Abstract
INTRODUCTION:
RNS60 is a novel immune-modulatory agent that has shown neuroprotective effects in ALS pre-clinical models. RNS60 is administered by weekly intravenous infusion and daily nebulization. The objective of this pilot open-label trial was to test the feasibility, safety, and tolerability of long-term RNS60 administration in people with ALS.
METHODS:
The planned treatment duration was 23 weeks and the primary outcomes were safety and tolerability. Secondary outcomes included PBR28 PET imaging and plasma biomarkers of inflammation.
RESULTS:
Sixteen participants with ALS received RNS60 and 13 (81%) completed 23 weeks of RNS60 treatment. There were no serious adverse events and no participants withdrew from the trial due to drug-related adverse events. There were no significant changes in the biomarkers.
DISCUSSION:
Long-term RNS60 administration was safe and well-tolerated. A large, multi-center, phase II trial of RNS60 is currently enrolling participants to test the effects of RNS60 on ALS biomarkers and disease progression.
Keywords: ALS, motor neuron disease (MND), clinical trial, neuroinflammation, PBR28
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease. Loss of motor neurons leads to progressive paralysis of voluntary muscles and death, usually within three to five years after symptom onset.1 Although there are two drugs approved by the US Food and Drug Administration (FDA) to treat ALS, riluzole and edaravone, the availability of new treatments for ALS continues to be an unmet medical need.2,3
Neuroinflammation is increasingly implicated in ALS pathogenesis4–9 including activation of circulating monocytes9–13, lymphocytic infiltration14 and microglial activation in the central nervous system (CNS).7,8 Mouse models of ALS show that activation of microglia and influx of T-lymphocytes into the CNS occur prior to symptom onset4,9,15 and brain imaging in ALS patients reveals that neuro-inflammation localizes to the motor regions.7,8,16 Emerging evidence points to T-lymphocytes as key players in ALS progression. Among different lymphocytic sub-populations, regulatory T-lymphocytes (Tregs) are CD4+CD25highFOXP3+ cells that normally suppress proinflammatory responses and hold neuroinflammation in check.5 In ALS patients, Treg suppressive function is impaired and the Treg transcription factor FOXP3 is reduced. Reduction in Treg function and FOXP3 expression correlates with faster ALS progression.17–19
RNS60 is a novel immune-modulatory agent with neuroprotective properties in several in vitro and in vivo models of neurodegeneration20–26, including pre-clinical models of ALS.27 In SOD1G93A transgenic mice, RNS60 treatment resulted in up-regulation of FOXP3-expressing Tregs and activation of protective astrocytes and microglia which rescued the motor neurons and ameliorated disease progression.27 These findings prompted translational efforts to human disease.
The preliminary safety of RNS60 was previously established in a series of phase I trials of healthy volunteers and people with asthma where it exhibited a robust safety profile (studies were conducted by Revalesio, Corp. between 2010 and 2015; unpublished data; see NCT01511302; NCT01057498; NCT01264783; NCT02490865). The administration regimen of RNS60 is unique as it is administered by weekly intravenous (I.V.) infusion and daily nebulization. This raised the question of whether this dosing regimen would be feasible and safe in ALS patients who commonly experience progressive loss of mobility, respiratory compromise, and difficulty traveling. As an important first step towards developing RNS60 as a potential therapeutic agent for ALS, we conducted a pilot clinical trial to test the long-term feasibility, safety, and tolerability of RNS60 treatment in people with ALS. Secondary outcomes included bio-fluid and neuro-imaging biomarkers to test the impact of the drug on inflammatory pathways.
Methods
This study was an investigator-initiated, open-label, pilot clinical trial that enrolled participants at Massachusetts General Hospital (MGH) between October 2015 and February 2017. The Last Patient Last Visit (LPLV) occurred on 10/25/2017. Partners Human Research Committee approved this study. The trial was registered on clinicaltrials.gov (NCT02525471).
Participant Selection Criteria
Eligible participants had a diagnosis of possible, probable laboratory-supported, probable, or definite ALS by revised El Escorial Criteria.28 As this was a pilot study, inclusion criteria were broad by design. There were no restrictions in disease duration, vital capacity (VC), use of assisted ventilation or presence of a feeding tube. Exclusion criteria included active infection, abnormal liver or kidney function, clinically significant unstable medical condition (other than ALS), and pregnancy. People with unstable psychiatric disease orcognitive impairment were excluded per Principal Investigator judgement.
Two brain imaging studies were planned for each participant: pre-treatment (between Screening and Baseline) and post-treatment (between the Week 18 and Week 23 visits). Brain imaging consisted of positron emission tomography (PET) using the radioligand [11C]-PBR28 that binds to the translocator protein TSPO. Blood samples were collected at screening for genotyping for the Ala147Thr polymorphism in the TSPO gene, which imparts a tri-modal pattern of binding affinity to the radioligand.29 Participants with low-affinity binding (Thr/Thr) were excluded as they tend to have very little detectable [11C]-PBR28 signal. Participants with high- (Ala/Ala) and mixed- (Ala/Thr) affinity binding, who demonstrate high and intermediate signal levels respectively, were included, and their binding affinity status was modeled as a covariate in the analyses. In order to be maximally sensitive to potential treatment-related changes in [11C]-PBR28 signal and to avoid floor effects, participants with an Upper Motor Neuron Burden (UMN-B) score of less than 25 were also excluded.7 Additional exclusion criteria for the brain imaging studies were exposure to immunomodulatory medications within 30 days of the Screening Visit, current use of tobacco or tobacco products, any contraindication to undergo neuroimaging studies such as presence of cardiac pacemaker, metallic clips or metallic particles in the body, or claustrophobia.
Intervention
Eligible participants received RNS60 through two routes: by intravenous infusion at MGH once a week (infusion dose: 375 mL, infused over approximately 40 minutes) and by nebulization at home the remaining 6 days a week (4 mL/day). Participants were provided with a nebulizer for home use at the beginning of the study. RNS60 for home use was dispensed every 7–8 weeks and participants were asked to return empty study drug containers weekly to determine compliance.
Study Overview
Participants signed the approved informed consent form at screening prior to any research related activities. Medical history, detailed ALS history, physical and neurological examinations, medication review, vital signs, and laboratory tests were performed to determine eligibility. All eligible participants received active drug starting with the first infusion at the baseline visit (Day 0), which occurred within 42 days of screening (Supplementary Figure 1) and at-home nebulization the following day. Follow-up visits included weekly in-person visits at the infusion center up to Week 23 for a total of 24 infusions. A final phone call occurred four weeks after receiving the last dose of study drug. Participants who completed the trial (23 weeks of treatment) were offered the option of remaining on RNS60 for a total of up to 47 weeks of treatment (optional open label extension).
Clinical Measurements
Clinical measurements included safety labs, slow vital capacity (SVC), the ALS Functional Rating Scale- Revised (ALSFRS-R)30 questionnaire, collection of Adverse Events (AE) and concomitant medication use, and measurements of muscle strength. Clinical measurements were done at Baseline, Week 11, and Week 23, and were repeated at Week 35 and Week 47 for participants who opted for extended treatment. AEs, concomitant medication changes, vital signs including weight and drug accountability were measured weekly. Isometric strength was tested using Accurate Test of Limb Isometric Strength (ATLIS) designed to precisely measure strength of 12 muscle groups in the arms and legs using an adjustable chair and frame with a fixed wireless load cell.31 The average arm and leg scores are presented by converting raw force values to percent of predicted values using similar methods used to calculate SVC.32
Biofluid biomarker assays
Blood draws for biomarker assays were performed at Baseline, Week 11, and Week 23 in the subset of 11 participants who also underwent brain imaging studies. Samples were processed on site, aliquoted, cryopreserved at −80°C, and shipped to the IRCCS Mario Negri Institute for Pharmacological Research for analysis. The following markers were measured: IL-17 in plasma and intracellular FOXP3 mRNA expression in whole blood. IL-17 plasma concentration was measured by Alpha technology using an AlphaLISA kit (#AL219C) from Perkin Elmer following the manufacturer’s procedure. The AlphaLISA signals were detected using an Ensight Multimode Plate Reader (PerkinElmer). FOXP3 mRNA levels were measured by RT-PCR.
Real Time PCR
Total RNA from blood was extracted and purified with PAXgene Blood RNA Kit (Qiagen) following manufacturer’s instruction. RNA samples were treated with DNase I and reverse transcription was performed with a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Real-time PCR was performed using the Taq Man Gene expression assay (Applied Biosystems), following the manufacturer’s instructions, on cDNA specimens using 1X Universal PCR master mix (Thermo Fisher Scientific) and 1X mix containing Forkhead box P3 (FOXP3; Hs01085834_m1; Thermo Fisher Scientific) probe. Relative quantification was calculated from the ratio between the cycle number (Ct) at which the signal crossed a threshold set within the logarithmic phase of FOXP3 gene and that of the reference β-actin gene (Hs01060665_g1; Thermo Fisher Scientific). Each sample was run in triplicate and the mean value was used as individual data for 2-ΔCt analysis.
Brain imaging
Brain imaging studies included simultaneous magnetic resonance- positron emission tomography (MR-PET) imaging using the radiotracer [11C]-PBR28. [11C]-PBR28 was produced in-house as previously described.33 The radiotracer was administered as an intravenous bolus (mean administered dose was 473.6 MBq at Baseline and 468.4 MBq at follow-up). Standardized uptake value (SUV) computed from data collected 60 to 90 min post time of injection were normalized to whole brain mean to create an SUV ratio (SUVR60–90min).7,34 This whole-brain normalization approach accounts for global difference in PET signal across participants, thereby allowing increased sensitivity to detect regional effects.7,34
Statistical Analysis
Primary outcomes were safety, as measured by the number of and severity of all adverse events, and tolerability, defined as the ability to complete the 23-week treatment period on study drug. Adverse events (AEs) were coded to preferred terms from the MedDRA library (version 16.1). As an additional safety measure, disease progression measured by ALSFRS-R was evaluated with a Wilcoxon signed-rank test. All analyses were carried out using R (version 3.4.3).
Secondary outcomes included levels of IL-17 in plasma and FOXP3 mRNA expression in whole blood. Linear mixed models were used for the plasma and whole blood measures as the outcome. Fixed effect for time (baseline or Week 23), random slope and intercept for each participant were used to estimate a 23-week change.
Neuroimaging analyses were similar to those previously reported in Loggia et al.34 Briefly, SUVR60–90min images were fed into a whole brain voxel-wise between-groups analysis (post-treatment vs. pre-treatment). Non-parametric permutation inference35 using a paired design was performed using 5,000 permutations and threshold-free cluster enhancement.36 The resultant statistical maps were family-wise error (FWE) adjusted (pFWE < 0.05) to correct for multiple comparisons. In addition to this voxel-wise analysis, we performed a Region-of-Interest (ROI) analysis. The ROI was defined based on FreeSurfer cortical reconstruction of the standard template MNI152. The ROI encompasses the precentral and paracentral cortex and underlying sub-cortical white matter (motor region) bilaterally.37 [11C]-PBR28 SUVR60–90min was then computed within this ROI. Pre- to post- change scores for [11C]-PBR28 SUVR60–90min extracted from this ROI was compared using a Wilcoxon signed-rank test.
Results
Study Population
Twenty-four volunteers were assessed for eligibility; 18 eligible individuals were enrolled due to 6 screen failures (Figure 1). Of the 18 eligible individuals, 2 were never dosed with RNS60 because they developed unrelated medical problems and withdrew consent prior to baseline. Their data from screening was excluded from analysis, leaving a dataset of 16 study participants (Figure 1). Demographic and clinical characteristics of the study participants are listed in Table 1.
Figure 1. CONSORT diagram: participant enrollment and follow-up for the trial.
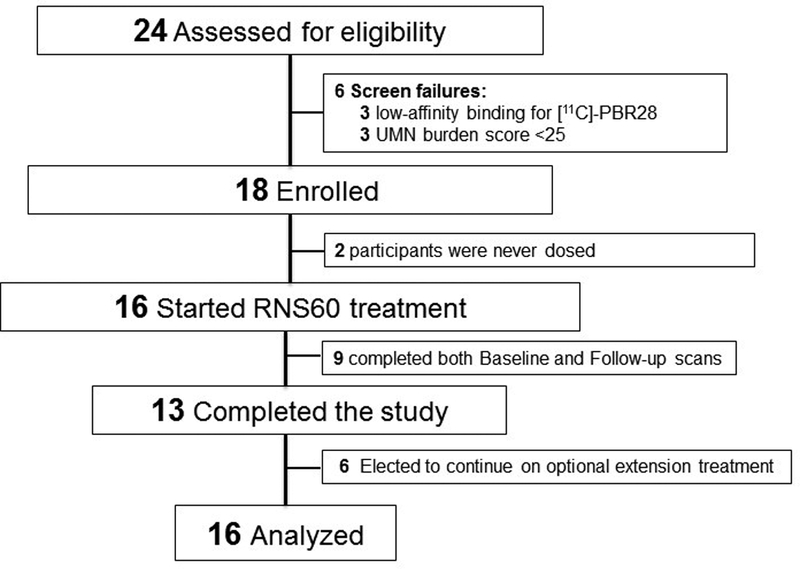
Twenty-four volunteers with ALS were assessed for eligibility. Eighteen enrolled in the trial but two were never dosed as they withdrew consent between Screening and Baseline due to unrelated medical problems. The remaining 16 participants received RNS60 and contributed data.
Table 1.
Baseline demographics and clinical characteristics of study participants
| Demographics and clinical characteristics | All Participants(N=16) |
|---|---|
| Male gender (N, %) | 8, 50% |
| White race (N, %) | 14, 88% |
|
Age (years) Mean ± SD (Min-Max) |
52.9 ± 11.3 (24.5 –69.8) |
| Bulbar onset (N, %) | 3, 18.8% |
| Riluzole use (N, %) | 16, 100% |
|
Months since symptom onset
Mean ± SD (Min-Max) |
30.3 ± 17.8 (7.1 – 68.4) |
|
ALSFRS-R Total Score Mean ± SD (Min-Max) |
34.1 ± 6.2 (23 – 44) |
|
SVC (%) Mean ± SD (Min-Max) |
63.8 ± 27.6 (28 – 99) |
|
ATLIS Arm Measures (%) Mean ± SD (Min-Max) |
35.9 ± 21.8 (0 – 64) |
|
ATLIS Leg Measures (%) Mean ± SD (Min-Max) |
53.9 ± 31.0 (4 – 95) |
|
Compliance with infusion visits at the study site (%) Mean ± SD (Min-Max) |
99 ± 1 (96 – 100) |
|
Compliance with home nebulization (%) Mean ± SD (Min-Max) |
96 ± 6 (76 – 100) |
| EEC definite (N, %) | 7, 43.75% |
| EEC probable (N, %) | 3, 18.75% |
| EEC probable lab-supported (N, %) | 4, 25% |
| EEC possible (N, %) | 2, 12.5% |
Data are presented as either percentages or means (±SD). Ranges are indicated in parenthesis as applicable.
Abbreviations: ALSFRS-R= ALS functional rating scale revised. ATLIS: Accurate Test of Limb Isometric Strength (results are presented as percent of predicted values)32. BMI: body mass index (measured in kg/m2). EEC: El Escorial Criteria. N= number of subjects. SD=standard deviation. SVC= slow vital capacity (results are presented as percent of predicted values for age, gender and height).
Safety and Tolerability
The trial met its primary endpoints for safety and tolerability. The most common AEs included falls (75%), headaches (50%), nasopharyngitis (38%) and contusions (31%) (all AEs that occurred in at least 12% of participants (N=2) are summarized in Supplementary Table 1 by MedDRA preferred term). No serious adverse events related to RNS60 occurred and no participant withdrew from the trial due to drug-related adverse events. Eighty-one percent of study participants (N=13) completed 23 weeks of RNS60 treatment (Figure 1). Withdrawals were due to disease progression (N=2) and relocation to a different state (N=1). Compliance with I.V. infusion visits and daily home nebulization were very high (Table 1). There were no clinically significant changes in vital signs or standard laboratory tests. As expected, ALSFRS-R and muscle strength declined over the 23 weeks of observation (Table 2). There were no statistically significant changes in SVC during the study period (Table 2). Individual ALSFRS-R and SVC trajectories are shown in Supplementary figure 2.
Table 2.
Disease progression (expressed as change per month) at Week 23.
| Outcome of disease progression | All Participants (N=16) |
|---|---|
|
ALSFRS-R Total Score
Mean ± SD (Min-Max) |
−1.1 ± 1.72† (−2.3, −0.2) |
|
SVC
Mean ± SD (Min-Max) |
−0.6 ± 4.41 (−4.9, 1.9) |
|
ATLIS Arm Measures (%) Mean ± SD (Min-Max) |
−1.7 ± 3.12† (−4.2, 0) |
|
ATLIS Leg Measures (%) Mean ± SD (Min-Max) |
−1.7 ± 5.61† (−8, 1) |
Data are presented as means (±SD) (expressed as change per month). Minimum and maximum changes are shown in parenthesis.
Abbreviations: ALSFRS-R= ALS functional rating scale revised. ATLIS: Accurate Test of Limb Isometric Strength (results are presented as percent of predicted values)32. N= number of subjects. SD=standard deviation. SVC= slow vital capacity (results are presented as percent of predicted values for age, gender and height).
Denotes statistically significant Wilcoxon for change at Week 23 compared to Baseline (p < 0.05).
Of the 13 participants who completed the trial, 6 elected to remain on study drug (optional extension) and 4 remained on RNS60 for a total of 47 weeks. The two withdrawals from the extension treatment were due to difficulties traveling to the study site (N=1) and scheduling difficulties at the time of initiation of edaravone treatment (N=1).
Brain imaging
Of the 11 eligible participants, 9 completed both scans (2 did not return for the post-treatment scan due to interval development of orthopnea). Of the 9 participants who had both baseline and follow-up scans, five were high- and four were mixed-affinity binders. Whole-brain voxel-wise analysis revealed no significant difference in [11C]-PBR28 SUVR60–90 min between pre-treatment scans and post-treatment scans. ROI analysis did not show statistically significant changes in [11C]-PBR28 uptake when pre-treatment scans were compared to post-treatment results. (Figure 2).
Figure 2. [11C]-PBR28 uptake within the Region of Interest (ROI).
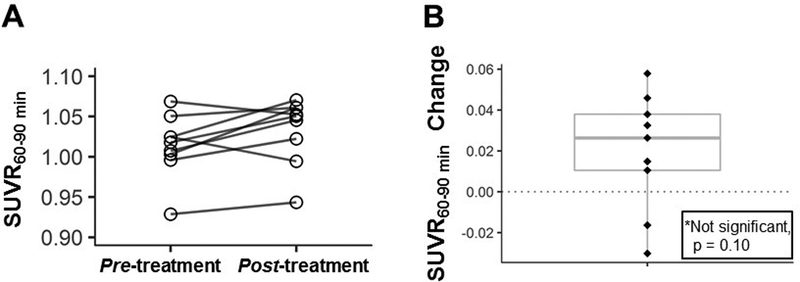
Panel A: [11C]-PBR28 uptake (expressed as SUVR60–90min) for each individual participant.
Panel B: individual changes in [11C]-PBR28 uptake (expressed as SUVR60–90min) from pre-treatment to post-treatment scans. There were no statistically significant changes in [11C]-PBR28 uptake over the course of the Core Study in the 9 participants who had both pre-treatment and post-treatment scans with boxplot showing median and interquartile range (Wilcoxon signed-rank test; mean change in SUVR60–90min was 0.02 [p= 0.10]).
Bio-fluid biomarkers
Biomarkers included IL-17 plasma levels (N=10) and FOXP3 mRNA expression in whole blood, a marker of Treg function (N=8). There were no statistically significant changes in IL-17 levels or FOXP3 expression through the course of the Core Study (estimated mean change in IL-17: −25.4 pg/mL [p= 0.3]; estimated mean change in FOXP3: −0.02 [p= 0.9]) (Figure 3).
Figure 3. Exploratory inflammatory biomarkers.
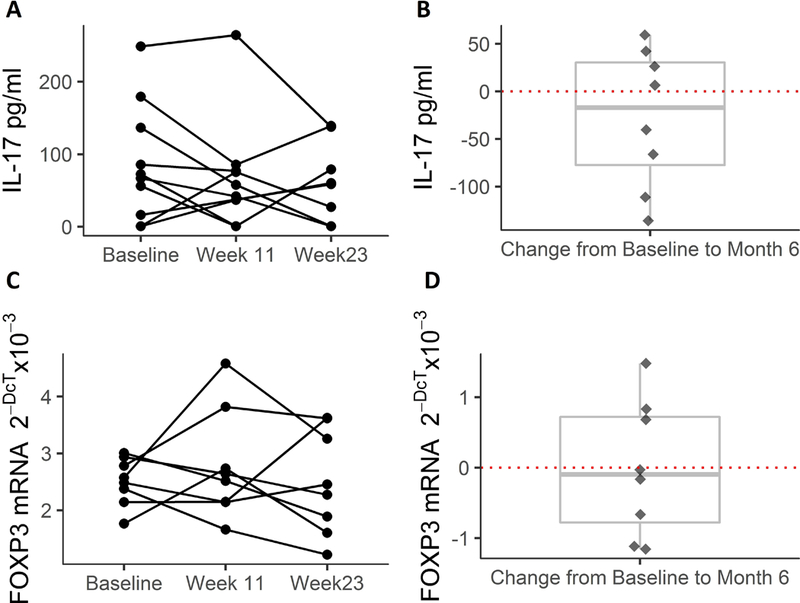
Panel A: Individual levels of IL-17 in plasma (N=10).
Panel B: individual changes in IL-17 from pre-treatment to post-treatment. There were no statistically significant changes over the course of the study with boxplot showing median and interquartile range (Wilcoxon signed-rank test).
Panel C: Individual levels of FOXP3 mRNA expression (a marker of Tregs) in whole blood (N=8).
Panel D: individual changes in FOXP3 mRNA expression from pre-treatment to post-treatment. There were no statistically significant changes over the course of the study with boxplot showing median and interquartile range (Wilcoxon signed-rank test).
Discussion
The trial met its primary endpoints, demonstrating the feasibility, safety, and tolerability of administering RNS60 long-term in people with ALS.
Tolerability and compliance were high, demonstrating the feasibility of this regimen in a broad ALS population. Of note, our trial started before the approval of edaravone in the U.S.2 Since then, the ALS community has developed considerable experience with long-term I.V. drug administration though this route of administration was already used successfully in a previous trial38. Future drug development efforts of RNS60 should include consideration of home infusions and long-term intravenous access as is now done routinely for edaravone therapy in the U.S.
RNS60 treatment was safe and well-tolerated. No serious adverse events related to RNS60 occurred and no participant withdrew from the trial due to drug-related adverse events. While this trial did not include a placebo group, the frequency of AEs recorded over a long-term observation period was consistent with what is expected in a cohort of patients with a progressive neurodegenerative disease.
This study cannot provide any conclusions about the effects of RNS60 on ALS progression since this pilot trial was limited by the small sample size and lack of placebo group. This trial did not have the statistical power to detect changes in disease progression measured by the ALSFRS-R or muscle strength analyses. We used ATLIS to measure muscle strength for the first time in the context of an ALS drug trial. This novel outcome measure may be valuable in future trials The vital capacity was relatively stable over the study period. Whether this finding reflects the therapeutic effect of the drug or is due to chance is unknown. A larger study is needed to evaluate the clinical efficacy of RNS60 for the treatment of people with ALS.
The addition of biomarkers to track inflammation in this pilot trial was uninformative. In mouse models of autoimmune encephalomyelitis, RNS60 upregulated FOXP3 (a marker of Tregs), reduced IL-17 levels, and ameliorated disease phenotype.21,25 Recently, RNS60 treatment was shown to prevent the downregulation of FOXP3 mRNA in SOD1G93A transgenic mice and this was associated with slowing of disease progression.27 The number of Treg cells inversely correlates with the rate of progression in ALS patients. Furthermore, increased levels of IL-17 have been detected in serum and CSF of ALS patients compared to healthy controls.19,39–41 In the present study, there was no change in blood levels of FOXP3 mRNA or IL-17 during the treatment period. The small sample size may have contributed to these non-significant findings and a larger, placebo-controlled trial of RNS60 is underway to test the effects of the drug on these biomarkers (NCT03456882). We used neuroimaging to track neuro-inflammation in the motor regions. Both the ROI and exploratory whole brain approaches did not reveal a significant difference in the pre vs. post treatment timepoints of this small pilot study (N=9). The ROI approach focused on motor areas that were previously shown to exhibit increased [11C]-PBR28 uptake in ALS compared to healthy controls and to correlate with upper motor neuron burden severity.7,16 This study successfully demonstrated that longitudinal PET imaging is feasible in the context of ALS clinical trials, however, this trial was underpowered for the imaging outcome as well. Our longitudinal data suggest that a sample size of 30 ALS subjects has 80% power to detect a 2% reduction in PBR28 PET signal after a successful treatment.42 This imaging technology could be used to increase the efficiency of the drug development by detecting target engagement and guiding dose-selection. It could also be used to select participants who are more likely to respond to RNS60 (i.e., patients with higher neuro-inflammation at baseline). A larger study is needed to investigate whether treatment with RNS60 leads to significant differences in [11C]-PBR28 uptake, reflecting changes in neuro-inflammation.
In conclusion, we demonstrated the feasibility, safety, and tolerability of long-term administration of RNS60 in people with ALS. This study builds on promising data in ALS pre-clinical models27 and represents the first translational step in the development of RNS60 as a potential therapeutic for ALS. A large, multi-center, international, placebo-controlled phase II trial of RNS60 is currently enrolling participants to test the effects of RNS60 on ALS biomarkers and disease progression (NCT03456882).
Supplementary Material
Acknowledgements:
This work was conducted with support from the MGH ALS Therapy Fund. Study drug was donated by Revalesio, Corp. Revalesio, Corp. was not involved in the study design or execution. The authors wish to thank the several individuals who have contributed to this work by providing logistical and technical support. In particular, we would like to thank: all NCRI study coordinators, research nurses and physical therapists for outstanding support of study activities; the NCRI Project Management, Data Management and Systems groups for trial management; Dr. Katharine Nicholson for help with study visits; Dr. Ettore Beghi, Dr. Elisabetta Pupillo, Dr. James Berry, and Dr. Miriam Moscovitch Lopatin for their help in coordinating shipping and analysis of the bio-samples; the Athinoula A. Martinos Center for Biomedical Imaging Radiopharmacy and the NMR technicians for exceptional assistance with neuroimaging. Finally, we would like to thank all study participants and their families and caregivers for their dedication and contribution to ALS research.
David Schoenfeld, PhD and James Chan, MA conducted all statistical analyses (MGH Biostatistics Center). Drs. Paganoni, Atassi, and Schoenfeld had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Abbreviations
- AEs
adverse events
- ALS
amyotrophic lateral sclerosis
- ALS-FRS-R
ALS Functional Rating Scale – Revised
- ATLIS
Accurate Test of Limb Isometric Strength
- CNS
central nervous system
- CSF
cerebrospinal fluid
- (c)DNA
(complementary)Deoxyribonucleic acid
- Ct
cycle number
- FDA
food and drug administration
- FOXP3
forkhead box P3
- FWE
family-wise error
- IL-17
interleukin 17
- IRCCS
Istituto di Ricovero e Cura a Carattere Scientifico
- I.V.
intravenously
- LPLV
Last Patient Last Visit
- MGH
Massachusetts General Hospital
- MND
motor neuron disease
- (MR)-PET
(magnetic resonance) - positron emission tomography
- (m)RNA
(messenger) ribonucleic acid
- ROI
region of interest
- RT-PCR
real time polymerase chain reaction
- SUV(r)
standardized uptake value (ratio)
- T regs
regulatory T cells
- TSPO
Translocator protein
- (S)VC
(slow) vital capacity
- UMN-B
upper motor neuros - burden
Footnotes
Disclosures:
Sabrina Paganoni has received research funding from the Salah Foundation, the ALS Association, ALS Finding a Cure, the American Academy of Neurology, and Amylyx.
Mohamad J. Alshikho reports no disclosures.
Sarah Luppino reports no disclosures.
Lindsay Pothier reports no disclosures.
James Chan reports no disclosures.
David Schoenfeld reports no disclosures.
Patricia L. Andres has provided consulting for Amylyx Pharmaceuticals. Named inventor of ATLIS, patented by Massachusetts General Hospital.
Suma Babu reports no disclosures.
Nicole R. Zürcher reports no disclosures.
Marco L. Loggia reports no disclosures.
Robert L. Barry reports no disclosures.
Silvia Luotti reports no disclosures.
Giovanni Nardo reports no disclosures.
Maria Chiara Trolese reports no disclosures.
Serena Pantalone reports no disclosures.
Caterina Bendotti reports no disclosures.
Valentina Bonetto reports no disclosures.
Fabiola De Marchi reports no disclosures.
Bruce Rosen reports no disclosures.
Jacob Hooker reports no disclosures.
Merit Cudkowicz has provided consulting for Cytokinetics, Astra Zeneca, Lilly, Genentech, Biogen-IDEC, Voyager, and Biohaven.
Nazem Atassi has provided consulting for Biogen and Mitsubishi Tanabe.
References:
- 1.Hardiman O, Al-Chalabi A, Brayne C, Beghi E, van den Berg LH, Chio A, et al. The changing picture of amyotrophic lateral sclerosis: lessons from European registers. Journal of neurology, neurosurgery, and psychiatry. 2017;88(7):557–563. [DOI] [PubMed] [Google Scholar]
- 2.Writing G, Edaravone ALSSG. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–512. [DOI] [PubMed] [Google Scholar]
- 3.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012(3):CD001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57(7):1282–1289. [DOI] [PubMed] [Google Scholar]
- 5.Appel SH, Zhao W, Beers DR, Henkel JS. The microglial-motoneuron dialogue in ALS. Acta Myol. 2011;30(1):4–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Kano O, Beers DR, Henkel JS, Appel SH. Peripheral nerve inflammation in ALS mice: cause or consequence. Neurology. 2012;78(11):833–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zurcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. NeuroImage Clinical. 2015;7:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601–609. [DOI] [PubMed] [Google Scholar]
- 9.Lincecum JM, Vieira FG, Wang MZ, Thompson K, De Zutter GS, Kidd J, et al. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet. 2010;42(5):392–399. [DOI] [PubMed] [Google Scholar]
- 10.Vaknin I, Kunis G, Miller O, Butovsky O, Bukshpan S, Beers DR, et al. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PLoS One. 2011;6(11):e26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122(9):3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu IM, Phatnani H, Kuligowski M, Tapia JC, Carrasco MA, Zhang M, et al. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci U S A. 2009;106(49):20960–20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell reports. 2013;4(2):385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50(1):30–36. [DOI] [PubMed] [Google Scholar]
- 15.Henkel JS, Beers DR, Siklos L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31(3):427–437. [DOI] [PubMed] [Google Scholar]
- 16.Alshikho MJ, Zurcher NR, Loggia ML, Cernasov P, Chonde DB, Izquierdo Garcia D, et al. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology. 2016;87(24):2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beers DR, Henkel JS, Zhao W, Wang J, Huang A, Wen S, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134(Pt 5):1293–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W, Beers DR, Liao B, Henkel JS, Appel SH. Regulatory T lymphocytes from ALS mice suppress microglia and effector T lymphocytes through different cytokine-mediated mechanisms. Neurobiol Dis. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO molecular medicine. 2013;5(1):64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, et al. Suppression of nuclear factor-kappaB activation and inflammation in microglia by physically modified saline. The Journal of biological chemistry. 2012;287(35):29529–29542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondal S, Martinson JA, Ghosh S, Watson R, Pahan K. Protection of Tregs, suppression of Th1 and Th17 cells, and amelioration of experimental allergic encephalomyelitis by a physically-modified saline. PloS one. 2012;7(12):e51869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K. Protection of dopaminergic neurons in a mouse model of Parkinson’s disease by a physically-modified saline containing charge-stabilized nanobubbles. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2014;9(2):218–232. [DOI] [PubMed] [Google Scholar]
- 23.Modi KK, Jana A, Ghosh S, Watson R, Pahan K. A physically-modified saline suppresses neuronal apoptosis, attenuates tau phosphorylation and protects memory in an animal model of Alzheimer’s disease. PloS one. 2014;9(8):e103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao VT, Khan D, Jones RG, Nakamura DS, Kennedy TE, Cui QL, et al. Potential Benefit of the Charge-Stabilized Nanostructure Saline RNS60 for Myelin Maintenance and Repair. Scientific reports. 2016;6:30020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondal S, Rangasamy SB, Ghosh S, Watson RL, Pahan K. Nebulization of RNS60, a Physically-Modified Saline, Attenuates the Adoptive Transfer of Experimental Allergic Encephalomyelitis in Mice: Implications for Multiple Sclerosis Therapy. Neurochemical research. 2017;42(5):1555–1570. [DOI] [PubMed] [Google Scholar]
- 26.Jana M, Ghosh S, Pahan K. Upregulation of Myelin Gene Expression by a Physically-Modified Saline via Phosphatidylinositol 3-Kinase-Mediated Activation of CREB: Implications for Multiple Sclerosis. Neurochemical research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallarola A, Sironi F, Tortarolo M, Gatto N, De Gioia R, Pasetto L, et al. RNS60 exerts therapeutic effects in the SOD1 ALS mouse model through protective glia and peripheral nerve rescue. Journal of neuroinflammation. 2018;15(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. [DOI] [PubMed] [Google Scholar]
- 29.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169(1–2):13–21. [DOI] [PubMed] [Google Scholar]
- 31.Andres PL, Skerry LM, Munsat TL, Thornell BJ, Szymonifka J, Schoenfeld DA, et al. Validation of a new strength measurement device for amyotrophic lateral sclerosis clinical trials. Muscle & nerve. 2012;45(1):81–85. [DOI] [PubMed] [Google Scholar]
- 32.Andres PL, English R, Mendoza M, Florence J, Malkus E, Schierbecker J, et al. Developing normalized strength scores for neuromuscular research. Muscle & nerve. 2013;47(2):177–182. [DOI] [PubMed] [Google Scholar]
- 33.Imaizumi M, Kim HJ, Zoghbi SS, Briard E, Hong J, Musachio JL, et al. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neurosci Lett. 2007;411(3):200–205. [DOI] [PubMed] [Google Scholar]
- 34.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain : a journal of neurology. 2015;138(Pt 3):604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human brain mapping. 2002;15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 37.Paganoni S, Alshikho MJ, Zurcher NR, Cernasov P, Babu S, Loggia ML, et al. Imaging of glia activation in people with primary lateral sclerosis. NeuroImage Clinical. 2018;17:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cudkowicz ME, Titus S, Kearney M, Yu H, Sherman A, Schoenfeld D, et al. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(11):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rentzos M, Rombos A, Nikolaou C, Zoga M, Zouvelou V, Dimitrakopoulos A, et al. Interleukin-17 and interleukin-23 are elevated in serum and cerebrospinal fluid of patients with ALS: a reflection of Th17 cells activation? Acta neurologica Scandinavica. 2010;122(6):425–429. [DOI] [PubMed] [Google Scholar]
- 40.Fiala M, Chattopadhay M, La Cava A, Tse E, Liu G, Lourenco E, et al. IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. Journal of neuroinflammation. 2010;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beers DR, Zhao W, Wang J, Zhang X, Wen S, Neal D, et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI insight. 2017;2(5):e89530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alshikho MJ, Zurcher NR, Loggia ML, Cernasov P, Reynolds B, Pijanowski O, et al. Integrated MRI and [(11) C]-PBR28 PET Imaging in Amyotrophic Lateral sclerosis. Ann Neurol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


