI-associated hydrostatic pressure elevation due to hypervolemia or impairment of forward flow. A critical limitation in determining whether right-sided hydrostatic pressure or impaired right ventricular systolic function drives AKI has been the technical difficulty in assessment of performance of the right ventricle in patients who have undergone CTS.
Speckle-tracking echocardiography (STE) is gaining acceptance as a reliable, reproducible, and practical indicator of right ventricular systolic function.10, 11 This novel measurement facilitates strain imaging, allowing for quantification of the shortening (negative strain) and lengthening (positive strain) of specific myocardial segments. While most speckle-tracking strain investigations of the right ventricle have been performed in ambulatory patients with pulmonary hypertension, this technique is resistant to confounding by artifact produced by movement of the ultrasound probe on the chest wall and motion of the heart within the chest cavity, and allows for image processing in non-parallel image planes – all common pitfalls of transthoracic echocardiography (TTE) following CTS.12 Speckle-tracking derived RV free wall peak longitudinal systolic strain (RV-PLSS) is a highly reproducible measure, well-validated for a number of nonoperative populations, with normal value of −19%. Abnormal preoperative values for a related measure, the RV global strain (RV-GLS), demonstrate association with postoperative mortality in CTS patients, demonstrating the emerging importance and value of RV speckle-tracking in the CTS population.13
We therefore performed a study using the speckle-tracking method to investigate the association between RV systolic function and postoperative AKI in the CTS population. We determined echocardiographic measures of RV systolic function and other potential patient risk factors that could contribute to the development of postoperative AKI a priori. We hypothesized that in cardiac surgery patients, reduced postoperative RV systolic function, (as measured by speckle-tracking derived RV-PLSS) is associated with AKI.
Methods
Study Design
This study was approved by the Institutional Review Board of Oregon Health & Science University. We performed a retrospective chart review of adult subjects undergoing on-pump cardiac surgical procedures at a single, 576-bed academic medical center between 2008 and 2015. We designed a targeted dataset extraction through the institutional corporate data warehouse. We requested data from subjects who had undergone coronary artery bypass grafting (CABG), cardiac valve repair/replacement, and combined CABG/valve procedures, identified by billing codes which matched these procedures. Subjects were excluded from the dataset extraction if age was <18 or there was prior history of chronic kidney disease greater than stage three according to KDIGO guidelines.14 Additional exclusions from the dataset included intraoperative cardiotomy of the right ventricle, subjects requiring delayed closure of sternotomy for any reason, and urgent and emergent surgical procedures.
We then identified subjects in the echocardiography storage system for which a TTE was performed within 48 hours following chest closure. We collected the following demographic data and covariables, many of which have been previously shown to be risk factors for AKI following CTS:6, 7 Age, sex, preoperative weight, and body mass index (BMI)_were recorded from the electronic medical record system. The most recent preoperative serum creatinine value was obtained from either the institutional lab record or outside laboratory reports. The estimated GFR was calculated from these variables according to the CKD-EPI equation.15 The presence of peripheral arterial disease (PAD, chronic obstructive pulmonary disease (COPD,) and diabetes mellitus (DM.), was recorded from ICD-9 codes and review of the electronic medical record problem list and history. For example, ICD-9 codes for either emphysema or chronic bronchitis would be considered positive for the presence of COPD. Preoperative left ventricular ejection fraction (LVEF) and Tricuspid Annular Plane Systolic Excursion (TAPSE) were recorded if reported. Preoperative echo parameters were recorded only after processing of postoperative images to avoid the introduction of bias. Procedural data was recorded from the cardiac perfusion record, and included procedure type (CABG, valve, combined) and duration of cardiopulmonary bypass and aortic cross clamping times. We also chose to include the volume and route of cardioplegia delivered, which have not previously been reported in relation to postoperative AKI.
Echocardiography
Transthoracic echocardiograms (TTE) imaging the four standard echocardiographic windows (parasternal long and short axes, apical, and subcostal) were performed in the intensive care unit by licensed sonographers or fellowship-trained anesthesiology critical care physicians with significant transthoracic echocardiography experience. TTE studies were performed within 48 hours following chest closure in all subjects. All TTE studies were postprocessed and analyzed by a single investigator (SY), using dedicated, vendor-independent, speckle tracking software (EchoInsite Right Ventricle Software Suite, Epsilon Imaging, Michigan, USA.) Using this software, a region of interest was manually applied to define the RV endocardial border at the septum, apex, and free wall in end-diastole.The longitudinal strain was then derived by the software automatically by tracking frame-to-frame displacement of the speckle pattern through end-systole. Manual adjustments were again made at end-systole to increase the accuracy of tracking. While the entire RV was assessed via this method, out study region of interest was the RV free wall, and septal segments were excluded from analysis. The longitudinal strain of the three free wall segments (basal, mid, and apical) were averaged to arrive at a final value for RV free wall PLSS. Association between AKI and RV-PLSS was the primary endpoint of this study. Studies were reviewed to identify the highest quality apical 4-chamber or apical RV-dedicated views. In addition to RV-PLSS of the free wall, we reported STE derived TAPSE, tricuspid systolic velocity (S’,) and fractional area change (FAC) as calculated using the same speckle-tracking software analyzing the RV-focused echo clips. The left ventricle ejection fraction (LVEF) and right ventricular systolic pressure (RVSP) values were determined from the postoperative TTE as well. If the velocity of the tricuspid regurgitant jet was recorded but not RVSP, a nominal right atrial pressure of 8 mmHg was used to estimate RVSP.. Images were graded as being poor, fair, or good quality based on criteria listed in Table 1.
Table 1.
Grading criteria for postoperative transthoracic echocardiograms.
| Good | Visualization of the entire RV free wall, including apical, mid, and basal segments throughout the entire cardiac cycle without significant artifact. Minor or no arrhythmias present. |
| Fair | Visualization of two of three segments of RV free wall throughout majority of cardiac cycle. Mild to moderate imaging artifact and/or arrhythmias may be present. |
| Poor | Visualization of one or two segments of RV free wall during systolic cycling. Significant imaging artifact and/or presence of arrhythmias common. |
Identifying AKI
In accordance with Kidney Disease Improving Global Outcomes (KDIGO) guidelines, hourly urine output and relative rise in serum creatinine were used as measures for the presence of AKI in the postoperative period. Urine output and serum creatinine values were recorded for 48 hrs postoperatively. Urine output was recorded from the electronic medical record indexed to body weight, and averaged over 6-hour periods beginning immediately postoperatively. These 6h periods were used to determine KDIGO staging. Baseline (immediate preoperative value) serum creatinine, and the highest recorded value of serum creatinine during the same 6 hour periods was recorded to determine of the change in serum creatinine (ΔsCr.) The primary outcome was defined as KDIGO Stage 1 AKI or greater, specifically, ΔsCr at least 1.5 times baseline, or ≥ 0.3 mg/dL, or urine output of less than 0.5ml/kg/hr for a 6-hour period. Staging criteria for KDIGO AKI can be found in the KDIGO Clinical Practice Guideline for Acute Kidney Injury.16
Statistical Analysis
The main predictor of interest was the RV-PLSS. In bivariate analysis, we compared baseline characteristics between subjects with and without AKI using the two-sided t-test with assumption of unequal variance for continuous variables and chi-square statistics for categorical variables Because some patients underwent TTE after they developed AKI, to limit potential confounding which could result from TTE results obtained during the resolution of the AKI outcome, we performed bivariate analysis to test the relationship of creatinine, urine output (using Student’s t-test) and KDIGO stage (using McNemar’s test) before and after the TTE. We then compared the association between RV-PLSS (the explanatory variable) and the change in creatinine (the response variable) using simple linear regression. In secondary analyses, we performed linear regression with TAPSE, S’, FAC, postoperative LVEF, and RVSP as explanatory variables. Following this we performed logistic regression, with development of any-stage KDIGO AKI as the dependent variable, and RV-PLSS, TAPSE, S’, FAC, postoperative LVEF, and RVSP as independent variables Statistical analysis was performed using STATA Version 14 [StataCorp. 2015. College Station, TX.]
Results
Study population
Electronic medical record review using Current Procedural Terminology codes for included cardiac surgery procedures during the time period of 2008–2015 yielded a list of 279 subjects with a postoperative TTE performed within 48 hours following chest closure. Those records underwent chart review for collection of baseline demographics and review of echocardiography findings. Inadequate image quality precluding strain analysis excluded 80 subjects, yielding the final study population of 199 subjects.
Demographics
Baseline demographics and procedural data for our study population are presented in Table 1. Subjects were 66.0±12.7 years old, mostly male, and mostly Caucasian (63.3% and 89.9%, respectively.) The mean preoperative BMI was 30.0±6.20 kg/m2. Comorbidities of PAD, COPD, and DM were consistent with previously reported prevalence in the CTS population. The majority of subjects had normal left ventricular function at the time of surgery with only 7 subjects (3.5%) presenting preoperatively with an LVEF < 35%. Preoperative right ventricular function parameters were not consistently reported. Mean preoperative eGFR for all subjects was 73.9±21.4 mL/min and mean serum creatinine was 1.03±0.40 mg/dL. There were 97 CABG procedures, 58 valve procedures, and 44 combined procedures. Mean duration of CPB was 130±45.4 min, with mean aortic cross clamp duration 96.1±35.4 min.
Echocardiography
Grading of postoperative TTE demonstrated 78 ‘good’ quality clips, 98 ‘fair’ quality clips, and 23 ‘poor’ quality clips. The mean RV-PLSS, for all subjects was −17.2±4.26%, with frequency distribution demonstrated in Figure 1. The mean value for PLSS when considering only ‘good’ quality clips was not significantly different from the group as a whole (−17.9±3.72%.) Speckle-tracking derived TAPSE, S’, and FAC were 1.02±0.35cm, 6.82±2.45cm/s, and 34.4±7.58%, respectively. RVSP was reported in 171/199 postoperative TTEs, with a mean value of 38.2±9.72 mmHg, and 11.1% (19/171) of this cohort demonstrated a postoperative LVEF < 35%. Twenty-eight subjects did not have a postoperative RVSP or LVEF reported.
Figure 1.
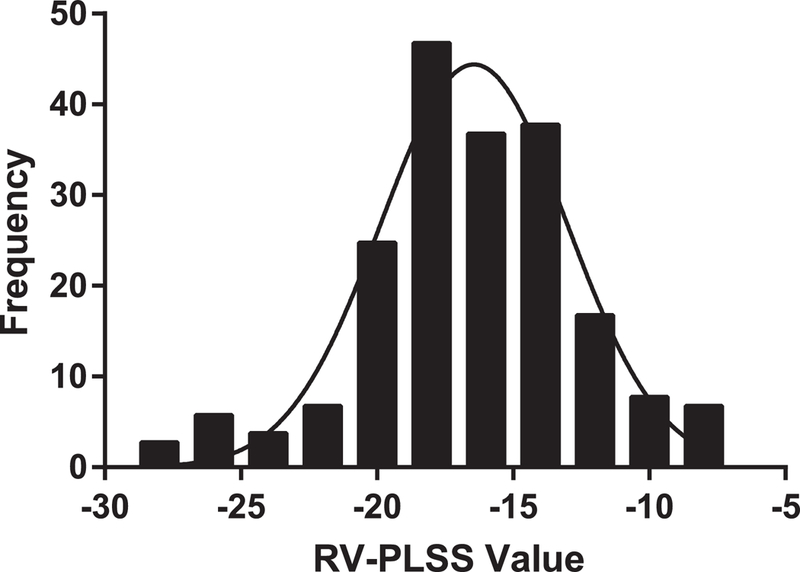
Frequency distribution of right ventricular peak longitudinal systolic strain (RV-PLSS) values in 199 subjects who were postoperative from cardiothoracic surgery. A normal distribution fit to the data with r2 =0.922 is superimposed. The mean RV-PLSS in our subjects with postoperative transthoracic echocardiographic imaging after cardiothoracic surgery is −17.2±4.26%, indicating RV systolic dysfunction is common in this population.
AKI Incidence
Mean serum creatinine was 1.10±0.33 mg/dL on the morning of POD #1, and 1.40±0.57 mg/dL on the morning of POD #2. Hourly urine output over averaged 6-hour periods for the 48 hours postoperatively demonstrated a nadir the afternoon of POD #1 (~24 hrs postoperatively) with mean value of 0.69±0.53 ml/kg/hr.
Incidence of any-stage KDIGO AKI, the primary outcome, was 87% (173/199). The incidence of specific KDIGO AKI Stages were: Stage I 37% (73/199), Stage II 45% (89/199), and Stage III 6% (11/199.)
Differences between AKI and non -AKI subjects
Because the incidence of any-stage AKI was 87% of the study population, baseline demographics were compared between those subjects with KDIGO AKI Stage ≥ 1 and those without, with results reported in Table 3. Amongst the cohort with KDIGO AKI Stage ≥ 1, age (68.9 vs 60.1 years; p<0.01,) preoperative BMI (30.8 vs 28.6 kg/m2; p=0.02,) and preoperative serum creatinine (1.07 vs 0.97 mg/dL; p=0.04) were higher in the AKI group, although preoperative eGFR was not different between AKI and non-AKI patients (72.9±20.8 mL/min vs. 80.8±24.4 mL/min, p=0.09). There were no significant differences in any procedure-specific variables including type of procedure, duration on aortic cross-clamp and CPB, or cardioplegia route and volume.
Table 3.
Comparative demographics and baseline recordings between subjects developing AKI in the postoperative period versus those without
| With AKI | Without AKI | p-value | ||
|---|---|---|---|---|
| Study Subjects, n (%) | 173 (86.9) | 26 (13.1) | -- | |
| Age, yrs, mean (S.D.) | 66.9 (12) | 59.8 (16) | 0.04 | |
| Sex, male, n (%) | 113 (65.2) | 14 (53.8) | 0.26 | |
| BMI, mean (S.D.) | 30.4 (6.1) | 27.8 (6.4) | 0.04 | |
| Race, white, n (%) | 159 (91.9) | 22 (85.6) | 0.26 | |
| Preoperative serum creatinine, mg/dL, mean (S.D.) |
1.04 (0.42) | 0.96 (0.26) | 0.14 | |
| Preoperative eGFR, ml/min, mean (S.D.) |
72.9 (20.8) | 80.8 (24.4) | 0.09 | |
| Presence of PAD, n (%) | 2 (1.1) | 2 (7.6) | 0.84 | |
| Presence of DM, n (%) | 63 (36.4) | 4 (15.3) | 0.06 | |
| Presence of COPD, n (%) | 10 (5.7) | 2 (7.6) | 0.70 | |
| Preoperative LVEF <35%, n (%) | 6 (3.5) | 1 (3.9) | 0.94 | |
| Surgery Type, n (%) | ||||
| CABG | 85 (49.1) | 12 (46.2) | 0.79 | |
| Valve | 49 (28.4) | 9 (34.6) | ||
| Combined | 39 (22.5) | 5 (19.2) | ||
| Aortic cross-clamp duration, min, mean (S.D) |
95.8 (35.9) | 87.5 (43.7) | 0.36 | |
| CPB Duration, min, mean (S.D.) | 130.6 (45.5) | 125.3 (44.9) | 0.58 | |
| Cardioplegia Route, n (%) | ||||
| Antegrade | 41 (23.7) | 5 (19.2) | 0.50 | |
| Retrograde | 2 (1.1) | 1 (3.8) | ||
| Combined | 129 (74.6) | 17 (65.4) | ||
| Cardioplegia Volume, ml (S.D.) | 3164 (1315) | 2624 (1646) | 0.12 | |
eGFR = estimated glomerular filtration rate; PAD = peripheral arterial disease; DM = diabetes mellitus; COPD = chronic obstructive pulmonary disease; LVEF = left ventricular ejection fraction; CPB = cardiopulmonary bypass; CABG = coronary artery bypass graft
Speckle-tracking strain derived RV function values were compared between AKI and non-AKI subjects and reported in Table 4. There were no differences in RV-PLSS, TAPSE, S’, or FAC between the AKI and non-AKI subjects. The proportion of subjects with postoperative LVEF<35% was not different in AKI and non-AKI subjects. However, RVSP was elevated in the AKI group (40.1±10.3 vs 34.8±7.5 mmHg; p<0.01). Linear regression of RVSP to the maximal change in serum creatinine postoperatively, demonstrated significant correlation but poor fit (p=0.035; R2=0.030.) Because the linear relationship was subtle, logistic regression of RVSP to the categorical outcome of AKI versus no AKI was performed. When adjusted for confounding by age and BMI this revealed a significant finding but similarly poorly explanatory model (OR 1.03, p=0.001; psuedoR2=0.137) To limit the possibility of bias due to TTE measurements being performed during AKI recovery, we analyzed the timing of AKI findings relative to the timing of TTE. The urine output was not different before and after TTE (0.93±0.04 mL/kg/hr before, 0.95±0.04 mL/kg/hr after TTE, p=0.83). Creatinine was higher after TTE than before (1.2 ± 0.3 mg/dL after, and 1.1±0.2 mg/dL before, p<0.0001). The mean KDIGO stage for was 0.6±0.8 before TTE, and 1.8±1.0 after (p<0.0001). These data strongly suggest that few patients experienced improvement in AKI or renal function during or prior to their TTE.
Table 4.
Comparison of postoperative transthoracic echocardiographic values between subjects developing AKI in the postoperative period versus those without
| With AKI | Without AKI | p-value | |
|---|---|---|---|
| RV-PLSS, %, mean (S.D.) | −17.1 (4.2) | −16.9 (3.6) | 0.75 |
| TAPSE, cm, mean (S.D.) | 1.01 (0.35) | 0.99 (0.32) | 0.72 |
| S’, cm/s, mean (S.D) | 6.8 (2.4) | 6.8 (2.2) | 0.75 |
| RV FAC, %, mean (S.D.) | 34.5 (7.7) | 33.6 (6.6) | 0.52 |
| Postop LVEF <35%, n (%) | 14 (8.1) | 5 (19.2) | 0.11 |
| RVSP, mmHg, mean (S.D.) | 38.9 (9.9) | 34.6 (7.9) | 0.02 |
RV-PLSS = Right ventricular peak longitudinal systolic strain; TAPSE = tricuspid annular plane systolic excursion; S’ = Tricuspid systolic velocity; RV FAC = right ventricle fractional area change; LVEF = left ventricle ejection fraction; RVSP = right ventricle systolic pressure
Discussion
The principal finding of this study is that postoperative RV-PLSS does not associatewith the development of AKI in the immediate postoperative period. A strength of this study is the use of novel means to measure RV myocardial systolic performance, which has previously been difficult to measure, as opposed to RV distention or volume overload.17–19 Use of speckle-tracking measures was highly successful in this challenging population, with 71% (199/279) feasibility. To our knowledge, this is the first reported evaluation of RV-PLSS following CTS, and our mean value of −17% provides a starting point for understanding the expected mean RV-PLSS in elective CTS patients who receive a postoperative TTE. This mean value for RV-PLSS is less negative (i.e. lower longitudinal deformation present) than previous reports on RV-PLSS for both normal and dysfunctional right ventricles in nonsurgical patients, and suggests altered RV mechanics following CTS and cardiotomy in particular, as consistent with the observations of other investigators.11, 12, 20 The surprising lack of association between RV myocardial systolic performance (as measured by RV-PLSS, S’, TAPSE, and FAC in our study) and development of AKI suggests that other factors may be determinative of AKI risk. However, RV myocardial systolic performance is predictive of other postoperative complications, including mortality, and may be helpful for this reason nonetheless 13.
Among TTE-derived measures, elevated RVSP was the only association with AKI discovered in our investigation. Prior studies have demonstrated that elevated CVP is a risk factor for AKI after CTS, presumably through a mechanism of reduced renal perfusion pressure by means of passive congestion.8, 9 Whether elevated CVP in these investigations was a result of RV systolic dysfunction or in spite of preserved function is difficult to ascertain, though the absence of association with RV myocardial systolic performance in our study supports the latter hypothesis. Elevated RVSP in the absence of RV systolic failure implicates a potential role for hypervolemic congestion, increased pulmonary vascular resistance, and/or decreased RV compliance (i.e. RV diastolic dysfunction,) and future studies may be directed at determining whether pulmonary resistance or RV diastology is the larger determinant of reduced renal perfusion. Aside from perfusion mechanics, another possible mechanism for an association between elevated RVSP and AKI could be an as yet undiscovered signaling mechanism between the pulmonary vasculature/endothelium and that of the kidney through neural, hormonal, or chemical communication.
Limitations
Our investigation has several limitations. This retrospective study may be confounded, and cannot be used to infer causation. The incidence of AKI in our postoperative subjects is greater than that commonly reported in the same population.21 It is possible that local practice or documentation variation could explain this difference.
A second limitation is that TTEs are not routinely performed on postoperative CTS patients at our institution without an indication and order from either the attending intensivist or surgeon. Therefore, our study population may therefore be biased toward those failing to progress as expected through the postoperative period. Our TTE’s were all performed postoperatively, and therefore our conclusions are limited to the relationship between postoperative TTE data and postoperative AKI. It should be noted that RVSP in this study is estimated from TTE measurements, and not directly measured by pressure transducer.
The permissive timing of postoperative TTEs is another limitation in our study. In a study designed to evaluate causation TTEs would be performed immediately upon arrival to the ICU, with the identification of AKI beginning subsequent to performance of the TTE. While this was not the case in our study, creatinine and KDIGO stage both increased in aggregate after TTE, suggesting that for most patients, AKI worsened, rather than improved after TTE. The timing of postoperative TTEs could have influenced the results of both speckle-tracking strain results and the finding of an association with elevated RVSP, yet the increased administration of cardioplegia solution would have preceded the development in AKI postoperatively in all cases.
Conclusion
This investigation does not support the hypothesis that postoperative diminished right ventricular myocardial systolic performance associates with postoperative AKI in CTS patients. This suggests that further investigation of cardiorenal connections in this arena may best be focused on hydrostatic pressure, pulmonary vascular resistance, or perhaps RV diastolic function. This is supported by the finding that elevated postoperative right ventricular systolic pressure associates with postoperative AKI and the novel discovery that the volume of cardioplegia administered intraoperatively associates with subsequent development of AKI.
Table 2.
Demographics and baseline recordings for all study subjects
| Study Subjects, n | 199 |
| Age, yrs, mean (S.D.) | 60.0 (12.7) |
| Sex, male, n (%) | 126 (63.3) |
| BMI, mean (S.D.) | 30.0 (6.20) |
| Race, white, n (%) | 181 (91.0) |
| Preoperative serum creatinine, mg/dL, mean (S.D.) | 1.03 (0.40) |
| Preoperative eGFR, ml/min, mean (S.D.) | 73.9 (21.4) |
| Presence of PAD, n (%) | 4 (2.0) |
| Presence of DM, n (%) | 67 (33.7) |
| Presence of COPD, n (%) | 12 (6.0) |
| Preoperative LVEF <35%, n (%) | 7 (3.5) |
| Aortic cross-clamp duration, min, mean (S.D) | 96.1 (35.4) |
| CPB Duration, min, mean (S.D.) | 130 (45.4) |
| Cardioplegia Route, n (%) | |
| Antegrade | 46 (23.5) |
| Retrograde | 3 (1.5) |
| Combined | 146 (75) |
| Cardioplegia Volume, ml (S.D.) | 3075 (1354) |
| Surgery Type, n (%) | |
| CABG | 97 (48.7) |
| Valve | 58 (29.1) |
| Combined | 44 (22.1) |
eGFR = estimated glomerular filtration rate; PAD = peripheral arterial disease; DM = diabetes mellitus; COPD = chronic obstructive pulmonary disease; LVEF = left ventricular ejection fraction; CPB = cardiopulmonary bypass; CABG = coronary artery bypass graft
Acknowledgments
Funding:
Funding for this study provided by NIH grant DK090754 awarded to Michael P. Hutchens. This material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
List of Abbreviations
- AKI
Acute Kidney Injury
- BMI
Body Mass Index
- CABG
Coronary Artery Bypass Graft
- COPD
Chronic Obstructive Pulmonary Disease
- CPB
Cardiopulmonary Bypass
- CTS
Cardiothoracic Surgery
- CVP
Central Venous Pressure
- DM
Diabetes Mellitus
- FAC
Fractional Area Change
- KDIGO
Kidney Disease Improving Global Outcomes
- LVEF
Left Ventricular Ejection Fraction
- PAD
Peripheral Arterial Disease
- RV-PLSS
Right Ventricular Peak Longitudinal Systolic Strain
- RVSP
Right Ventricular Systolic Pressure
- S’
Tricuspid Systolic Velocity
- TAPSE
Tricupsid Annular Plane Systolic Excursion
- TTE
Transthoracic Echocardiogram
- ΔsCr
Change in Serum Creatinine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations:
Declarations of interest: none.
Research Ethics:
Ethics review and approval for this study provided by the Oregon Health & Science University Institutional Review Board.
Contributor Information
Shaun Yockelson, Department of Anesthesiology and Perioperative Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd Mail Code UH-2, Portland, OR, 97239 , 541-954-7507..
Stephen B. Heitner, OHSU Knight Cardiovascular Institute, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd Mail Code UHN-62, Portland, OR, 97239..
Sarah Click, School of Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd Mail Code L-349, Portland, OR, 97239..
Gemechu Geleto, School of Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd Mail Code L-349, Portland, OR, 97239..
Miriam M. Treggiari, Department of Anesthesiology and Perioperative Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd Mail Code UH-2, Portland, OR, 97239..
Michael P. Hutchens, Department of Anesthesiology and Perioperative Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd Mail Code UH-2, Portland, OR, 97239. Staff Physician, Portland VA Medical Center, Portland, OR..
References
- 1.Lassnigg A: Minimal Changes of Serum Creatinine Predict Prognosis in Patients after Cardiothoracic Surgery: A Prospective Cohort Study. Journal of the American Society of Nephrology 15:1597–1605, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Machado MN, Nakazone MA, Maia LN: Prognostic value of acute kidney injury after cardiac surgery according to kidney disease: improving global outcomes definition and staging (KDIGO) criteria. PLoS One 9:e98028, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legouis D, Galichon P, Bataille A, et al. : Rapid Occurrence of Chronic Kidney Disease in Patients Experiencing Reversible Acute Kidney Injury after Cardiac Surgery. Anesthesiology 126:39–46, 2017. [DOI] [PubMed] [Google Scholar]
- 4.O’Neal JB, Shaw AD, Billings FTt: Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 20:187, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco C, Haapio M, House AA, et al. : Cardiorenal syndrome. J Am Coll Cardiol 52:1527–1539, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Huen SC, Parikh CR: Predicting acute kidney injury after cardiac surgery: a systematic review. Ann Thorac Surg 93:337–347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seabra VF, Alobaidi S, Balk EM, et al. : Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 5:1734–1744, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gambardella I, Gaudino M, Ronco C, et al. : Congestive kidney failure in cardiac surgery: the relationship between central venous pressure and acute kidney injury. Interact Cardiovasc Thorac Surg 23:800–805, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Guinot PG, Abou-Arab O, Longrois D, et al. : Right ventricular systolic dysfunction and vena cava dilatation precede alteration of renal function in adult patients undergoing cardiac surgery: An observational study. Eur J Anaesthesiol 32:535–542, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Grant AD, Smedira NG, Starling RC, et al. : Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 60:521–528, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Meris A, Faletra F, Conca C, et al. : Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr 23:823–831, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Teske AJ, De Boeck BW, Olimulder M, et al. : Echocardiographic assessment of regional right ventricular function: a head-to-head comparison between 2-dimensional and tissue Doppler-derived strain analysis. J Am Soc Echocardiogr 21:275–283, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Ternacle J, Berry M, Cognet T, et al. : Prognostic value of right ventricular two-dimensional global strain in patients referred for cardiac surgery. J Am Soc Echocardiogr 26:721–726, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Stevens PE: Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85:49–61, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, et al. : A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med 150:604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellum JA, Lameire N: Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical Care 17:204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad F, Couture P, Tousignant C, et al. : The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg 108:407–421, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Haddad F, Couture P, Tousignant C, et al. : The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg 108:422–433, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist P, Calcutteea A, Henein M: Echocardiography in the assessment of right heart function. Eur J Echocardiogr 9:225–234, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Raina A, Vaidya A, Gertz ZM, et al. : Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant 32:777–783, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1:19–32, 2006. [DOI] [PubMed] [Google Scholar]


