Abstract
Background & Aims:
Some patients with defecatory disorders (DD) have high anal pressures that may impede rectal evacuation. Αlpha-1 adrenoreceptors mediate as much as 50% of anal resting pressure in humans. We performed a randomized, placebo-controlled study of the effects of alfuzosin, an alpha1-adrenergic receptor antagonist, on anal pressures alone in healthy women and also on bowel symptoms in women with DD.
Methods:
In a double-blind study performed from March 2013 through March 2017, anal pressures were evaluated before and after 36 women with DD (constipation for at least 1 year) and 36 healthy women (controls) were randomly assigned (1:1) to groups given oral alfuzosin (2.5 mg immediate release) or placebo. Thereafter, patients were randomly assigned (1:1) to groups given oral alfuzosin (10 mg extended release) or placebo each day for 2 weeks. Participants kept daily diaries of bowel symptoms for 2 weeks before (baseline) and during administration of the test articles (treatment). Weekly questionnaires recorded the overall severity of constipation symptoms, bloating, abdominal pain, nausea, and vomiting; overall satisfaction with treatment of constipation was evaluated at weeks 2 and 4. The primary endpoint was the change in the number of spontaneous (SBMs) and complete SBMs (CSBMs) between the treatment and baseline periods. We evaluated relationships between stool form, passage, and complete evacuation.
Results:
Alfuzosin reduced anal resting pressure by 32 ± 3 mmHg vs 16 ± 3 mmHg for placebo (P=.0001) and anal pressure during evacuation by 26 ± 3 mmHg vs 16 ± 3 mmHg for placebo, (P=.03). However alfuzosin did not significantly increase the rectoanal gradient, SBMs or CSBMs compared with placebo. Both formulations of alfuzosin were well tolerated. Hard stools and the ease of passage during defecation accounted for 72% and 76% of the variance in the satisfaction after defecation, respectively, during baseline and treatment periods.
Conclusion:
In a randomized trial, alfuzosin reduced anal pressure at rest and during simulated evacuation in healthy and constipated women, compared with placebo, but did not improve bowel symptoms in constipated women. This could be because the drug does not improve stool form or dyssynergia, which also contribute to DD. ClinicalTrials.gov number, NCT 01834729
Keywords: Anismus, anal manometry, sympathetic nervous system, biofeedback
INTRODUCTION
A substantial proportion of constipated patients have defecatory disorders (DD),1 which are caused by inadequate rectal propulsive forces and/or impaired anal relaxation during defecation 2 and should be treated with pelvic floor biofeedback therapy.2 However, a Cochrane review suggested that more evidence is required to support the efficacy of biofeedback therapy for DD.3 Biofeedback therapy is not widely available or universally covered by insurance programs. Aside from measures to aid evacuation (e.g., enemas and suppositories), there are no effective therapeutic options for DD; sacral nerve stimulation and pelvic floor botulinum toxin injection are ineffective.4 Hence, additional options are necessary.5
Pelvic floor biofeedback therapy addresses somatic dysfunctions in DD (i.e., impaired volitional development of rectal propulsive forces and/or pelvic floor relaxation).6–8 However, normal defecation also entails visceral processes such as colonic high amplitude propagated contractions, rectal sensation, and relaxation of the internal anal sphincter.6, 7 Some patients with DD have visceral dysfunctions, including diminished propagated colonic motor sequences, reduced rectal sensation, and high anal resting pressure, which is predominantly maintained by the internal anal sphincter.9, 10 Even after adjusting for outlet obstruction and slow colon transit, high anal resting pressure is the only independent predictor of healthcare utilization in patients with DD.11 It is conceivable that high anal pressures impede rectal evacuation, particularly in patients with reduced anal relaxation during defecation.
In humans, monkeys, and dogs, the internal sphincter is functionally innervated by sympathetic motor nerves.6 The sympathetic nervous system maintains up to 50% of anal resting pressure in humans.12 Sympathetic stimulation doubled spontaneous contraction in the monkey internal anal sphincter.13 Similar to the bladder outlet,14 it is conceivable that α1-adrenoreceptors exert sympathetic tone on the internal anal sphincter. Indeed, a small study observed that the α1-antagonist indoramin reduced anal resting pressure by 30% in patients with anal fissures.15 In primates, α1-adrenoreceptors also relax the rectum (personal communication, Dr. Kathleen Keef). Hence, α1-antagonists may also increase rectal contractility and thereby facilitate defecation.
Alfuzosin is an α1-adrenoreceptor antagonist that is approved for treating impaired voiding. Our hypotheses were that alfuzosin will reduce anal pressure at rest and during evacuation, and also increase the rectoanal gradient during evacuation in healthy women and women with DD and improve bowel symptoms in women with DD.
METHODS
Study Design
This was a double-blind, randomized, placebo-controlled, parallel-group study conducted at Mayo Clinic in the United States. This study was approved by the Institutional Review Board at Mayo Clinic, registered on ClinicalTrials.gov (NCT 01834729), and conducted between March 2013 and March 2017. All authors had access to the study data, reviewed, and approved the final manuscript. At baseline (Part A), anal pressures were recorded before and after randomization to alfuzosin or placebo, on the same day, in all participants. Thereafter, in Part B, bowel habits were recorded before (2 weeks) and during (2 weeks) randomized treatment with placebo or alfuzosin in constipated patients. The randomization to alfuzosin or placebo in Part B was balanced based on randomization in Part A.
Participants
Thirty-eight healthy women (age 41 ± 3y, BMI 26 ± 1 kg/m2, Mean ± SEM) and 36 women (age 40 ± 2y, BMI 26 ± 1 kg/m2) with constipation for 1 year or longer participated in this study. The healthy women did not have a functional bowel disorder, anxiety or depression evaluated with a questionnaire.16 The patients had symptoms of chronic constipation or constipation-predominant IBS,17 a digital rectal examination, and anorectal tests, performed in the clinical practice, suggestive of a DD.18, 19 The key exclusion criteria were: pregnancy, clinical evidence of significant systemic disease, symptomatic orthostatic hypotension, medications that substantially alter GI transit, except as permitted as rescue agents, prolonged Q-Tc interval (500 msec or longer) on an electrocardiogram, renal insufficiency, rectal inflammation or cancer, and a history of pelvic radiation, rectosigmoid surgery or inflammatory bowel disease. During the baseline and treatment periods, the use of rescue medications (bisacodyl tablet or suppository or Fleet’s enema rectally) was permitted when 72 h or longer had passed since the patient’s previous BM. Fiber supplements were continued during the trial.
Anorectal Manometry
After administering 2 sodium phosphate enemas (Fleets®, C.B. Fleet, Lynchburg, VA), rectal and anal pressures were measured, at rest, during squeeze, and simulated evacuation with high-resolution anorectal manometry (Medtronic Inc, Los Angeles, CA) before and after alfuzosin.20, 21 Pressures were interpreted by comparison to the 10th-90th percentile values from a database of 75 healthy women who had a seated balloon expulsion time less than 61 seconds (Supplementary Methods).
Other Anorectal Tests
When clinically necessary, other anorectal tests were performed using established techniques. The findings suggested an evacuation disorder in 11 of 12 patients who had a MR proctogram, 13 of 15 who had a barium proctogram, and 13 of 14 who had anal sphincter surface electromyography (EMG) (Supplementary Material).18, 22
Alfuzosin
The α1 adrenergic antagonist alfuzosin is 144-fold more selective for the prostate than vascular tissue, rarely causes orthostatic hypotension, and barely penetrates the blood-brain barrier.14, 23 The Part A study used an immediate release formulation of alfuzosin (2.5 mg, Xatral™, Sanofi-Aventis), which is not available in the United States and was imported from the United Kingdom. This formulation has a mean bioavailability of 64%, a tmax of 1.0 – 1.5 hours, and a terminal thalf of 3–5 hours.14, 23 The post-drug manometry was repeated 1.5 hours after the drug was given. Part B used the extended release formulation of alfuzosin (10 mg, Uroxatral™, Sanofi-Aventis, Bridgewater, NJ). Alfuzosin was administered under an investigator-initiated IND (117098) from the FDA.
Paper-Based Efficacy Assessments and Endpoints
A daily diary recorded the time of each BM, its form (7-point Bristol Stool Form Scale (BSFS); 1=“hard lumps” to 7=“watery”), whether the BM was associated with satisfaction after defecation (yes/no); the ease of passage (i.e., 1 - “manual disimpaction”, 2 -“enema needed”, 3 - “straining needed”, 4 - “normal”, 5 - “urgent without pain”, 6 - “urgent with pain”, 7 - “incontinence”); and use of rescue medications. Daily patient reports also included the severity of most severe and overall average abdominal pain recorded on a scale ranging from 0, “none” to 6, “very severe”. Weekly questionnaires recorded the overall severity of constipation symptoms in the past week and the worst bloating, abdominal pain, nausea, and vomiting in the last 24 hours, all scored from 0 (“None “) to 6 (“Very Severe”). The overall satisfaction with treatment of constipation was evaluated at the end of the pre-drug and treatment periods, i.e., at 2 and 4 weeks respectively.
Primary endpoints
For Part A, the primary efficacy endpoint was the change in anal resting pressure for alfuzosin compared to placebo. The secondary endpoints were the change in anal pressure and rectoanal gradient during evacuation for alfuzosin versus placebo.
For Part B, the primary efficacy endpoints were the change in the number of spontaneous bowel movements (SBMs) and complete spontaneous bowel movements (CSBMs) between treatment and baseline periods, averaged over 2 weeks each. A bowel movement was considered spontaneous if no laxative, enema or suppository was taken in the preceding 24 hours. SBMs that were associated with a sensation of complete bowel emptying were CSBMs.
Pre-specified secondary endpoints were (i) stool form, ease of passage, satisfaction after defecation, abdominal pain severity recorded with daily diaries, (ii) overall relief and severity of constipation recorded on weekly diaries, and (iii) global patient reported outcomes measured at 2 and 4 weeks. Bowel movements that occurred within 24 h after rescue medications were excluded from the efficacy endpoints.
Statistical Analysis
An EXCEL spreadsheet of treatment assignments (balanced on age, hysterectomy and BMI using a block size of 4), was generated by computer and sent to the research pharmacy. Study personnel were blinded until the study was completed. Drug effects on anal pressures in healthy and constipated women and separately between healthy and constipated women were analyzed with paired parametric or non-parametric tests as appropriate. Comparisons between parameters were evaluated with Spearman’s correlation coefficients.
Bowel diary data were first averaged per day and then separately for baseline (weeks 1 and 2) and treatment (weeks 3 and 4) periods. Treatment groups were compared using analysis of covariance (ANCOVA) with the corresponding baseline (run-in) value as the covariate. Data were analyzed per intent-to-treat analysis using all subjects randomized. Missing values were imputed using the corresponding overall mean in all subjects with non-missing data, and an adjustment in the ANCOVA error degrees of freedom (i.e., subtracting one df for each missing value imputed) to obtain an appropriate error residual variance.
Among patients with DD, the relationships among various bowel symptoms is poorly understood. Hence, as secondary analyses, logistic regression models with repeated measures (to account for multiple observations within subject) evaluated the factors (i.e., stool form and passage) contributing to a sense of complete evacuation, separately for the 2 week baseline and treatment periods. All analyses used SAS® software (version 9.3, Cary NC).
Sample size assessment
The coefficient of variation in anal resting pressure in healthy women and patients with DD was respectively 20% and 11% (unpublished data). Part A had 80% power to detect a treatment effect size on of approximately 15–20% in healthy women and DD using a two sample t-test with a two-sided alpha level of 0.05. For part B, differences between treatment groups of ~4 stools per week, differences in stool consistency scores of ~1.1 and ease of passage scores of 0.8 could be detected with 80% power with a 2-sided alpha level of 0.05.24
RESULTS
Patient disposition, demographics, and baseline characteristics
Thirty six healthy women completed Part A (Supplementary Figure 1). Of 35 constipated women who completed Part A, 31 also completed Part B. The age and BMI were not significantly different between healthy and constipated participants (Table 1). Among patients, 15 (42%) had constipation-predominant IBS, 21 (58%) had functional constipation, and 13 (36%) had significant anxiety and/or depression.
Table 1.
Summary of patient demographics and baseline characteristics
| Parameter a | Healthy (N= 38) | Constipation (N=36) | |||||
|---|---|---|---|---|---|---|---|
| Placebo (N=l 8) | Alfuzosin (N=20) | p valueb | Placebo (N=18) | Alfuzosin(N=18) | p valuec | p valued | |
| Demographic data | |||||||
| Age (yr) | 32 (26 – 51) | 45 (28 – 53) | 0.8 | 36 (26 – 50) | 40 (35 – 44) | 0.7 | 0.6 |
| Body-mass index§ (kg/m2) | 24 (24 – 29) | 27 (23 – 29) | 0.8 | 25 (21 – 30) | 25 (19 – 29) | 0.8 | 0.5 |
| Baseline data | |||||||
| Functional constipation, N (%) | 0 | 0 | 9 (50%) | 12 (67%) | |||
| IBS-C, N (%) | 0 | 0 | 9 (50%) | 6 (33%) | |||
| Depression, N (%) | 0 | 0 | 3 (17%) | 2 (11%) | |||
| Anxiety, N (%) | 0 | 0 | 4 (22%) | 4 (22%) | |||
| Anorectal pressures, mm Hg | |||||||
| Anal pressure at rest | 85 (57 – 91) | 91 (83 – 98) | 0.1 | 83 (76 – 100) | 81 (73 – 90) | 0.2 | 0.7 |
| Anal pressure at squeeze | 225 (188 – 265) | 251 (200 – 277) | 0.4 | 224 (191 – 282) | 194 (148 – 235) | 0.05 | 0.2 |
| Rectal pressure – simulated evacuation | 30 (25 – 45) | 24 (13 – 39) | 0.4 | 32 (28 – 42) | 21 (8 – 34) | 0.04 | 0.7 |
| Rectal pressure increment – simulated evacuation | 27 (19 – 41) | 26 (11 – 34) | 0.5 | 34 (22 – 38) | 17 (6 – 33) | 0.1 | 0.6 |
| Anal pressure – simulated evacuation | 74 (59 – 85) | 82 (65 – 93) | 0.2 | 86 (70 – 103) | 78 (71 – 91) | 0.7 | 0.5 |
| Rectoanal gradient – simulated evacuation | −44 (−53 – −26) | −59 (−67 – −39) | 0.05 | −51 (−61 – −32) | −65 (−81 – −38) | 0.3 | 0.3 |
| Anal relaxation – simulated evacuation, %e | 23 (4 – 33) | 19 (9 – 25) | 0.8 | 2 (−7 – 16) | 9 (−11 – 17) | 0.8 | 0.05 |
| Anal relaxation – simulated evacuation after rectal distension,% | 13 (−6 – 30) | 11 (3 – 26) | 0.5 | 0 (−17 – 10) | −6 (−30 – 14) | 0.7 | 0.02 |
| Two or more abnormal manometry parameters during evacuation, N (%) | 4 (22%) | 5 (25%) | 0.8 | 6 (33%) | 8 (44%) | 0.5 | 0.1 |
| Balloon expulsion time > 60s, N (%) | 1 (6%) | 2 (10%) | 0.6 | 7 (39%) | 9 (50%) | 0.5 | 0.0005 |
| Abnormal MR or barium proctogram, N (%) | 0 | 0 | 7 (39%) | 7 (39%) | |||
| Abnormal anorectal surface EMG exam, N (%) | 0 | 0 | 8 (44%) | 5 (28%) | |||
| Abnormal test(s) indicative of DD, N (%) | 5 (28%) | 7 (35%) | 0.6 | 17 (94%) | 17 (94%) | 1 | <0.0001 |
Values are medians(interquartile range) unless specified otherwise. Statistical comparisons were only performed for parameters that were evaluated in all participants.
Comparison of placebo versus alfuzosin in healthy participants
Comparison of placebo versus alfuzosin in constipated patients.
Comparison of healthy and constipated patients
Positive larger values represent greater anal relaxation.
Of 35 patients who completed the manometry, 16 patients (46%) had an abnormal BET (Table 1). In 17 of 19 patients with a normal BET, the diagnosis of a DD was confirmed with HRM alone (3 patients), HRM and barium or MR proctogram (2 patients), HRM and anal EMG (3 patients), barium proctogram (1 patient), barium and MR proctogram (4 patients), anal EMG alone (3 patients), or anal EMG with barium proctogram (1 patient). Hence, 34 patients had objective features of a DD; 20 patients had two or more abnormal tests. In the remaining 2 patients, the clinical features, and the anal manometry and/or rectal balloon expulsion test performed in the clinical practice but not in the research study suggested a DD. Other diagnostic tests were not performed in these 2 patients.
Effects of alfuzosin on anal pressures and the rectal balloon expulsion test
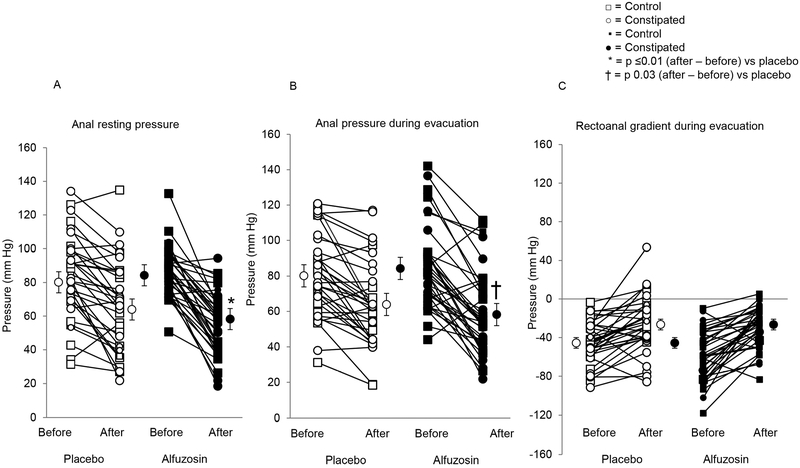
Alfuzosin reduced anal resting pressure versus placebo (32 ± 3 versus 16 ± 3 mmHg for placebo, P=.0001) (Figures 1 and 2, Table 2). During simulated evacuation, alfuzosin reduced anal pressure by 26 ± 3 mmHg versus 16 ± 3 mmHg for placebo (P=.03). Drug effects on anal pressures at rest and during simulated evacuation were correlated for placebo (r=0.57, P=0.0005) and alfuzosin (r=0.61, P<0.0001). By contrast, drug effects on anal squeeze pressures were not significant. The effects of alfuzosin on anal pressures were not significantly different between healthy and constipated participants. The rectal balloon expulsion time was prolonged in 5 of 18 patients before and in 3 patients after treatment with placebo. In the alfuzosin group, the corresponding numbers were respectively 6 and 3 patients.
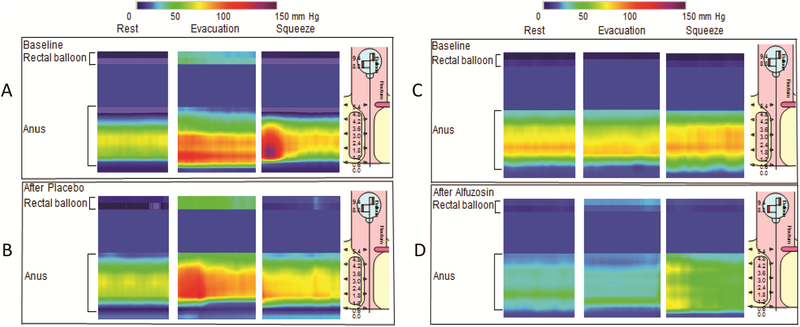
Figure 1. Drug effects on anal pressures in constipated patients.
Compared to rest, anal pressures were greater, during squeeze and to a greater extent, reflecting paradoxical contraction, during evacuation, before (A) and after placebo (B) in one patient. In another patient, alfuzosin reduced anal pressure at rest and during evacuation (C and D). In both patients, anal pressure increased during voluntary contraction (squeeze), before and after placebo and alfuzosin.
Figure 2. Drug effects on anal resting pressure (Panel A), anal pressure during simulated evacuation (Panel B), and rectoanal gradient (Panel C).
Compared to placebo, alfuzosin significantly decreased anal resting pressure and pressure during simulated evacuation.
Table 2.
Comparison of effects of alfuzosin and placebo on rectoanal pressures
| Healthy women | Constipated patients | |||
|---|---|---|---|---|
| Parameter | Placebo | Alfuzosin | Placebo | Alfuzosin |
| Anal resting pressure | −12 (−31, −4) | −32 (−43, −18)a | −21 (−29, −10) | −32 (−50, −17)a |
| Anal squeeze pressure | −31 (−47, 11) | −5 (−39, 4) | −29 (−51, 10) | −20 (−32, 3) |
| Anal squeeze increment | −3 (−37, 3) | 20 (6, 38) | 3 (−27, 22) | 6 (−3, 32) |
| Simulated evacuation - rectal pressure | 2 (−7, 5) | 2(−1, 11) | 0 (−10, 3) | 3 (−5, 11) |
| Simulated evacuation - anal pressure | −16 (−23, −8) | −29 (−37, −12) | −15 (−23, −3) | −28 (−43, −22)a |
| Simulated evacuation - anal relaxation | 9 (−3, 16) | −20 (−27, 19) | −7 (−20, 8) | −11 (−22, 5) |
| Simulated evacuation - rectoanal gradient | 19 (9, 28) | 26 (16, 40) | 16 (1, 34) | 27 (14, 47) |
| Rectal balloon expulsion time | 0 (−2, 0) | −1 (−5, 0) | 0 (−17, 0) | −2 (−13, 0) |
Values are Difference (After – Before Drug) in mmHg except for balloon expulsion time (seconds) and anal relaxation during simulated evacuation (%). Values are medians (interquartile range)
p ≤ 0.01 versus corresponding difference for placebo
Effects on Bowel Habits
Of the 31 patients who returned bowel diaries, 25 had less than 3 CSBMs per week at baseline, averaged over weeks 1 and 2. The remaining 6 patients had 7 (2 patients), 8 (2 patients), 9 (1 patient), and 14 (1 patient) CSBMs (Supplementary Figure 2). Among these patients, the proportion of all BMs that were CSBMs is 0.6 or less, which may explain their lack of satisfaction with defecation. Also, weekly diaries in these 6 patients suggested that they were bothered, in particular, by abdominal pain and/or bloating. To avoid confounding by other measures used to treat constipation, drug effects on stool form, consistency, and ease of passage were only evaluated for SBMs; these effects were not significant (Table 3). Drug effects on the number of CSBMs and laxative use were not significant (Table 3).
Table 3.
Effects of treatment on symptoms
| Placebo | Alfuzosin | |||
|---|---|---|---|---|
| Baseline (weeks 1 and 2) | Treatment period (weeks 3 and 4) | Baseline (weeks 1 and 2) | Treatment period (weeks 3 and 4) | |
| Daily bowel diary | ||||
| Spontaneous bowel movements per weeka | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Stool consistency (Bristol stool form score)a | 3.7 ± 0.4 | 3.4 ± 0.3 | 3.6 ± 0.3 | 3.4 ± 0.3 |
| Ease of passage (scale 1–7)a | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.1 |
| Incomplete evacuation (% bowel movements)a | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.05 | 0.7 ± 0.1 |
| Complete spontaneous bowel movements/weekb | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.05 | 0.7 ± 0.1 |
| Proportion of complete spontaneous bowel movements/weekb | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.03 | 0.1 ± 0.1 |
| Most severe abdominal pain (0–6)c | 2.9 ± 0.3 | 2.6 ± 0.3 | 3.0 ± 0.3 | 3.3 ± 0.4 |
| Overall severity of abdominal symptoms in 24 hrs. (0–6)c | 2.7 ± 0.3 | 2.4 ± 0.3 | 2.6 ± 0.3 | 2.8 ± 0.3 |
| Use of stool softeners or bulk laxatives, N (%) | 5 (2%) | 7 (3%) | 15 (8%) | 8 (4%) |
| Biweekly instrument# | ||||
| Satisfaction with treatment of constipation (0–4)e | 0.8 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.4 |
| Weekly instruments# | ||||
| Overall severity of constipation symptoms in the last 1 week (0–6)f | 3.1 ± 0.3 | 2.4 ± 0.3 | 3.0 ± 0.2 | 3.2 ± 0.3 |
| Overall relief of constipation symptoms in the last 1 week (0–6)g | 3.2 ± 0.3 | 2.4 ± 0.2 | 2.9 ± 0.2 | 2.8 ± 0.2 |
| Worst bloating in last 24 hrs (0–10)d | 5.4 ± 0.7 | 4.6 ± 0.8 | 5.4 ± 0.5 | 5.3 ± 0.7 |
| Worst abdominal pain in last 24 hrs (0–10)d | 3.7 ± 0.7 | 3.3 ± 0.7 | 4.8 ± 0.6 | 5.4 ± 0.7 |
| Worst nausea in last 24 hrs (0–10)d | 1.8± 0.7 | 1.1 ±0.5 | 1.7 ± 0.5 | 2.2 ± 0.7 |
| Worst vomiting in last 24 hrs (0–10)d | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.03 ± 0.03 | 0.4 ± 0.3 |
Values are mean ± SE
Spontaneous bowel movements only
Spontaneous bowel movements associated with satisfaction after defecation
Assessed using 7 point ordinal scale: 0=none, 1=very mild, 2=mild, 3=moderate, 4=moderately severe, 5=severe, 6=very severe
Assessed using 11 point ordinal scale: 0=none, 10=worse
Assessed using 5 point ordinal scale: 0=none, 1=a little bit, 2=moderately, 3=quite a bit, 4=extremely
Assessed using 7 point ordinal scale: 0=none, 1=very mild, 2=mild, 3=moderate, 4=moderately severe, 5=severe, 6=very severe
Assessed using 7 point ordinal scale: 0=completely relieved, 1=considerably relieved, 2=somewhat relieved, 3=unchanged, 4=somewhat worse, 5=considerably worse, 6=significantly worse
Relationship between Stool Form, Passage, and Satisfactory Evacuation
Prompted by the finding that 6 of 31 patients (19%) had 3 or more weekly CSBMs we explored the relationships among these bowel symptoms. Drug effects on stool form and ease of passage were correlated for alfuzosin (r=0.78, P=0.001) but not placebo (r= −0.02, P=0.9) (Supplementary Figure 3).
After excluding 31 bowel movements that were not spontaneous and 13 that were incontinent, 950 bowel movements were considered in the multivariable models that evaluated satisfaction after defecation (Table 4). Hard stools (i.e., BSFS of 1) were associated with increased odds of unsatisfactory defecation during the baseline (Model 1) and treatment periods (Table 4). Likewise, bowel movements with abnormal passage were associated with unsatisfactory defecation during the baseline (Model 2) and treatment periods (Model 5). Taken together, stool form and passage were associated with an area under the curve (AUC) respectively of 0.72 and 0.76 during the baseline (Model 3) and treatment periods (Model 6). In both these multivariable models, ease of passage remained significant after adjusting for stool form.
Table 4.
Multivariable models of the factors that predict incomplete evacuation
| Baseline period (2 weeks) | Treatment period (2 weeks) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Bristol stool form score | ||||||
| 1 (hard lumps) | 3.97 (1.6–9.8) | 2.08 (0.8–5.3) | 4.57 (1.2–18.1) | 2.95 (0.6–13.8) | ||
| 2 (lumpy sausage) | 1.62 (0.7–3.6) | 1.19 (0.6–2.3) | 1.46 (0.7–3) | 1.16 (0.54–2.5) | ||
| 3 (cracked sausage) | 1.4 (0.7–2.7) | 0.95 (0.5–1.7) | 1.05 (0.6–2) | 0.85 (0.49–1.5) | ||
| 4 (smooth sausage) | Reference group | Reference group | Reference group | Reference group | ||
| 5 (soft lumps) | 1.59 (0.9–2.8) | 1.63 (0.89–3) | 0.83 (0.5–1.3) | 0.77 (0.5–1.2) | ||
| 6 (mushy) | 0.83 (0.3–2.1) | 0.85 (0.4–2) | 0.63 (0.2–1.5) | 0.6 (0.2–1.5) | ||
| 7 (watery) | 0.87 (0.2–3.2) | 0.45 (0.1–1.4) | 0.87 (0.33–2.3) | 0.91 (0.3–2.7) | ||
| Stool passage a | ||||||
| Normal passage | Reference group | Reference group | Reference group | Reference group | ||
| Abnormal passage | 3.63 (2.4–5.6) | 3.13 (2–4.9) | 3.33 (1.7–6.4) | 2.56 (1.4–4.7) | ||
| C-statistic | 0.66 | 0.67 | 0.72 | 0.72 | 0.71 | 0.76 |
All values are means (95% CI)
Normal passage = “normal or urgency without pain”. Abnormal passage = Bowel movements that entailed manual disimpaction, straining, or urgency with pain. Bowel movements associated with fecal incontinence or required an enema were not considered in this analysis.
Adverse Effects
Among participants who received alfuzosin the mean BP was not different (P=0.3) after (83 ± 2 mmHg) versus before (85 ± 2 mmHg) drug administration. Likewise, the heart rate was not different (P=0.7) after (64 ± 2 mmHg) versus before alfuzosin (63 ± 2 mmHg).
Side effects included orthostatic hypotension in 1 patient treated with immediate-release and headache and fatigue in 1 patient treated with extended-release alfuzosin. One patient randomized to placebo reported abdominal cramps.
DISCUSSION
In this study, the α1-adrenergic antagonist, alfuzosin reduced anal resting pressure by 30–40% in healthy and constipated women probably by inhibiting the α1-adrenergic tonic excitatory input to the internal anal sphincter.13 This reduction is comparable to the effects of spinal anesthesia, which also inhibits tonic excitatory adrenergic input to the anal sphincter, and reduced anal resting pressure from an average of 72 mmHg to 40 mmHg in humans.12 In parallel with reduced anal resting pressure, alfuzosin also significantly reduced the anal pressure during evacuation. However, alfuzosin’s effects on the rectoanal gradient, CSBMs, other bowel symptoms, and satisfaction with bowel habits were not statistically significant.
Why did alfuzosin reduce anal pressures but not improve bowel symptoms? Peak plasma concentrations are higher (average of 20 ng/ml) for the alfuzosin immediate-release preparation (approximate average of 14 ng/ml) used in Part A than the extended-release formulation (10mg) used in Part B; however the area under the curve is comparable.14 Plasma alfuzosin concentrations are greater after meals (approximately 12 ng/ml) than under fasting conditions (approximately 4 ng/ml). Some patients may not have complied with the recommendation that alfuzosin should be taken with meals. Over time, compensatory mechanisms may restore anal resting pressure, as demonstrated after resection of the lumbar colonic and hypogastric nerves in dogs.25 Finally, alfuzosin does not improve other features of DD, i.e., dysfunction of voluntary muscles (i.e., external anal sphincter and puborectalis) and hard stools.
Biofeedback therapy reduced dysynergia, improved satisfaction with bowel habits, and reduced laxative use in patients with DD.26–28 However, even among patients with DD who were treated with biofeedback therapy provided by exceptionally skilled practitioners, the improvement in bowel symptoms was sustained in only 64% of patients.29 Two of 3 pivotal controlled trials of biofeedback therapy did not record CSBMs because patients were permitted to use stool softeners or milk of magnesia.27, 28 Another pivotal trial did not report CSBMs because daily bowel diaries were not maintained during the treatment period.26
Of 31 patients who completed bowel diaries, six, who had 3 or more CSBMs per week, would not be eligible to participate in most therapeutic trials for chronic constipation. However, among these patients, only 60% or less of all BMs were CSBMs, which may explain their lack of satisfaction. These data suggest that it may be worthwhile to consider not only the absolute number of CSBMs but also the proportion of BMs that are CSBMs. Germane to the assessment of CSBMs, it is important to recognize that stool form and size affect the ease of defecation 30, 31 and the satisfaction after defecation 31 in healthy and constipated patients. However, the factors that contribute to satisfaction after defecation are only partly understood. Indeed, in a community sample of people who were asymptomatic (53%), had diarrhea (26%) or constipation (21%), stool form, frequency, straining to begin and end defecation, and rectal urgency explained only 24% of the inter-subject variation in the satisfaction after defecation. By comparison, in this study, stool form and passage explained a larger proportion (i.e., over 70%) of the area under the curve for complete evacuation in patients with DD. Considered individually, both hard stools and ease of passage were associated with unsatisfactory defecation. However, in the combined models, only ease of passage remained significant, which suggests that hard stools are harder to evacuate, predisposing to less satisfaction after defecation. Consistent with this hypothesis, patients treated with alfuzosin reported that it was easier to pass softer stools. Some DD patients may have increased rectal sensation related to IBS,32 which may also partly explain the reduced satisfaction after defecation. Supporting this explanation, constipated subjects complained of unsatisfactory defecation not only with hard but also with soft, formed stools.31
Both hard stools and DD are associated with slow colon transit.33,34, 35 Hence, controlled trials in DD should consider treating all patients with scheduled laxatives or secretagogues, supplemented with biofeedback therapy or another approach targeted to toward pelvic floor dysfunction. For such trials, only bowel movements that are preceded by as needed laxative(s) over and above regularly prescribed agents should be considered as non-CSBMs.
There were several strengths of this placebo-controlled study,. To our knowledge, this is the first randomized-controlled trial of an orally-administered drug in patients with idiopathic DD. Correspondingly, there were limitations. Men were not studied. In 6% of patients, research anorectal tests did not confirm the prior diagnosis of DD, which may reflect intra-individual variability in anorectal tests. In order to simplify study procedures, the baseline bowel diary was not preceded by a run-in period. Six of 31 patients had 3 or more CSBMs during the baseline period. The treatment phase only lasted 2 weeks.
In summary, alfuzosin was safe and well tolerated and reduced anal pressure at rest and during simulated evacuation in healthy and constipated women. Although alfuzosin did not significantly improve bowel habits compared to placebo, there may be a role for this agent when biofeedback therapy is unavailable or ineffective, especially in patients who have a DD and dysfunctional voiding.36 Future studies should evaluate the effects of alfuzosin over a longer duration and as an adjunct to biofeedback therapy or medications in DD, particularly in patients with high anal resting pressures.
Supplementary Material
What you need to know:
Background:
High anal pressures impede rectal evacuation in some patients with defecatory disorders. Alpha-1 adrenoreceptors mediate as much as 50% of anal resting pressure.
Findings:
When compared to placebo, Alfuzosin, an alpha adrenergic antagonist, significantly reduced anal resting pressure and anal pressure during evacuation, in healthy and constipated women, but not bowel habits in constipated women.
Implications for patient care:
Alfuzosin is safe and well tolerated. Further studies evaluating the role of alfuzosin in combination with biofeedback treatment and/or laxatives are necessary.
Acknowledgments
Financial Support: The project described was supported by USPHS NIH Grant R01 DK78924 and Grant Number 1 UL1 RR024150* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Competing Interests: none
REFERENCES
- 1.Noelting J, Eaton J, Choung RS, et al. The Incidence Rate and Characteristics of Clinically Diagnosed Defecatory Disorders in the Community. Neurogastroenterology and Motility 2016;28:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao S, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology 2016;150:1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database of Systematic Reviews 2014;3:CD008486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Rao SSC, Shin AS. Surgical Interventions and the Use of Device-Aided Therapy for the Treatment of Fecal Incontinence and Defecatory Disorders. Clinical Gastroenterology & Hepatology 2017;15:1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neshatian L The assessment and management of defecatory dysfunction: a critical appraisal. Current Opinion in Gastroenterology 2018;34:31–37. [DOI] [PubMed] [Google Scholar]
- 6.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterology & Motility 2006;18:507–19. [DOI] [PubMed] [Google Scholar]
- 7.Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Digestive Diseases & Sciences 2012;57:1445–64. [DOI] [PubMed] [Google Scholar]
- 8.Bharucha AE, Pemberton JH, Locke GR, 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinning PG, Bampton PA, Andre J, et al. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. 2004;127:49–56. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology 2005;128:1199–1210. [DOI] [PubMed] [Google Scholar]
- 11.Staller K, Barshop K, Kuo B, et al. Resting anal pressure, not outlet obstruction or transit, predicts healthcare utilization in chronic constipation: a retrospective cohort analysis. Neurogastroenterology & Motility 2015;27:1378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenckner B, Ihre T. Influence of autonomic nerves on the internal and sphincter in man. Gut 1976;17:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobine CA, Fong M, Hamilton R, et al. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterology & Motility 2007;19:937–45. [DOI] [PubMed] [Google Scholar]
- 14.Roehrborn CG. Alfuzosin: overview of pharmacokinetics, safety, and efficacy of a clinically uroselective alpha-blocker. Urology 2001;58:55–63; discussion 63–4. [DOI] [PubMed] [Google Scholar]
- 15.Pitt J, Dawson PM, Hallan RI, et al. A double-blind randomized placebo-controlled trial of oral indoramin to treat chronic anal fissure. Colorectal Disease 2001;3:165–8. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 17.Bharucha AE, Locke GR, Seide B, et al. A New Questionnaire for Constipation and Fecal Incontinence. Alimentary Pharmacology & Therapeutics. 2004;20:355–364. [DOI] [PubMed] [Google Scholar]
- 18.Noelting N, Eaton J, Thapa P, et al. Incidence Rate and Characteristics of Clinically Diagnosed Defecatory Disorders in the Community. Gastroenterology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratuapli S, Bharucha AE, Harvey D, et al. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterology and Motility 2013;25:e813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal Values For High-Resolution Anorectal Manometry In Healthy Women: Effects Of Age And Significance Of Rectoanal Gradient. American Journal of Gastroenterology 2012;107:1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratuapli S, Bharucha AE, Noelting J, et al. Phenotypic Identification and Classification of Functional Defecatory Disorders Using High Resolution Anorectal Manometry Gastroenterology 2013;144:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tirumanisetty P, Prichard D, Fletcher JG, et al. Normal values for assessment of anal sphincter morphology, anorectal motion, and pelvic organ prolapse with MRI in healthy women. Neurogastroenterology & Motility 2018;02:02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salva P, Bianchetti G, Morselli P, et al. Pharmacokinetics of alfuzosin after single oral administration to healthy volunteers, of three different doses. Biopharmaceutics & Drug Disposition 1992;13:583–90. [DOI] [PubMed] [Google Scholar]
- 24.Zinsmeister A, Burton D, Camilleri M. Pharmacodynamic and clinical endpoints for functional colonic disorders: statistical considerations. Digestive Diseases and Sciences 2012;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizutani M, Neya T, Ono K, et al. Histochemical study of the lumbar colonic nerve supply to the internal anal sphincter and its physiological role in dogs. Brain Res 1992;598:45–50. [DOI] [PubMed] [Google Scholar]
- 26.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia.[see comment]. Gastroenterology 2006;130:657–64. [DOI] [PubMed] [Google Scholar]
- 27.Rao SSC, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clinical Gastroenterology & Hepatology 2007;5:331–8. [DOI] [PubMed] [Google Scholar]
- 28.Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternative treatments for fecal incontinence. Diseases of the Colon & Rectum 2009;52:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao SSC, Valestin J, Brown CK, et al. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. American Journal of Gastroenterology 2010;105:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannister JJ, Davison PA, Timms JA, et al. Effect of stool size and consistency on defecation. Gut 1987;28:1246–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharucha AE, Seide BM, Zinsmeister AR, et al. Insights into normal and disordered bowel habits from bowel diaries. American Journal of Gastroenterology 2008;103:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corsetti M, Cesana B, Bhoori S, et al. Rectal Hypersensitivity to Distention in Patients with Irritable Bowel Syndrome: Role of Distention Rate. Clinical Gastroenterology and Hepatology 2004;2:49–56. [DOI] [PubMed] [Google Scholar]
- 33.Saad RJ, Rao SS, Koch KL, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. The American journal of gastroenterology 2010;105:403–11. [DOI] [PubMed] [Google Scholar]
- 34.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterology & Motility 2004;16:589–96. [DOI] [PubMed] [Google Scholar]
- 35.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic Variation Of Colonic Motor Functions In Chronic Constipation. Gastroenterology 2010;138:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klingele CJ, Lightner DJ, Fletcher JG, et al. Dysfunctional urinary voiding in women with functional defecatory disorders. Neurogastroenterology and Motility 2010. 22:1094–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.