Abstract
Objective:
Cross-sectional data indicate that systemic inflammation is important in oesophageal adenocarcinoma. We conducted a prospective study to assess whether prediagnostic circulating markers of inflammation were associated with oesophageal adenocarcinoma and to what extent they mediated associations of obesity and cigarette smoking with cancer risk.
Design:
This nested case-control study included 296 oesophageal adenocarcinoma cases and 296 incidence-density matched controls from seven prospective cohort studies. We quantitated 69 circulating inflammation markers using Luminex-based multiplex assays. Conditional logistic regression models estimated associations between inflammation markers and oesophageal adenocarcinoma, as well as direct and indirect effects of obesity and smoking on risk of malignancy.
Results:
Soluble tumor necrosis factor receptor 2 (sTNFR2) (odds ratioquartile 4 vs 1=2.67, 95% confidence interval: 1.52–4.68) was significantly associated with oesophageal adenocarcinoma. Additional markers close to the adjusted significance threshold included C-reactive protein, serum amyloid A, lipocalin-2, resistin, interleukin (IL)3, IL17A, soluble IL6 receptor, and soluble vascular endothelial growth factor receptor 3. Adjustment for body mass index, waist circumference, or smoking status slightly attenuated biomarker-cancer associations. Mediation analysis indicated that sTNFR2 may account for 33% (p=0.005) of the effect of waist circumference on oesophageal adenocarcinoma risk. Resistin, plasminogen activator inhibitor 1, C-reactive protein and serum amyloid A were also identified as potential mediators of obesity-esophageal adenocarcinoma associations. For smoking status, only plasminogen activator inhibitor 1 was a nominally statistically significant (P<0.05) mediator of cancer risk.
Conclusion:
This prospective study provides evidence of a link between systemic inflammation and oesophageal adenocarcinoma risk. In addition, this study provides the first evidence that indirect effects of excess adiposity and cigarette smoking, via systemic inflammation, increase the risk of oesophageal adenocarcinoma.
Keywords: Esophageal Neoplasms, Inflammation, Prospective Studies, Adipose Tissue, Serum, Cigarette Smoking
Introduction
Inflammation is a hallmark of cancer [1] and cross-sectional data indicate that it is of key importance in the development of oesophageal adenocarcinoma [2, 3]. With regards to oesophageal adenocarcinoma, inflammatory mechanisms are inferred from the risk factor profile which includes gastroesophageal reflux disease (GERD) [4], obesity [5], and cigarette smoking [6]. While GERD is understood to have direct carcinogenic effects on oesophageal mucosa, it is unknown whether systemic inflammation may partly explain associations of obesity and smoking with oesophageal adenocarcinoma. The direct mechanical effect of central obesity on oesophageal adenocarcinoma risk is widely accepted; central adiposity amplifies intra-gastric pressure and disturbs normal sphincter function, culminating in a higher propensity for GERD and subsequent increased risk of malignant transformation [7, 8]. However, evidence is accumulating for an indirect inflammatory effect of central (android) adiposity in relation to the risk of oesophageal adenocarcinoma [9]. Body mass index (BMI, kg/m2) and waist circumference are strong correlates of visceral adipose tissue [10], a highly metabolic fat type that has the potential to have far-reaching systemic effects [11]. Mechanisms underlying the association between cigarette smoking and oesophageal adenocarcinoma possibly include genotoxic effects [12], promotion of GERD [13], and promotion of systemic inflammation and immune dysfunction [14–17]. Elucidating whether systemic inflammation is a mechanism that underlies these exposures on cancer risk is important so that we can have a clearer picture of pathogenesis providing knowledge for risk reduction strategies and highlighting molecular pathways for therapeutic intervention.
There have been many case-control studies of systemic inflammation markers and oesophageal adenocarcinoma risk [2, 3], but interpreting this literature is difficult due to reverse causation as well as cancer treatment and survivorship factors. One prior prospective study assessed a small number of inflammation-related biomarkers in relation to oesophageal adenocarcinoma within a cohort Barrett’s oesophagus patients[18, 19]. Because oesophageal adenocarcinoma is a rare malignancy, most cohort studies to date have accrued fewer than 100 cases. Therefore, we designed a nested case-control study using data from seven prospective cohorts within the National Cancer Institute (NCI) Cohort Consortium to evaluate whether pre-diagnostic circulating biomarkers of inflammation are associated with risk of oesophageal adenocarcinoma and to what extent they mediate carcinogenic effects of obesity and smoking.
Methods
Study Population
This nested case-control study was designed within seven prospective cohort studies: the Cancer Prevention Study-II Nutrition Cohort (CPS-II) [20], the Carotene and Retinol Efficacy Trial (CARET) [21], the European Prospective Investigation into Cancer and Nutrition (EPIC) [22], the Multiethnic Cohort Study (MEC) [23], the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) [24], the Prostate Cancer Prevention Trial (PCPT) [25], and the Women’s Health Initiative (WHI) [26]. All cohorts, apart from EPIC, were conducted within the US population. We refer the reader to the citations provided for cohort-specific details. Each cohort identified all individuals with a first cancer diagnosis (excluding non-melanoma skin cancer [NMSC]) of oesophageal adenocarcinoma (International Classification of Diseases (ICD)-10: C150–159 with histology codes consistent with adenocarcinoma, ICD for Oncology-3: 8140–8575) identified during follow-up and after serum collection. Each cohort used incidence-density sampling with replacement to match 1 control per case based on sex, race/ethnicity, date of birth (+/− 1 year), date of entry (+/− 1 year), exit date (>=date of diagnosis of index case and defined as diagnosis of cancer [exc. NMSC], death, loss to follow-up, or end of follow-up), and number of freeze-thaw cycles of serum available for analysis. Unthawed serum samples were preferred and all sera were stored at a minimum of −70°C between initial collection and laboratory analysis. Sequential relaxation rules for the matching criteria were: expand date of entry criterion in increments of +/−1 year until a control is matched or until date of entry +/−3 years is reached; expand date of birth criterion in increments of +/−1 year until a control is matched or until date of birth +/−3 years is reached; expand freeze-thaw cycles criterion in increments of +/−1 cycle until a control is matched with no maximal limit. Additional minor study-specific matching criteria were also used pertinent to the design of each study. All studies provided questionnaire data on participant characteristics and exposures including BMI. CPS-II, EPIC, MEC, PCPT, and WHI also provided data for 94% of participants on waist circumference, which was either measured by study staff or self-measured with provision of a tape measure and instructions from the study (CPS-II, MEC).
Laboratory methods
We measured 69 unique biomarkers of inflammation, immunity and metabolism using seven Luminex bead-based multiplex assays (EMD Millipore Corp., MA) and 235 μl of prediagnostic serum per subject (395 ul for quality control subjects analyzed in duplicate). These assays provided a broad scope of markers that have been implicated in metabolic dysfunction or disease and require a minimal amount of serum, hence our previous application of such assays to a range of hypotheses [27–33]. The Luminex assay is comprised of analyte-specific capture antibodies conjugated to beads of defined spectral properties. After binding and washing, analyte-specific, biotinylated detector antibodies were added which were used in combination with a streptavidin-conjugated fluorescent protein and a detection system (Bio-Plex 200 Analyzer, Bio-Rad Laboratories, Hercules, CA / FLEXMAP 3D, Luminex, IL, Chicago) to provide quantitation. Concentrations of biomarkers were estimated using a four- or five-parameter standard curve, depending on the panel, using Bioplex Manager 6.1 software (BioRad, Hercules, CA). Within each cohort, 10% of cases and/or controls were assessed in duplicate for estimating coefficients of variation and intraclass correlation coefficients. Individually matched cases and controls, and duplicate quality control samples, were assayed in the same batch, sequentially.
Statistical analysis
Pearson pairwise correlations were estimated for each biomarker pair. This correlation matrix was used to estimate the effective number of independent variables through matSpDlite [34] and the corresponding adjusted statistical significance threshold for alpha=0.05. Odd ratios (OR) and 95% confidence intervals (CI) were estimated by conditional logistic regression models based on the matched sets to assess biomarkers in relation to oesophageal adenocarcinoma. Each biomarker was assessed categorically according to the percentage of values above the lower limit of detection: biomarkers detected in 75% or more of subjects were categorized into quartiles; biomarkers detected in 50–<75% of subjects were categorized into tertiles; and biomarkers detected in <50% of subjects were dichotomized (above versus below the lower limit of detection). Cut-points were determined using the entire study population.
We conducted crude models (inherently adjusted for matching factors of study, sex, race/ethnicity, date of birth, date of entry, exit date, and number of freeze-thaw cycles of serum available for analysis), and then assessed whether education and marital status altered associations between inflammation markers and oesophageal adenocarcinoma risk. Next we assessed whether adjustment for, or stratification by, BMI, waist circumference, smoking status and cigarettes per day altered associations.
Effect modification was assessed using a likelihood ratio test comparing models with and without an exposure-modifier cross-product interaction term. We estimated the marginal effects of potential mediators in relation to oesophageal adenocarcinoma risk, before conducting formal mediation analysis to estimate direct effects of these exposures (e.g., obesity) and indirect effects via categorized inflammation markers on oesophageal adenocarcinoma risk [35]. Models restricted to men were assessed (there were too few women for women-only models). Models stratified by time between blood draw and oesophageal adenocarcinoma diagnosis and models excluding cases diagnosed within 3 years of blood draw were conducted to assess reverse causation or time-dependent effects. Biomarkers showing strong associations (low p values and monotonic/threshold effects) with oesophageal adenocarcinoma were modeled together to assess for independence of association. All statistical analyses were conducted using Stata v14 (StataCorp LLC, College Station, TX).
Results
There were 296 cases and 296 controls included in this study (Table 1). The mean age of participants with oesophageal adenocarcinoma was 63 years, the male-to-female ratio was 3.4:1, and 92% self-identified as white. The incidence-density matched control population was very similar. Non-matching characteristics of mean BMI and mean waist circumference were slightly higher in cases (28.7 kg/m2 and 97.1cm) compared with controls (27.2 kg/m2 and 93.5cm). Median time between blood draw and cancer diagnosis was 6.5 years (inter-quartile range: 3.6–9.5). Matched sets were perfectly matched on number of freeze-thaw cycles, and 58% of serum samples were previously unthawed with the remaining 42% having undergone a single freeze-thaw cycle prior to analysis.
Table 1.
Study Population and Participant Demographics
| Variables | Controls n (%)* | Cases n (%)* | Total population n (%)* |
|---|---|---|---|
| Study | |||
| Cancer Prevention Study-II Nutritional Cohort (CPS-II) | 24 (8.11) | 24 (8.11) | 48 (8.11) |
| Carotene and Retinol Efficacy Trial (CARET) | 51 (17.23) | 51 (17.23) | 102 (17.23) |
| European Prospective Investigation into Cancer and Nutrition (EPIC) | 72 (24.32) | 72 (24.32) | 144 (24.32) |
| Multiethnic Cohort Study (MEC) | 22 (7.43) | 22 (7.43) | 44 (7.43) |
| Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) | 63 (21.28) | 63 (21.28) | 126 (21.28) |
| Prostate Cancer Prevention Trial (PCPT) | 26 (8.78) | 26 (8.78) | 52 (8.78) |
| Women’s Health Initiative (WHI) | 38 (12.84) | 38 (12.84) | 76 (12.84) |
| Number of participants | 296 (100.00) | 296 (100.00) | 592 (100.00) |
| Male | 228 (77.03) | 228 (77.03) | 456 (77.03) |
| Female | 68 (22.97) | 68 (22.97) | 136 (22.97) |
| Mean age at blood draw (SD) | 63.4 (7.7) | 63.4 (7.8) | 63.4 (7.8) |
| Race | |||
| White/Assumed White | 271 (91.55) | 273 (92.23) | 544 (91.89) |
| Black | 6 (2.03) | 4 (1.35) | 10 (1.69) |
| Asian or Pacific Islander | 9 (3.04) | 9 (3.04) | 18 (3.04) |
| Missing/Other | 10 (3.38) | 10 (3.38) | 20 (3.38) |
| Ethnicity | |||
| Non-Hispanic | 234 (79.05) | 234 (79.05) | 468 (79.05) |
| Hispanic | 8 (2.70) | 8 (2.70) | 16 (2.70) |
| Unclear/Missing | 54 (18.24) | 54 (18.24) | 108 (18.24) |
| Education | |||
| Less than High School | 42 (14.19) | 58 (19.59) | 100 (16.89) |
| High School Graduate | 46 (15.54) | 49 (16.55) | 95 (16.05) |
| Post High School/Vocational | 37 (12.50) | 39 (13.18) | 76 (12.84) |
| Some College | 55 (18.58) | 66 (22.30) | 121 (20.44) |
| College Graduate | 46 (15.54) | 52 (17.57) | 98 (16.55) |
| Post College Degree | 56 (18.92) | 17 (5.74) | 73 (12.33) |
| Unknown | 14 (4.73) | 15 (5.07) | 29 (4.90) |
| Marital status | |||
| Single | 7 (2.36) | 14 (4.73) | 21 (3.55) |
| Married/Living together | 231 (78.04) | 228 (77.03) | 459 (77.53) |
| Divorced/Seperated | 24 (8.11) | 21 (7.09) | 45 (7.60) |
| Widowed | 19 (6.42) | 21 (7.09) | 40 (6.76) |
| Other/Unknown | 15 (5.07) | 12 (4.05) | 27 (4.56) |
| Smoking status | |||
| Never | 110 (37.16) | 55 (18.58) | 165 (27.87) |
| Former | 119 (40.20) | 169 (57.09) | 288 (48.65) |
| Current | 58 (19.59) | 64 (21.62) | 122 (20.61) |
| Unknown | 9 (3.04) | 8 (2.7) | 17 (2.87) |
| Mean cigarettes per day (SD) | 6.49 (12.21) | 5.54 (11.14) | 5.95 (11.61) |
| Mean BMI at blood draw (kg/m2) (SD) | 27.2 (4.0) | 28.7 (4.6) | 28.0 (4.4) |
| Mean waist circumference at blood draw (cm) (SD) | 93.5 (11.4) | 97.1 (11.8) | 95.3 (11.8) |
Unless otherwise indicated all values will be reported as n (%)
The heat map in Supplemental Table 1 shows all pairwise Pearson correlations using the full dataset. Although these correlations are pairwise, there were clear patterns of association within the correlation matrix that demonstrated the complex relationships of the circulating biomarkers of inflammation. The effective number of tests based on the correlation matrix was 42.2 with a corresponding Sidak adjusted statistical significance threshold of 0.00125.
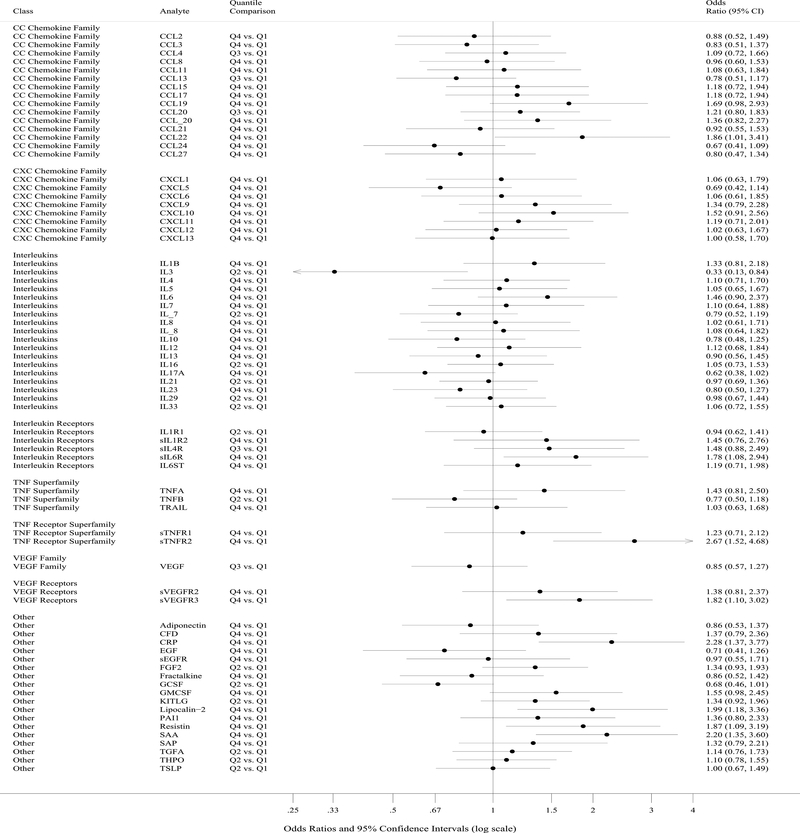
Soluble tumor necrosis factor receptor 2 (sTNFR2, ORQ4 vs Q1=2.67, p=0.0006) surpassed this threshold for statistical significance in relation to oesophageal adenocarcinoma risk while C-reactive protein (CRP, ORQ4 vs Q1=2.28, p=0.0014) was borderline (Table 2, Supplemental Table 2, and Figure). Additional notable biomarker-cancer relationships with low p values that did not surpass the specified threshold for statistical significance included serum amyloid A (SAA), lipocalin-2, resistin, interleukin (IL)3, IL17A, soluble IL6 receptor (sIL6R), and soluble vascular endothelial growth factor receptor 3 (sVEGFR3). All nine of these inflammation markers were included in a single multivariable model to assess the degree of independence in their associations with oesophageal adenocarcinoma. Estimates for lipocalin-2, resistin, and CRP in relation to oesophageal adenocarcinoma were attenuated while estimates for the other biomarkers in the model were unaffected (Supplemental Table 3). Eleven biomarkers had coefficients of variation greater than 30% (Supplemental Table 4), yet only one of these was among the top nine biomarkers associated with oesophageal adenocarcinoma shown in Table 2 (lipocalin-2).
Table 2.
Selected associations between circulating inflammation markers and oesophageal adenocarcinoma.
| Analyte | Quantiles | Base Model | Base Model + BMI (continuous) | Base Model + Waist Circumference (continuous) | Base Model + Smoking Status (never/former/current) | Base Model + Cigarettes Per Day (continuous) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| IL3 | 0.3–0.3 pg/mL | referent | referent | referent | referent | referent | |||||
| 0.3–149.7 pg/mL | 0.33 (0.13–0.84) | 0.020 | 0.28 (0.10–0.76) | 0.013 | 0.23 (0.05–1.18) | 0.079 | 0.43 (0.16–1.16) | 0.095 | 0.35 (0.14–0.89) | 0.028 | |
| IL17A | 0.3–4.5 pg/mL | referent | referent | referent | referent | referent | |||||
| 4.5–7.8 pg/mL | 0.53 (0.33–0.85) | 0.009 | 0.52 (0.32–0.86) | 0.011 | 0.52 (0.26–1.05) | 0.070 | 0.60 (0.36–1.02) | 0.057 | 0.50 (0.30–0.83) | 0.007 | |
| 7.8–13.5 pg/mL | 0.49 (0.30–0.80) | 0.004 | 0.48 (0.28–0.83) | 0.008 | 0.42 (0.20–0.88) | 0.022 | 0.57 (0.33–0.99) | 0.046 | 0.45 (0.27–0.76) | 0.003 | |
| 13.5–297.6 pg/mL | 0.62 (0.38–1.02) | 0.058 | 0.58 (0.35–0.98) | 0.041 | 0.75 (0.38–1.47) | 0.397 | 0.74 (0.43–1.28) | 0.282 | 0.63 (0.37–1.07) | 0.089 | |
| sIL6R | 7.1–17.1 ng/mL | referent | referent | referent | referent | referent | |||||
| 17.1–21.6 ng/mL | 1.34 (0.84–2.14) | 0.218 | 1.33 (0.81–2.18) | 0.258 | 1.47 (0.77–2.81) | 0.244 | 1.49 (0.89–2.49) | 0.126 | 1.31 (0.80–2.14) | 0.285 | |
| 21.6–25.9 ng/mL | 1.50 (0.93–2.40) | 0.093 | 1.28 (0.78–2.11) | 0.336 | 1.51 (0.78–2.93) | 0.223 | 1.52 (0.91–2.54) | 0.110 | 1.61 (0.98–2.64) | 0.060 | |
| 25.9–45.5 ng/mL | 1.78 (1.08–2.94) | 0.025 | 1.66 (0.98–2.82) | 0.060 | 2.13 (1.06–4.27) | 0.033 | 1.80 (1.04–3.09) | 0.034 | 1.83 (1.09–3.07) | 0.022 | |
| sTNFR2 | 2.3–5.3 ng/mL | referent | referent | referent | referent | referent | |||||
| 5.3–6.4 ng/mL | 1.11 (0.67–1.82) | 0.694 | 1.03 (0.61–1.73) | 0.919 | 1.55 (0.74–3.28) | 0.247 | 0.88 (0.51–1.50) | 0.633 | 1.08 (0.65–1.79) | 0.777 | |
| 6.4–7.9 ng/mL | 1.73 (1.06–2.83) | 0.029 | 1.59 (0.95–2.65) | 0.076 | 2.27 (1.10–4.70) | 0.027 | 1.40 (0.82–2.39) | 0.222 | 1.72 (1.04–2.85) | 0.036 | |
| 7.9–20.9 ng/mL | 2.67 (1.52–4.68) | 0.001 | 2.27 (1.26–4.11) | 0.007 | 4.82 (2.03–11.45) | 0.000 | 1.95 (1.07–3.57) | 0.030 | 2.49 (1.40–4.44) | 0.002 | |
| sVEGFR3 | 0.3–1.0 ng/mL | referent | referent | referent | referent | referent | |||||
| 1.0–1.5 ng/mL | 1.44 (0.91–2.26) | 0.116 | 1.29 (0.80–2.09) | 0.293 | 1.16 (0.62–2.18) | 0.648 | 1.40 (0.86–2.31) | 0.179 | 1.61 (1.00–2.59) | 0.049 | |
| 1.5–2.0 ng/mL | 1.26 (0.79–2.00) | 0.330 | 1.14 (0.71–1.85) | 0.584 | 1.00 (0.55–1.82) | 0.993 | 1.32 (0.79–2.18) | 0.285 | 1.22 (0.76–1.97) | 0.412 | |
| 2.0–11.7 ng/mL | 1.82 (1.10–3.02) | 0.020 | 1.71 (1.01–2.90) | 0.047 | 2.00 (1.01–3.97) | 0.048 | 1.64 (0.95–2.83) | 0.078 | 1.68 (1.00–2.83) | 0.052 | |
| CRP | 0.0–3.7 μg/mL | referent | referent | referent | referent | referent | |||||
| 3.7–7.6 μg/mL | 1.28 (0.78–2.09) | 0.330 | 1.20 (0.71–2.00) | 0.496 | 1.25 (0.62–2.54) | 0.537 | 1.07 (0.62–1.84) | 0.807 | 1.28 (0.77–2.12) | 0.343 | |
| 7.6–19.0 μg/mL | 1.72 (1.06–2.81) | 0.029 | 1.42 (0.85–2.38) | 0.183 | 1.19 (0.59–2.37) | 0.628 | 1.38 (0.82–2.35) | 0.228 | 1.57 (0.95–2.59) | 0.079 | |
| 19.0–250.0 μg/mL | 2.28 (1.37–3.77) | 0.001 | 1.93 (1.14–3.27) | 0.014 | 2.46 (1.23–4.94) | 0.011 | 1.81 (1.05–3.14) | 0.033 | 2.31 (1.37–3.90) | 0.002 | |
| Lipocalin-2 | 0.6–175.9 ng/mL | referent | referent | referent | referent | referent | |||||
| 175.9–244.4 ng/mL | 1.77 (1.11–2.83) | 0.016 | 1.97 (1.20–3.24) | 0.007 | 2.32 (1.22–4.42) | 0.010 | 1.84 (1.09–3.10) | 0.022 | 1.93 (1.17–3.20) | 0.011 | |
| 244.4–339.7 ng/mL | 1.23 (0.75–2.03) | 0.410 | 1.46 (0.86–2.47) | 0.162 | 1.83 (0.89–3.77) | 0.099 | 1.01 (0.59–1.74) | 0.971 | 1.26 (0.75–2.11) | 0.391 | |
| 339.7–1421.8 ng/mL | 1.99 (1.18–3.36) | 0.009 | 2.33 (1.33–4.09) | 0.003 | 3.00 (1.44–6.23) | 0.003 | 1.78 (1.01–3.14) | 0.045 | 2.01 (1.18–3.45) | 0.011 | |
| Resistin | 1.3–22.1 ng/mL | referent | referent | referent | referent | referent | |||||
| 22.1–29.0 ng/mL | 1.27 (0.77–2.08) | 0.347 | 1.24 (0.74–2.09) | 0.408 | 1.73 (0.84–3.56) | 0.139 | 1.26 (0.74–2.15) | 0.399 | 1.22 (0.72–2.06) | 0.460 | |
| 29.0–37.3 ng/mL | 1.25 (0.74–2.11) | 0.402 | 1.28 (0.74–2.21) | 0.382 | 2.39 (1.09–5.24) | 0.030 | 1.04 (0.59–1.84) | 0.879 | 1.17 (0.68–2.03) | 0.571 | |
| 37.3–103.6 ng/mL | 1.87 (1.09–3.19) | 0.022 | 1.90 (1.08–3.33) | 0.026 | 2.76 (1.30–5.89) | 0.008 | 1.70 (0.95–3.02) | 0.073 | 1.73 (1.00–3.01) | 0.051 | |
| SAA | 0.6–1.6 μg/mL | referent | referent | referent | referent | referent | |||||
| 1.6–3.6 μg/mL | 1.47 (0.88–2.46) | 0.138 | 1.33 (0.78–2.26) | 0.298 | 1.49 (0.70–3.14) | 0.299 | 1.52 (0.88–2.64) | 0.135 | 1.37 (0.81–2.31) | 0.246 | |
| 3.6–7.2 μg/mL | 1.55 (0.96–2.48) | 0.071 | 1.31 (0.80–2.16) | 0.283 | 1.38 (0.71–2.67) | 0.339 | 1.58 (0.94–2.64) | 0.082 | 1.51 (0.93–2.46) | 0.099 | |
| 7.2–2344.0 μg/mL | 2.20 (1.35–3.60) | 0.002 | 1.90 (1.13–3.19) | 0.015 | 2.15 (1.06–4.37) | 0.033 | 1.99 (1.18–3.38) | 0.010 | 2.05 (1.23–3.40) | 0.006 | |
Summary results provided in each column are not directly comparable across columns because not all study participants had all covariates of interest. Qualified comparative statements in the text of this manuscript are based on a comparison of each of these models to a model restricted to—but not adjusted for—the covariate under examination (results not shown).
Figure. Summary plot of associations between circulating markers and oesophageal adenocarcinoma risk comparing the highest with the lowest quantile.
Each circle represents an odds ratio estimate and the width of the intersecting horizontal line the 95% confidence interval. Circulating markers are ordered by class and the quantile comparison is shown for each given marker.
Adjustment for education (categorical) or marital status (categorical) did not alter estimates of associations of biomarkers in relation to oesophageal adenocarcinoma (results not shown). Adjustment for BMI, waist circumference or smoking status slightly attenuated effects in some of these relationships (Table 2) when compared with models restricted to, but not adjusted for, participants with the covariate under examination (results not shown). Meanwhile, adjustment for cigarettes per day had negligible effects whether modeled as a continuous or categorical metric. Given these results, we conducted formal mediation analyses to estimate direct effects of BMI, waist circumference and smoking status on oesophageal adenocarcinoma risk and indirect effects via circulating inflammation markers. Given little evidence of effect modification by BMI, waist circumference and smoking status (results not shown), we did not assess higher-order interactions within the mediation models. We estimated marginal effects of cigarette smoking (ORever vs never=3.57, 95%CI:2.20, 5.79), BMI (ORper 5 kg/m2=1.51, 95%CI:1.23, 1.85), and waist circumference (ORper standard deviation (SD) [11.76 cm]=1.54, 95%CI:1.18, 2.01) on risk of oesophageal adenocarcinoma using univariate conditional logistic regression models. Next, from the mediation analyses we tabulated the top ten inflammation mediators ranked by percentage of indirect effect on oesophageal adenocarcinoma risk (Table 3, Supplemental Tables 5 & 6). sTNFR2 accounted for 33% (p=0.005) of the effect of waist circumference on oesophageal adenocarcinoma risk. Resistin (13.6%, p=0.04) was also a significant (p<0.05) mediator of the waist circumference-esophageal adenocarcinoma association, while plasminogen activator inhibitor 1 (PAI1, 17.7%, p=0.02), CRP (16.8%, p=0.02) and SAA (12.3%, p=0.045) were significant mediators of the BMI-esophageal adenocarcinoma association. PAI1 (7.7%, p=0.03) was the sole significant mediator of the smoking effect on cancer risk. Five of the top ten mediators for waist circumference and BMI were the same (C-C motif chemokine 19 [CCL19], CRP, PAI1, resistin, sTNFR2), while the smoking status analysis had IL12 (shared with BMI) and sTNFR1 (shared with waist circumference) among its top ten mediators.
Table 3.
Mediation analysis showing top mediators of the relation between waist circumference (per standard deviation [11.75 cm]) and oesophageal adenocarcinoma risk
| Mediator | Controls (n) | Cases (n) | Total Effect of Waist Circumference |
Direct Effect of Waist Circumference |
Indirect Effect of Waist Circumferencevia Mediator |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | % Indirect | |||
| sTNFR2 | 163 | 163 | 1.61 (1.21, 2.13) | 9.9E-04 | 1.37 (1.03, 1.83) | 2.9E-02 | 1.17 (1.05, 1.31) | 0.005 | 33.0 |
| CFD | 162 | 162 | 1.54 (1.17, 2.03) | 2.1E-03 | 1.44 (1.08, 1.93) | 1.4E-02 | 1.07 (0.97, 1.17) | 0.16 | 15.2 |
| Resistin | 162 | 162 | 1.66 (1.24, 2.20) | 5.7E-04 | 1.55 (1.17, 2.04) | 2.1E-03 | 1.07 (1.00, 1.14) | 0.04 | 13.6 |
| PAI1 | 162 | 162 | 1.56 (1.18, 2.06) | 2.0E-03 | 1.47 (1.11, 1.94) | 7.0E-03 | 1.06 (1.00, 1.13) | 0.07 | 13.2 |
| sTNFR1 | 163 | 163 | 1.61 (1.22, 2.13) | 9.1E-04 | 1.53 (1.16, 2.01) | 2.9E-03 | 1.06 (0.98, 1.14) | 0.16 | 11.4 |
| KITLG | 153 | 153 | 1.73 (1.28, 2.34) | 4.0E-04 | 1.63 (1.21, 2.18) | 1.2E-03 | 1.06 (1.00, 1.13) | 0.06 | 11.2 |
| sIL6R | 163 | 163 | 1.55 (1.18, 2.03) | 1.6E-03 | 1.48 (1.13, 1.94) | 4.7E-03 | 1.05 (0.99, 1.10) | 0.10 | 10.3 |
| CCL19 | 165 | 165 | 1.59 (1.21, 2.10) | 9.1E-04 | 1.52 (1.16, 2.00) | 2.5E-03 | 1.05 (0.98, 1.12) | 0.15 | 10.1 |
| CRP | 163 | 163 | 1.45 (1.10, 1.91) | 8.2E-03 | 1.40 (1.06, 1.86) | 1.9E-02 | 1.04 (0.96, 1.11) | 0.34 | 9.4 |
| TNFA | 165 | 165 | 1.61 (1.22, 2.12) | 7.6E-04 | 1.54 (1.18, 2.02) | 1.6E-03 | 1.04 (0.98, 1.11) | 0.17 | 8.9 |
Note that the total effect varies slightly due to minor differences in the sample sizes due to assay failure. The percent indirect effect is estimated as 100 × (log(OR_indirect) / log(OR_total)).
Restriction to men did not affect the relationships between circulating biomarkers and risk of oesophageal adenocarcinoma (data not shown).
Analyses stratified by time between blood draw and cancer diagnosis indicated that associations of lipocalin-2, resistin, SAA and sVEGFR3 with cancer risk were stronger closer to cancer diagnosis; all other circulating biomarkers (IL3, CRP, IL17A, sIL6R, sTNFR2) did not differ by time between blood draw and cancer diagnosis (Supplemental Tables 7 and 8). Similar observations were evident when excluding the 67 matched pairs in which the oesophageal adenocarcinoma case was diagnosed within three years of blood draw, while other biomarker-cancer relations strengthened, specifically IL1B (ORQ4 vs Q1=2.51, p=0.009), IL6 (ORQ4 vs Q1=2.00, p=0.033), adiponectin (ORQ4 vs Q1=0.45, p=0.018), and PAI1 (ORQ4 vs Q1=2.09, p=0.041) (Supplemental Table 9).
Posthoc analyses in which sTNFR2 and TNFA were modelled together caused an attenuation of TNFA’s effect (ORQ4 vs Q1=1.06, 95%CI:0.58, 1.96) while sTNFR2’s relation with oesophageal adenocarcinoma strengthened (ORQ4 vs Q1=2.81, 95%CI:1.54, 5.12; results not tabulated). When tertiles of the metabolites sTNFR2 and TNFA were cross-classified, the strongest risks were observed for sTNFR2-high/TNFA-medium (OR=2.66, 95%CI:1.29, 5.47) and for sTNFR2-high/TNFA-high (OR=1.95, 95%CI:1.01, 3.76), when each was compared with sTNFR2-low/TNFA-low (results not tabulated).
Discussion
In this study, sTNFR2 was significantly, positively associated with risk of oesophageal adenocarcinoma and partly mediated the association between BMI and this malignancy. There was also evidence for other biomarker-esophageal adenocarcinoma associations, as well as biomarkers acting as mediators between inflammation-associated exposures (excess adiposity/smoking) and cancer risk. This study highlights the importance of systemic inflammation in the etiology of oesophageal adenocarcinoma.
There have been only two previous prospective analyses of inflammation-related biomarkers and oesophageal adenocarcinoma [18, 19], both of which were conducted within the Seattle Barrett’s Esophagus Study (SBES) comprised of almost 400 Barrett’s oesophagus subjects. Hardikar et al [18] assessed CRP, IL6, sTNFR1 & 2, and F2-isoprostanes in relation to oesophageal adenocarcinoma risk and found weak evidence for positive associations of CRP (hazard ratio (HR)= 1.98, 95%CI:1.05, 3.73), IL6 (HR=1.95, 95%CI:1.03, 3.72) and sTNFR2 (HR= 1.90, 95%CI:0.98, 3.67) with cancer risk when comparing these exposures as dichotomized variables based on median splits. Although tests for trend across quartiles were not statistically significant, statistical power was limited due to accrual of just 45 oesophageal adenocarcinomas cases. These tentative findings from Hardikar et al support some of the associations we describe herein from our study of seven prospective cohorts.
Potential biologic mechanisms underlying our primary association between sTNFR2 and oesophageal adenocarcinoma risk are not obvious due to the complexity of TNFRs and their roles in multiple cellular processes. The central characteristic of ligands in the tumor necrosis factor-alpha (TNFA) superfamily is the ability to promote pro-inflammatory signaling. TNFA is the primary ligand for TNFR2 and is found in soluble as well as membrane-bound forms. TNFA can induce a variety of downstream pathways with a complex biology that may include apoptotic and/or cell stimulatory signals [36–38]. Soluble TNFRs are shed forms of the extracellular portion of these receptors which can bind and sequester circulating TNFA, reducing its bioavailability, resulting in an altered endocrinological state. The posthoc cross-classification results—in which the strongest associations with oesophageal adenocarcinoma were observed for subjects with high TNFR2 and high/medium TNFA—may point to the importance of uncontrolled chronic inflammation in this disease process. TNFR2 protein is found at low concentrations on oesophageal stratified squamous epithelium [39] while evidence from the Gene Expression Omnibus (GEO) Profiles database [40] is indicative of TNFR2 gene expression in Barrett’s oesophagus (GEO accessions GDS1321 [41], GDS4350 [42], GDS3472 [43]). TNFR2 is also present on stimulated T lymphocytes [44] which, from cross-sectional data, have been proposed to be a prominent tissue infiltrate throughout oesophageal adenocarcinogenesis [45, 46]. In addition, bile acids have been suggested to elicit a proinflammatory response (including upregulation of TNFA) [47, 48] which could induce oxidative damage and further increase risk of malignancy.
Estimates of association between anthropometric variables and smoking status with oesophageal adenocarcinoma risk from this prospective study are largely in-line with those of prior studies [5, 6], albeit with a slightly stronger association with smoking status. The underlying mechanisms that link these key exposures with cancer risk remain largely unexplored, but this study has begun to reveal a systemic inflammatory link, particularly with central adiposity. Central adiposity is a strong correlate of visceral adipose tissue—a highly metabolic fat type that is linked with oesophageal adenocarcinoma and has an efflux of proinflammatory cytokines, including TNFA [11, 49, 50]. The TNF-signaling pathway is also implicated by the main finding of this study—sTNFR2, which also has a major role in the adipocytokine signaling pathway [51]. Further investigations of these pathways and other inflammation markers associated with oesophageal adenocarcinogenesis by this study are clearly warranted to help further elucidate biological mechanisms underlying these observations.
Strengths of this analysis included the use of prediagnostic specimens, the relatively large sample size, and the broad scope of this initial discovery study enabled by multiplex assays. Limitations included the lack of data on gastroesophageal reflux exposures (which has not been collected by most prospective studies), and the availability of only BMI and waist circumference as proxies of adipose patterning and composition. The sample size and sex ratio of this malignancy precluded assessment of women-only. Additional limitations, which would be expected to be non-differential and thus usually attenuate estimates towards the null, include use of a single blood specimen which precluded a deeper assessment of how these biomarkers were associated with malignancy, and the fact that some assays had less than the desired level of reproducibility.
In conclusion, this study is the first broad, well-powered prospective assessment of inflammation markers and oesophageal adenocarcinoma. It highlights the importance of a heightened systemic inflammatory state in the etiology of oesophageal adenocarcinoma and provides the first evidence that indirect effects of excess adiposity and cigarette smoking, via systemic inflammation, increase the risk of oesophageal adenocarcinoma.
Supplementary Material
Summary
What is already known about this subject?
Cross-sectional data indicate that systemic inflammation is an important component of oesophageal adenocarcinoma.
Mechanisms of how obesity and cigarette smoking are related to risk of oesophageal adenocarcinoma are not well understood.
What are the new findings?
Prediagnostic circulating soluble tumor necrosis factor receptor 2 (sTNFR2) was significantly associated with oesophageal adenocarcinoma and did not vary with time between blood draw and cancer diagnosis.
C-reactive protein, serum amyloid A, lipocalin-2, resistin, interleukin (IL)3, IL17A, soluble IL6 receptor, and soluble vascular endothelial growth factor receptor 3 also provided evidence of associations with oesophageal adenocarcinoma.
Formal mediation analysis indicated that sTNFR2 may account for 33% of the effect of waist circumference on oesophageal adenocarcinoma risk. Resistin, plasminogen activator inhibitor 1, C-reactive protein and serum amyloid A were also identified as potential mediators of obesity-esophageal adenocarcinoma associations.
Plasminogen activator inhibitor 1 was identified as a mediator of the smoking-esophageal adenocarcinoma relationship.
How might it impact on clinical practice in the foreseeable future?
Elucidating mechanisms of how excess adiposity and cigarette smoking increase risks of oesophageal adenocarcinoma may provide foundational evidence for the development of risk triage strategies and clinical interventions.
Acknowledgements
The authors express sincere appreciation to all participants, study-site principal investigators, biospecimen and clinical research associates that participated/contributed to each of the cohorts.
Funding:
Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
CARET is funded by National Cancer Institute, National Institutes of Health grants U01-CA63673 and UM1-CA167462
CPS-II: The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study Nutritional Cohorts.
The coordination of EPIC is financially supported by the European Commission (DG-SANCO), Imperial College London and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 (Norway); Health Research Fund (FIS), PI13/00061 to Granada, PI13/01162 to EPIC-Murcia, Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A11692 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom).
The MEC is funded by the National Cancer Institute National Institutes of Health, U.S. Department of Health and Human Services U01 CA164973.
PCPT is supported by the National Cancer Institute of the National Institutes of Health under Award Numbers 5UM1CA182883 and U10CA37429. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Abbreviations:
- BMI
body mass index
- CARET
Carotene and Retinol Efficacy Trial
- CCL19
C-C motif chemokine 19
- CI
confidence interval
- cm
centimeter
- CPS-II
Cancer Prevention Study-II Nutrition Cohort
- CRP
C-reactive protein
- EPIC
European Prospective Investigation into Cancer and Nutrition
- GEO
Gene Expression Omnibus
- GERD
gastroesophageal reflux disease
- ICD
International Classification of Diseases
- IL
interleukin
- MEC
Multiethnic Cohort Study
- NCI
National Cancer Institute
- NMSC
non-melanoma skin cancer
- OR
odds ratio
- PAI1
plasminogen activator inhibitor 1
- PCPT
Prostate Cancer Prevention Trial
- PLCO
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
- SAA
serum amyloid A
- SBES
Seattle Barrett’s Esophagus Study
- SD
standard deviation
- sTNFR
soluble tumor necrosis factor receptor
- sVEGFR3
soluble vascular endothelial growth factor receptor 3
- TNFA
tumor necrosis factor alpha
- WHI
Women’s Health Initiative
Footnotes
There are no financial disclosures from any of the authors.
SHORT LIST OF WHI INVESTIGATORS Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland. For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 2.Colleypriest BJ, Ward SG, Tosh D. How does inflammation cause Barrett’s metaplasia? Curr Opin Pharmacol 2009;9:721–6. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AM, Duong M, Healy L, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: epidemiology, etiology and new targets. Cancer Epidemiol 2011;35:309–19. [DOI] [PubMed] [Google Scholar]
- 4.Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal Reflux in Relation to Adenocarcinomas of the Esophagus: A Pooled Analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS ONE 2014;9:e103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol 2012;41:1706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: A systematic review and meta-analysis. Am J Gastroenterol 2006;101:2619–28. [DOI] [PubMed] [Google Scholar]
- 8.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol 2008;103:292–300. [DOI] [PubMed] [Google Scholar]
- 9.Reid BJ, Li X, Galipeau PC, et al. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer 2010;10:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camhi SM, Bray GA, Bouchard C, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013;9:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olliver JR, Hardie LJ, Gong Y, et al. Risk factors, DNA damage, and disease progression in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 2005;14:620–5. [DOI] [PubMed] [Google Scholar]
- 13.Ness-Jensen E, Lagergren J. Tobacco smoking, alcohol consumption and gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol 2017;31:501–8. [DOI] [PubMed] [Google Scholar]
- 14.Bennett JM, Glaser R, Andridge RR, et al. Long lasting effects of smoking: breast cancer survivors’ inflammatory responses to acute stress differ by smoking history. Psychoneuroendocrinology 2013;38:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandol SJ, Apte MV, Wilson JS, et al. The burning question: why is smoking a risk factor for pancreatic cancer? Pancreatology 2012;12:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruger K, Dischereit G, Seimetz M, et al. Time course of cigarette smoke-induced changes of systemic inflammation and muscle structure. Am J Physiol Lung Cell Mol Physiol 2015;309:L119–28. [DOI] [PubMed] [Google Scholar]
- 17.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009;9:377–84. [DOI] [PubMed] [Google Scholar]
- 18.Hardikar S, Onstad L, Song X, et al. Inflammation and oxidative stress markers and esophageal adenocarcinoma incidence in a Barrett’s esophagus cohort. Cancer Epidemiol Biomarkers Prev 2014;23:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duggan C, Onstad L, Hardikar S, et al. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2013;11:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer 2002;94:2490–501. [DOI] [PubMed] [Google Scholar]
- 21.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst 2004;96:1743–50. [DOI] [PubMed] [Google Scholar]
- 22.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113–24. [DOI] [PubMed] [Google Scholar]
- 23.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andriole GL, Levin DL, Crawford ED, et al. Prostate Cancer Screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst 2005;97:433–8. [DOI] [PubMed] [Google Scholar]
- 25.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013;310:1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trabert B, Eldridge RC, Pfeiffer RM, et al. Prediagnostic circulating inflammation markers and endometrial cancer risk in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Int J Cancer 2017;140:600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassig BA, Dai Y, Vermeulen R, et al. Occupational exposure to diesel engine exhaust and alterations in immune/inflammatory markers: a cross-sectional molecular epidemiology study in China. Carcinogenesis 2017;38:1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiels MS, Katki HA, Hildesheim A, et al. Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp TJ, Castro FA, Gao YT, et al. Application of multiplex arrays for cytokine and chemokine profiling of bile. Cytokine 2015;73:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitahara CM, Trabert B, Katki HA, et al. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev 2014;23:2840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purdue MP, Hofmann JN, Kemp TJ, et al. A prospective study of 67 serum immune and inflammation markers and risk of non-Hodgkin lymphoma. Blood 2013;122:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi AK, Kemp TJ, Pfeiffer RM, et al. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol Biomarkers Prev 2011;20:1902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyholt DR. Matrix Spectral Decomposition (matSpDlite) 2015. [Available from: https://neurogenetics.qimrberghofer.edu.au/matSpDlite/.
- 35.Breen R, Karlson KB, Holm A. Total, Direct, and Indirect Effects in Logit and Probit Models. Sociological Methods & Research 2013;42:164–91. [Google Scholar]
- 36.Blaser H, Dostert C, Mak TW, et al. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol 2016;26:249–61. [DOI] [PubMed] [Google Scholar]
- 37.Lebrec H, Ponce R, Preston BD, et al. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr Med Res Opin 2015;31:557–74. [DOI] [PubMed] [Google Scholar]
- 38.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev 2014;25:453–72. [DOI] [PubMed] [Google Scholar]
- 39.Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 40.Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res 2013;41:D991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimchi ET, Posner MC, Park JO, et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res 2005;65:3146–54. [DOI] [PubMed] [Google Scholar]
- 42.di Pietro M, Lao-Sirieix P, Boyle S, et al. Evidence for a functional role of epigenetically regulated midcluster HOXB genes in the development of Barrett esophagus. Proc Natl Acad Sci U S A 2012;109:9077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stairs DB, Nakagawa H, Klein-Szanto A, et al. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett’s esophagus. PLoS One 2008;3:e3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Subleski JJ, Hamano R, et al. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol 2010;40:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavanagh ME, Conroy MJ, Clarke NE, et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett 2016;370:117–24. [DOI] [PubMed] [Google Scholar]
- 46.Picardo SL, Maher SG, O’Sullivan JN, et al. Barrett’s to oesophageal cancer sequence: a model of inflammatory-driven upper gastrointestinal cancer. Dig Surg 2012;29:251–60. [DOI] [PubMed] [Google Scholar]
- 47.Huo X, Agoston AT, Dunbar KB, et al. Hypoxia-inducible factor-2α plays a role in mediating oesophagitis in GORD. Gut 2017;66:1542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun D, Wang X, Gai Z, et al. Bile acids but not acidic acids induce Barrett’s esophagus. Int J Clin Exp Pathol 2015;8:1384–92. [PMC free article] [PubMed] [Google Scholar]
- 49.Beddy P, Howard J, McMahon C, et al. Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br J Surg 2010;97:1028–34. [DOI] [PubMed] [Google Scholar]
- 50.Nelsen EM, Kirihara Y, Takahashi N, et al. Distribution of Body Fat and Its Influence on Esophageal Inflammation and Dysplasia in Patients With Barrett’s Esophagus. Clinical Gastroenterology and Hepatology 2012;10:728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanehisa M, Furumichi M, Tanabe M, et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 2017;45:D353–d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.