Abstract
Aims
Human papilloma virus (HPV) is the cause of different types of carcinoma. Despite the remarkable effectiveness of the HPV vaccines, there have been many complaints about their risk–benefit profile due to adverse events following immunization (AEFI). The purpose of this study is to analyse the safety profile of the HPV vaccine basing on real‐life data derived from reports of suspected AEFIs collected in the US Vaccine Adverse Events Reporting System (VAERS) and assess if the searches on Google overlap with spontaneous reporting.
Methods
We collected all the reports in VAERS between January 2007 to December 2017 related to the HPV vaccines. A disproportionality analysis using reporting odds ratio (ROR) with 95% confidence interval was performed.
Results
Over the 10‐year period, 55 356 reports of AEFI related to HPV vaccines were retrieved in VAERS, corresponding to 224 863 vaccine‐event pairs. The highest number of reports was related to Gardasil (n = 42 244). The two events more frequently reported and statistically significant for HPV vaccines were dizziness (n = 6259; ROR = 2.60; 95% confidence interval 2.53–2.66) and syncope (n = 6004; ROR = 6.28; 95% confidence interval 6.12–6.44). The trends of spontaneous reporting and Google searches overlap.
Conclusion
The AEFI analysis showed that the events most frequently reported were non‐serious and listed in the corresponding summary of product characteristics. Potential safety signals arose regarding less frequent AEFIs that would deserve further investigation. It is extremely important to disseminate correct and evidence‐based scientific information.
Keywords: adverse events following immunization, human papilloma virus, safety, vaccine, vaccine adverse event reporting system, vaccinovigilance
What is Already Known about this Subject
Human papilloma virus (HPV) prevalence in cervical cancer, the third most common cancer in women, is 99.7% worldwide. HPV 16 and 18 are the primary cause of 70% of all cervical cancers.
The safety profile of HPV vaccines has been proven to be good although there have been numerous controversies regarding their adverse events following immunization (AEFI) especially for postural orthostatic tachycardia syndrome and complex regional pain syndrome.
Currently, the risk–benefit profile for HPV vaccines remains favourable.
What this Study Adds
The reports of AEFIs collected showed that HPV vaccines are also used in ages other than those for which they are indicated.
The analysis of the safety profile of HPV vaccines confirms the AEFIs listed in the summaries of product characteristics, also highlighting possible new signals suxh as alopecia, hyperacusis and parosmia.
The evaluation of the results of Google searches and HPV vaccine spontaneous reporting trends shows that the two variables overlapped and there is a possible relationship between the web searches and the attitude towards spontaneous reporting for HPV vaccine related adverse events.
Introduction
Vaccines are among the greatest public health achievements, as they allow the eradication and/or prevention of many serious and lethal diseases. Human papilloma virus (HPV) vaccines are considered so important by the World Health Organization that are recommended to be included in national vaccination programmes 1. HPV vaccine was first marketed in the USA in 2006 (HPV4), followed by Italy, which recommended vaccination between 2007–2008 2.
It is estimated that HPV prevalence in cervical cancer, the third most common cancer in women, is 99.7% worldwide 3, 4. HPV is also one of the most common sexually transmitted infections 5. In Italy, it is estimated that in 2012 there were 1515 new cases of cervical carcinoma and 697 deaths 6. HPV 16 and 18 are the primary cause of 70% of all cervical cancers worldwide 7, and HPV 6 and 11 are present in over 90% of all anogenital warts 8. In recent years, both the number of new cases and the number of deaths from cervical cancer have been reduced. The standardized incidence rate in Italy decreased from 14 per 100 000 women in 1980 to 4 per 100 000 in 2012; and the standardized mortality rate from 7 per 100 000 women in 1980 to about 2 per 100 000 in 2012 9. This was possible due to the combined action of the early screening through the Papanicolaou test and prophylactic vaccination with HPV vaccines. Three HPV vaccines are now available: Cervarix (HPV 16 and 18), Gardasil (HPV 6, 11, 16, 18) and Gardasil 9 (HPV 6, 11, 16, 18, 31, 33, 45, 52, 58). Several studies have emphasized the efficacy and safety of these vaccines 10, 11, 12, 13, 14, 15. However, their safety profiles have been debated due to the growth of antivaccine movements, and there have been numerous controversies regarding their adverse events following immunization (AEFI). A systematic review investigating the perceived risk of vaccines in Europe 16 underlined the high number of safety concerns about HPV vaccination: in 29 articles analysed, the most common concerns were about the safety. On 26 May 2016, C. Gøtzsche and others of the Nordic Cochrane Centre, made a complaint over how the European Medicines Agency handled the safety assessment of HPV vaccines 17. After that, in July 2015, a referral procedure was started by European Medicines Agency 18 to better clarify the safety profile of these vaccines. In November 2015, the Committee for Medicinal Products for Human Use stated that the benefit–risk profile of HPV vaccines remains favourable and therefore recommends the maintenance of the marketing authorizations 19. The aim of this research was to contribute to the ongoing discussion of the safety profile of HPV vaccines basing on real‐life data derived from spontaneous reports of suspected AEFIs in the Vaccine Adverse Events Reporting System (VAERS). Furthermore, we intended to analyse the potential relationship of HPV vaccine Google searches on the trend of related spontaneous reporting of adverse drug reactions.
Methods
Study population and design
Data were retrieved in VAERS, the US vaccine safety surveillance database of AEFI created in 1990, co‐administered by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration 20. This system does not allow for establishing a causal association between the vaccine and the reported AEFI, but allows the detection of unusual or unexpected patterns of adverse event reporting. The VAERS reports include information age, sex, state/territory, vaccine characteristics, description and other information captured in VAERS include about the event (e.g. laboratory test, onset date, outcome) and the patient (medical history and concomitant therapies). Vaccines are reported in VAERS according to type and name, manufacturer, route of administration and other information, if available, as batch number or if the vaccine is a booster dose. Symptoms were coded using the Medical Dictionary for Regulatory Activities, which provides highly specific standardized medical terminology that facilitates international sharing of regulatory information 21. One or more symptoms can be reported for each VAERS report. Reports are also classified by the seriousness criteria of the Code of Federal Regulations 22. For the purpose of the present research, we analysed VAERS reports received from 01 January 2007 to 31 December 2017 (11 years) related to HPV vaccines. We first analysed the data using the CDC Wonder online computer interface 23. This database assists in the analysis of public health data through ad hoc queries. We collected all the reports related to HPV vaccines: HPV 2 (Cervarix), HPV 4 (Gardasil), HPV 9 (Gardasil 9) and HPV X (HPV vaccine not specified) in the last 10 years without filter for age, sex, seriousness, reporter or state/territory in order to have a complete view of all reported adverse events for these vaccines regardless of other factors. To allow an in‐depth analysis also considering the possible off‐label uses and related side effects, we also considered the reports concerning age ranges other than those for which the vaccines are indicated.
Data mining
We analysed all the reports related to HPV vaccines, first pooled and then separated by single vaccine. We categorized the data by vaccine type, age, sex, seriousness, onset interval (i.e. number of days from the time of vaccination to the time of the reported symptoms) and year of reporting. A comparison analysis was performed as follows: (i) all the HPV vaccine vs. other vaccines in the VAERS database; and (ii) each single HPV vaccine vs. others HPV vaccines of the database. The analysis was performed using the reporting odds ratio (ROR) with 95% confidence interval (CI) and P value ≤ 0.05, as statistical parameter to evaluate vaccine–event pairs distribution. This is a quantitative approach based on frequency analysis of 2 × 2 contingency table, developed for evaluating vaccine–reaction frequency compared to reference distributions of other vaccines from the whole database. If ROR < 1, it is assumed that there is no disproportionality and the distribution of the events following immunization is the same across vaccines; conversely, if ROR is >1 there is an increased frequency for the vaccine–event pair considered. For the most frequent events, it was evaluated whether or not they were listed in the summary of product characteristics (SmPCs) of the corresponding vaccine. Lastly, we reviewed all the death reports per year and per vaccine that included at least minimal identifying information for the patient (age and sex). All the reports based on indirect information (e.g. heard on TV/read in newspapers) were not considered in the analysis of deaths.
Google trend analysis
A second analysis evaluated the possible relationship between online searches and the number of spontaneous AEFIs reports. For this purpose, we used Google Trends 24, an online tracking tool that shows how often a search‐term is entered compared to the total search volume across various regions of the world. The analysis on Google Trends can be done by term or topic (i.e. a group of terms that share the same concept). The relative search volume is the query share of a particular term or topic normalized by the highest query share of that term/topic over the time series and presented on a scale from 0 to 100. Each point generated by Google Trends is divided by the highest point, which is conventionally set at 100.
For our aim, we searched the topic “human papilloma virus vaccine” in the same period analysed for spontaneous AEFIs reports in VAERS (2007–2017) in the USA, as almost all the reports were from USA.
We descriptively analysed the changes in web search queries during the study period and compared them to the number of reports per year.
Results
Descriptive analysis
During the study period, we retrieved a total number of 55 356 individual case safety reports (ICSRs) referred to HPV vaccines (corresponding to 224 863 drug‐reaction pairs) in the VAERS database: 77.1% concerned HPV 4 – Gardasil; 13.1% HPV 9 – Gardasil9; 7.1% HPV 2 – Cervarix; and for 2.8% the vaccine type was unknown (HPVX). Figure 1 shows the number of reports per year and the year of approval of the three vaccines in the USA. The number of reports concerning females was significantly higher than that for males (71.7% vs. 10.7%). Table 1 shows reports classified by age, sex, seriousness and fatal outcome. Almost 50% of the reports concerned females aged 6–17 years. Very few reports were related to age groups ≤5 years and ≥60 years (0.6% overall). The onset of AEFI ranged from day 0 (i.e. vaccination day) in 40.4% of the reports, up to over 120 days after the vaccination. Serious AEFIs were 7873 out of 55 356, (12.2%), and 406 (0.73%) had a fatal outcome.
Figure 1.
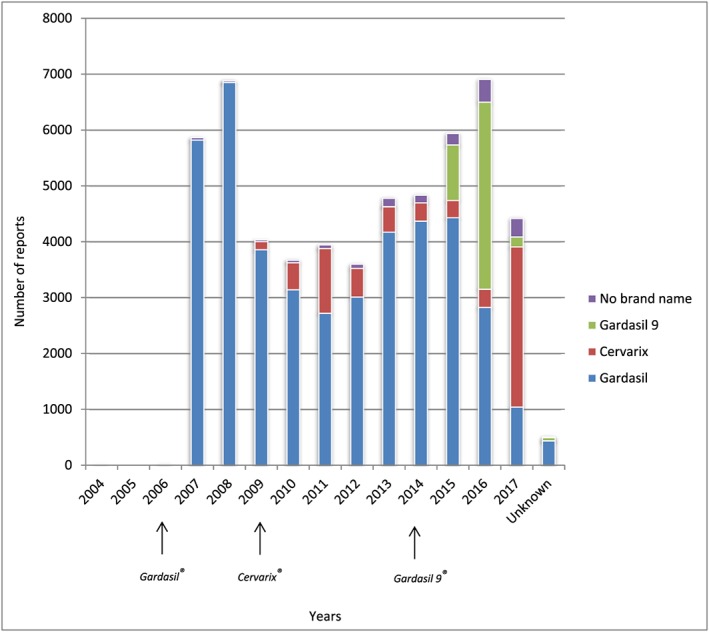
Number of reports per vaccine and per year and year of approval of the vaccines
Table 1.
Characteristics of the human papilloma virus vaccine reports retrieved in the Vaccine Adverse Events Reporting System
| Age group | Events Reported | Serious | Death | ||
|---|---|---|---|---|---|
| <6 months | 101 (0.18%) | F | 39 (0.07%) | 8 | 1 |
| M | 32 (0.06%) | 5 | 0 | ||
| U | 30 (0.05%) | 2 | 2 | ||
| 6 months–5 years | 203 (0.37%) | F | 77 (0.14%) | 4 | 0 |
| M | 56 (0.10%) | 3 | 0 | ||
| U | 70 (0.13%) | 0 | 0 | ||
| 6–17 years | 27 455 (49.60%) | F | 22 020 (39.78%) | 4489 | 105 |
| M | 4349 (7.86%) | 256 | 11 | ||
| U | 1086 (1.96%) | 20 | 4 | ||
| 18–29 years | 11 118 (20.08%) | F | 10 170 (18.37%) | 1525 | 37 |
| M | 743 (1.34%) | 64 | 4 | ||
| U | 205 (0.37%) | 5 | 2 | ||
| 30–59 years | 869 (1.57%) | F | 766 (1.38%) | 216 | 7 |
| M | 84 (0.15%) | 15 | 0 | ||
| U | 19 (0.03%) | 1 | 0 | ||
| ≥ 60 years | 45 (0.08%) | F | 26 (0.05%) | 0 | 0 |
| M | 14 (0.03%) | 0 | 0 | ||
| U | 5 (0.01%) | 0 | 0 | ||
| Unknown | 15 565 (28.12%) | F | 6593 (11.91%) | 1073 | 153 |
| M | 645 (1.17%) | 38 | 5 | ||
| U | 8327 (15.04%) | 149 | 75 | ||
| Total | 55 356 (100%) | 7873 (14.22%) | 406 (0.73%) | ||
F = female; M = male; U = unknown
The percentages have been calculated out of the total events reported (55 356)
Serious events reported = 7873; not serious events reported = 47 483; total = 55 356
death = 406; not death = 54 950; total = 55 356
Multiple events can be found in a single Vaccine Adverse Events Reporting System report
Disproportionality analysis
The analysis was performed on 224 863 drug–reaction pairs related to HPV vaccines. The events consisting of incorrect vaccine storage, routinely laboratory tests or incorrect administration were not considered because not pertinent to our discussion of the AEFIs. First, we analysed all the HPV vaccine together (HPV2, HPV4, HPV9, HPVX) vs. other vaccines in VAERS. The AEFIs most frequently reported and statistically significant for HPV vaccines were nonserious and listed in the corresponding SmPCs: dizziness (6259 reports), syncope (6004), headache (5562), nausea (4307) and fatigue (3212). HPV vaccine–syncope showed an ROR = 6.28 (95% CI 6.12–6.44). Table 2 shows the top 25 AEFIs for HPV vaccines.
Table 2.
Most‐reported adverse drug reactions for human papilloma virus vaccines compared to other vaccines in Vaccine Adverse Events Reporting System reports
| Events | No. of reports | ROR | SD | 95%CI |
|---|---|---|---|---|
| Dizziness | 6259 | 2.60 | 0.01 | 2.53–2.66 |
| Syncope | 6004 | 6.28 | 0.01 | 6.12–6.44 |
| Headache | 5562 | 1.62 | 0.01 | 1.58–1.67 |
| Nausea | 4307 | 1.74 | 0.02 | 1.69–1.80 |
| Fatigue | 3212 | 1.59 | 0.02 | 1.53–1.64 |
| Loss of consciousness | 3060 | 3.97 | 0.02 | 3.83–4.11 |
| Pallor | 2212 | 2.07 | 0.02 | 1.98–2.15 |
| Malaise | 2018 | 1.43 | 0.02 | 1.37–1.50 |
| Arthralgia | 1900 | 1.50 | 0.02 | 1.43–1.57 |
| Asthenia | 1894 | 1.34 | 0.02 | 1.28–1.40 |
| Hypoaesthesia | 1670 | 1.65 | 0.02 | 1.58–1.74 |
| Convulsion | 1660 | 2.31 | 0.02 | 2.20–2.42 |
| Fall | 1570 | 3.72 | 0.03 | 3.54–3.91 |
| Immediate postinjection reaction | 1568 | 1.87 | 0.03 | 1.78–1.96 |
| Paraesthesia | 1457 | 1.26 | 0.03 | 1.20–1.33 |
| Abdominal pain | 1351 | 2.89 | 0.03 | 2.74–3.05 |
| Tremor | 1183 | 1.56 | 0.03 | 1.48–1.66 |
| Muscular weakness | 1175 | 1.74 | 0.03 | 1.64–1.84 |
| Presyncope | 1013 | 6.50 | 0.03 | 6.10–6.92 |
| Hyperhidrosis | 992 | 1.29 | 0.03 | 1.21–1.38 |
| Activities of daily living impaired | 897 | 2.18 | 0.03 | 2.04–2.33 |
| Abdominal pain upper | 868 | 2.18 | 0.03 | 2.04–2.34 |
| Feeling abnormal | 823 | 1.71 | 0.04 | 1.60–1.83 |
| Back pain | 808 | 1.49 | 0.04 | 1.39–1.60 |
| Gait disturbance | 727 | 1.48 | 0.04 | 1.37–1.59 |
CI, confidence interval; ROR, reporting odds ratio; SD, standard deviation
Among the vaccine–reaction pairs with higher and statistically significant ROR vs. other vaccines we observed a number of reactions already investigated by the regulatory authorities 18, 19 such as postural orthostatic tachycardia syndrome (ROR = 44.02, 95% CI 37.88–51.15) and chronic fatigue syndrome (ROR = 9.19, 95% CI 7.77–10.86). Other reactions with high disproportionality were alopecia (ROR = 10.40, 95% CI 9.45–11.43), systemic lupus erythematosus (ROR = 7.24, 95% CI 6.13–8.54), hyperacusis (ROR = 7.13, 95% CI 6.10–8.33) and thrombosis (ROR = 6.44, 95% CI 5.24–7.91). Many events were related to epilepsy (n = 278, ROR = 3.53, 95% CI 3.13–3.99) and the associated seizure (n = 511, ROR = 2.09, CI 95% 1.91–2.28) and cognitive disorders (n = 293, ROR = 5.20, 95% CI 4.62–5.87). We then analysed each HPV vaccine vs the other HPV vaccines.
The most reported HPV vaccine was Gardasil, which resulted in statistical significance in 272 out of 2735 vaccine–reaction pairs (9.95%). Table 3 shows the 25 most reported and statistically significant AEFIs for Gardasil, most of which str already listed in the SmPC of the vaccine. A high number of reports were retrieved for alopecia (n = 420, ROR = 1.90, 95%CI 1.68–2.15) and gaze palsy (n = 313, ROR = 2.05 95%CI 1.76–2.39). A disproportionality was detected also for thrombosis (n = 103, ROR = 4.53, 95%CI 2.82–7.27) and abdominal distension (n = 117, ROR = 4.50 95% CI 2.94–6.89).
Table 3.
Most‐reported adverse drug reactions for each human papilloma virus vaccine and corresponding RORs
| Gardasil | Cervarix | Gardasil9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | n | ROR | 95% CI | Events | n | ROR | 95% CI | Events | n | ROR | 95% CI |
| Dizziness | 5053 | 1.28 | 1.24–1.31 | Headache | 672 | 1.14 | 1.06–1.23 | Injection site pain | 305 | 1.40 | 1.25–1.57 |
| Syncope | 4808 | 1.21 | 1.17–1.25 | Loss of consciousness | 528 | 1.72 | 1.57–1.87 | Injection site erythema | 297 | 1.88 | 1.67–2.11 |
| Nausea | 3448 | 1.25 | 1.21–1.30 | Malaise | 465 | 2.53 | 2.31–2.77 | Injection site swelling | 257 | 1.82 | 1.61–2.06 |
| Fatigue | 2521 | 1.18 | 1.13–1.23 | Pyrexia | 398 | 1.15 | 1.04–1.27 | Pallor | 234 | 1.16 | 1.02–1.33 |
| Injection site pain | 1931 | 1.07 | 1.02–1.12 | Pallor | 396 | 1.78 | 1.61–1.96 | Erythema | 178 | 1.28 | 1.11–1.49 |
| Vomiting | 1881 | 1.22 | 1.16–1.29 | Presyncope | 380 | 4.99 | 4.50–5.53 | Injection site warmth | 136 | 2.29 | 1.93–2.71 |
| Rash | 1501 | 1.52 | 1.44–1.61 | Pain | 354 | 1.31 | 1.18–1.46 | Peripheral swelling | 111 | 7.13 | 5.84–8.69 |
| Injection site erythema | 1494 | 1.18 | 1.11–1.24 | Fall | 311 | 2.02 | 1.80–2.26 | Seizure | 108 | 2.76 | 2.28–3.35 |
| Urticaria | 1463 | 1.60 | 1.51–1.69 | Arthralgia | 282 | 1.46 | 1.29–1.64 | Hyperhidrosis | 108 | 1.21 | 1.00–1.47 |
| Convulsion | 1442 | 1.90 | 1.79–2.02 | Asthenia | 277 | 1.42 | 1.26–1.61 | Head injury | 94 | 1.57 | 1.28–1.93 |
| Injection site swelling | 1300 | 1.08 | 1.02–1.15 | Hypoaesthesia | 246 | 1.43 | 1.26–1.63 | Chills | 82 | 1.31 | 1.05–1.63 |
| Erythema | 1293 | 1.48 | 1.39–1.57 | Muscular weakness | 208 | 1.81 | 1.58–2.08 | Skin warm | 77 | 1.86 | 1.48–2.33 |
| Dyspnoea | 1253 | 1.32 | 1.24–1.41 | Feeling abnormal | 195 | 2.57 | 2.22–2.96 | Injection site pruritus | 64 | 1.57 | 1.22–2.01 |
| Immediate post‐injection reaction | 1248 | 1.14 | 1.07–1.21 | Gait disturbance | 192 | 3.02 | 2.61–3.49 | Unresponsive to stimuli | 61 | 1.48 | 1.15–1.92 |
| Paraesthesia | 1240 | 1.81 | 1.70–1.94 | Tremor | 152 | 1.21 | 1.03–1.42 | Eye movement disorder | 57 | 4.16 | 3.16–5.47 |
| Abdominal pain | 1081 | 1.32 | 1.23–1.41 | Blood pressure decreased | 142 | 4.21 | 3.55–5.00 | Injection site induration | 55 | 1.55 | 1.19–2.04 |
| Myalgia | 1050 | 1.15 | 1.07–1.23 | Memory impairment | 126 | 3.38 | 2.82–4.05 | Muscle twitching | 44 | 1.43 | 1.06–1.93 |
| Pruritus | 924 | 1.70 | 1.57–1.83 | Sleep disorder | 120 | 3.54 | 2.94–4.27 | Injection site reaction | 41 | 1.61 | 1.18–2.20 |
| Activities of daily living impaired | 711 | 1.15 | 1.05–1.25 | Photophobia | 107 | 2.46 | 2.02–2.99 | Posture abnormal | 39 | 3.09 | 2.22–4.29 |
| Abdominal pain upper | 694 | 1.28 | 1.17–1.39 | Dyskinesia | 105 | 1.45 | 1.19–1.76 | Seizure like phenomena | 39 | 2.68 | 1.93–3.72 |
| Oedema peripheral | 683 | 2.60 | 2.35–2.87 | Depressed level of consciousness | 104 | 8.01 | 6.49–9.88 | Injection site mass | 38 | 1.49 | 1.07–2.06 |
| Back pain | 633 | 1.17 | 1.07–1.28 | Post vaccination syndrome | 102 | 280.23 | 120.08–654.00 | Cellulitis | 34 | 2.71 | 1.91–3.86 |
| Diarrhoea | 617 | 1.16 | 1.06–1.27 | Seizure | 101 | 2.09 | 1.71–2.55 | Injection site nodule | 27 | 3.29 | 2.20–4.91 |
| Chest pain | 598 | 1.56 | 1.42–1.72 | Somnolence | 98 | 1.87 | 1.52–2.28 | Injection site urticaria | 23 | 1.65 | 1.08–2.52 |
| Smear cervix abnormal | 593 | 4.57 | 4.02–5.20 | Visual impairment | 86 | 1.47 | 1.18–1.82 | Vaccination site swelling | 18 | 3.90 | 2.36–6.47 |
CI = confidence interval; ROR = reporting odds ratio
In comparison, Gardasil9 resulted in statistical significance in 92 out of 1277 vaccine‐reaction pairs compared to others HPV vaccines in VAERS. The top 25 most reported and significant AEFIs, shown in Table 3, were mainly related to the injection site (injection site pain, erythema, swelling, warmth, pruritus, induration, reaction, mass, nodule and urticaria). Most of the AEFIs were listed in the SmPC of Gardasil9. Peripheral swelling was reported seven times more for HPV9 (ROR = 7.13, 95% CI 5.84–8.69) than for the other HPV vaccines. Disproportionality was also observed for eye movement disorder (ROR = 4.16, 95% CI 3.16–5.47) and for the events related to seizure disorders (seizure, generalized tonic–clonic seizure and seizure‐like phenomena).
Disproportionalities also emerged for Cervarix that were statistically significant for 397 vaccine‐reaction pairs out of 2229. For this vaccine, the most reported events were headache (672 reports), loss of consciousness (528), malaise (465), pyrexia (398) and pallor (396). Table 3 shows the 25 more reported AEFIs for HPV2. Several AEFIs related to this vaccine and not listed in the SmPC showed a disproportionality compared to other HPV vaccines as shock (n = 57, ROR = 11.72, 95% CI 8,61–15.96), parosmia (15, ROR = 6.16, 95% CI 3.37–11.27), peripheral neuropathy (63, ROR = 5.76, 95% CI 4.40–7.53) and complex regional pain syndrome (43, ROR = 5.44, 95% CI 3.91–7.56).
Analysis of death reports
We selected the reports in VAERS that were related to HPV vaccines and reporting death as final outcome (406 reports). Most reports contained unverifiable information or unanswered follow‐up requests. For this reason, we then considered for the analysis only the reports that described the age and sex of the patient (167 reports), thus excluding those without useful information and probably unfounded. Of the discarded reports, most were related to information read on the web, reported by newspapers or by hearsay. Of the 167 reports included in the analysis, 130 (77.8%) concerned Gardasil and 151 out of 167 (90.4%) referred to females, especially females aged 6–29 years (94.7%). Almost all reports included other concomitant medications, vaccines or comorbidities in addition to HPV vaccines. The great majority of the reports presented other causes of death or did not have a well‐specified cause; in most cases, the causal relationship with the vaccine was excluded.
Google trends analysis
Figure 2 shows the trend of the number of reports per year and, at the same time, the volume of searches carried out on Google for the topic “human papilloma virus vaccine”. This tool also allowed detection of the related queries about the topic; one of the most inquired was about the side effects of the HPV vaccines. The interest for the topic was high in 2006, the year of Gardasil marketing in the USA. Then, the trend decreased until 2010; a further peak was recorded between 2010 and 2011. From 2011 to 2012 there has been a further decline in searches, followed by an increase in 2013 (third peak) and a further decrease between 2013 and 2014. From 2014 to 2016, the volume of searches increased again reaching the fourth peak in 2016. In parallel, analysing the number of reports received over time by VAERS, we see that the trend is overlapping, except between 2006–2007 where the number of HPV reports increased while the searches declined, and in 2008 where reports reached a peak while searches remained quite low. This analysis allowed highlighting of how there is a relationship between the volume of google searches and the number of reports of AEFIs retrieved in VAERS. The decline in interest in HPV vaccines between 2007 and 2010 is matched with the decrease in the number of reports of AEFIs. Between 2010 and 2011 both trends increased and both fell and then rose again in 2012 and 2013, respectively. In general, as the volume of searches on Google increases, the number of reports increases as well and vice versa, as can be finally observed in the period between 2014–2016.
Figure 2.
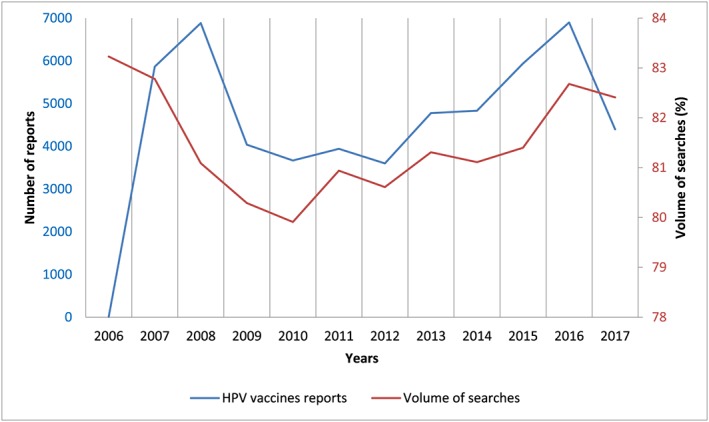
Number of reports per year and volume of searches of the “human papilloma virus vaccine” topic on Google Trends
Discussion
The monitoring of vaccine safety, as well as drugs, starts from premarketing studies and continues throughout the life cycle of the vaccine after marketing and, in case of withdrawal, even a few years after this. This emphasizes the systems in place to ensure patient safety.
Overall analysis
Overall, our analysis shows that the benefit–risk profile of HPV vaccines is largely favourable given the high number of cancer cases prevented against the above small rate of possible serious AEFIs. Based on National Vaccine Injury Compensation Program statistics reports 25, between 2006 and 2016, 101 405 935 doses of HPV vaccines were distributed in the USA. During the same period, 2513 serious reports were retrieved in VAERS, corresponding to a rate of two reports per 100 000 doses. The first vaccine marketed was Gardasil in 2006, which is also the most reported vaccine. All the HPV vaccine are indicated in girls and women ages 9–25 or 26 years 26, 27, 28, the age class that represented the majority of reports in VAERS (n = 38 573, 69.7%). Initially, vaccines were indicated for the female population and only afterwards the indication was also extended to males. The reports relating to male patients represent only 10.7% of total reports. The analysis of the fatal reports provided reassuring information: in all cases where the cause of death was understood, it was independent from the vaccine and none demonstrated certainty of causal association between vaccine administration and death.
Vaccine evaluation
Regarding the safety of these vaccines, our data are in line with those reported in the SmPCs of the vaccines and with other postmarketing studies available in the literature 29, 30. From our analysis, noteworthy was the association between HPV vaccines and syncope (ROR = 6.28), and with related events with a high ROR as loss of consciousness, fall and presyncope. These vaccine–reaction pairs have been already highlighted in other articles 31, 32 and investigated by the CDC 33. The CDC pointed out that more than half of the syncope reports concerned adolescents and were mainly related to the three vaccines administered in teenagers: HPV, Tdap and MCV4. Therefore, more than an adverse event after vaccination, syncope seems to be linked to the response to pain or anxiety resulting from the vaccination process.
Another vaccine–reaction pair that deserves attention is thrombosis (ROR = 6.44), especially with Gardasil. The peculiar age range for which HPV vaccines are indicated, appears to be superimposable to the larger part of the population that start to take hormonal contraceptives 34. From this perspective, it becomes difficult to discern the role of HPV vaccines in determining adverse reactions such as thromboembolism, pulmonary embolism or venous thrombosis, common side effects of hormonal contraceptives. A large cohort study of about one million adolescent girls from two Scandinavian countries showed no consistent evidence for a plausible association 35 for venous thromboembolic events after vaccination with HPV4.
Gardasil 9, by contrast, was associated with reports of predominantly mild adverse reactions mainly localized at the injection site. A similar result also emerged from a meta‐analysis of randomized clinical trials of Costa et al., who showed that adverse reactions such pain and erythema occurred significantly more in the HPV9 group than in HPV4 36.
Some new signals emerged from the present study such as alopecia (n = 491, ROR = 10.39), hyperacusis (n = 185, ROR = 7.13) and parosmia (n = 37, ROR = 4.77), which require further investigation with control groups.
As far as alopecia is concerned, Wise et al., as early as in 1997, reported cases of hair loss after routine immunizations 37. A few years later, Tuccori et al. 38 reported cases of telogen effluvium resulting from HPV vaccinations, also highlighting possible mechanisms to support the causative role of xenobiotics in the development of alopecia. Both the papers reported that it is difficult to determine a causal role of vaccines.
Overall, our data confirm what is already known and discussed for HPV vaccines and enriches the knowledge by investigating the adverse events reporting system and looking at potential association with Internet searches. It is impossible to determine which of the three vaccines is the safest, since they have been marketed at different times and the distributed doses are different. The added value in cancer prevention and therefore the reduction of mortality, along with their favourable safety profile, makes these vaccines a great medical discovery. However, vaccines are victims of their own success: they makes the diseases they prevent being perceived as extinguished even if this is not true. Generally, a higher standard of safety is expected for vaccine compared to other drugs as they are administered to healthy individuals. In addition, the disarray generated by media about such a sensitive topic may increase uncertainty, hesitation and reluctance towards vaccinations.
Strengths and limitations
Pharmacovigilance studies based on spontaneous reporting have limitations and need to be supported with more accurate observational studies.
First, quality of the data reported in pharmacovigilance databases may be incomplete due to difficulty gathering information from the reporters. In addition, there could be the possible existence of reports based on indirect information (e.g. heard on TV/read in newspapers) in VAERS that reduce the quality of the data collected. Moreover, the absence of an unvaccinated comparison group is another limitation to consider: this type of study does not allow assessing if a vaccine actually caused an AEFI or not. In particular, ROR computing does not allow the quantification of the risk of an AEFI but only suggests a statistical association between a drug and an adverse event.
Another limitation is represented by underreporting (i.e. lack of reports for all AEFIs that actually occur) and selective reporting, that contribute to misestimation of the number of AEFIs occurring. We should also consider the Weber effect, an epidemiological phenomenon stating that the number of reported AEFIs rises until the second year of marketing, peaks and then declines 39. Possible duplication of the reports represents another bias to which attention must be paid.
However, pharmacovigilance tools based on spontaneous reporting allow retrieval of real‐life data regarding the safety of medicinal products without the restricted inclusion criteria of the clinical trials. Post‐marketing research can be very useful for the evaluation of the drug safety, especially for the paediatric population.
Vaccine hesitancy
Very interesting data arose by comparing the trend of spontaneous reporting to the amount of research queries on Google. This showed overlap between the two trends. Considering the recent concerns on vaccination and the growth of antivaccination movements, it is important to ensure correct scientific information, and to keep in mind that the information found on the web not always is correct. In an article, 70% of subjects reported that what they found on the web influenced their decision towards vaccination 40. As reported by Kata 41, today everybody is an expert. This implies the risk of the possible dissemination of fake news regarding the safety of vaccines. In the context of spontaneous reporting, this attitude of relying on information that is not always correct can lead to an over‐reporting that creates even more unnecessary alarmism on vaccine safety. As reported by Eberth et al. 42, however, it does not necessarily mean that media coverage about a specific topic prompts people to report false adverse events. However, this may increase awareness about spontaneous reporting and it may lead to increased attention to the possible AEFIs and the importance of reporting them.
Conclusion
In infectious disease, vaccines significantly contribute to prolongation of life expectancy and provide a significant improvement of the quality of life. In the case of HPV, vaccination even reduces the risk of some forms of cancer with an acceptable safety profile. Our data are in agreement with the vaccine SmPCs and with the results of the safety investigations carried out by the regulatory authorities in recent years. From our research, some new signals emerged that need further investigation. There is a great importance of disseminating an evidence‐based scientific information performed with effective communication, in order to slow down and possibly reverse the decline in vaccination coverage as is happening with measles for instance.
Contributors
Substantial contributions to conception or design of the work (G.B., D.M.), or the acquisition (G.B., O.D.), analysis (G.B., D.M.) or interpretation of data for the work (G.B., D.M., O.D., A.V.). Drafting of the work (G.B., D.M.) or revising it critically for important intellectual content (O.D., A.V.). All authors approved the submitted final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing Interests
There are no competing interests to declare.
Bonaldo, G. , Vaccheri, A. , D'Annibali, O. , and Motola, D. (2019) Safety profile of human papilloma virus vaccines: an analysis of the US Vaccine Adverse Event Reporting System from 2007 to 2017. Br J Clin Pharmacol, 85: 634–643. 10.1111/bcp.13841.
All the authors declare no conflict of interest, no external funding source, and no editorial or manuscript support. The manuscript does not contain clinical studies or patient data. For this type of study, formal consent is not required.
References
- 1. World Health Organization . Human papillomavirus vaccines: WHO position paper, October 2014–Recommendations. [DOI] [PubMed]
- 2. European Centre for Disease Prevention and Control . ECDC guidance. Introduction of HPV vaccines in European Union countries: an update. 2012. Available at http://ecdc.europa.eu/en/publications/Publications/20120905_GUI_HPV_vaccine_update.pdf (last accessed 5 October 2018).
- 3. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–19. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 5. Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, et al Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013; 40: 187–193. [DOI] [PubMed] [Google Scholar]
- 6. Rossi S, Crocetti E, Capocaccia R, Gatta G, AIRTUM Working Group , Buzzoni C, et al Estimates of cancer burden in Italy. Tumori 2013; 99: 416–424. [DOI] [PubMed] [Google Scholar]
- 7. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al Human papillomavirus type distribution in invasive cervical cancer and high‐grade cervical lesions: a meta‐analysis update. Int J Cancer 2007; 121: 621–632. [DOI] [PubMed] [Google Scholar]
- 8. Von Krogh G. Management of anogenital warts [condylomata acuminata]. Eur J Dermatol 2001; 11: 598–603. [PubMed] [Google Scholar]
- 9. Reparto di Epidemiologia dei Tumori del Cnesps‐Iss . Approfondimento “Andamenti di incidenza e mortalità per cervicocarcinoma in Italia”, EpiCentro, 2013. Available at http://www.epicentro.iss.it/temi/materno/8marzoTumori.asp (last accessed 5 October 2018).
- 10. Kahn JA, Burk RD. Papillomavirus vaccines in perspective. Lancet 2007; 369: 2135–2137. [DOI] [PubMed] [Google Scholar]
- 11. The FUTURE II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. N Engl J Med 2007; 356: 1915–1927. [DOI] [PubMed] [Google Scholar]
- 12. Ault KA, Future II Study Group . Effect of prophylactic human papillomavirus L1 virus‐like‐particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007; 369: 1861–1868. [DOI] [PubMed] [Google Scholar]
- 13. Garland SM, Hernandez‐Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356: 1928–1943. [DOI] [PubMed] [Google Scholar]
- 14. Joura EA, Leodolter S, Hernandez‐Avila M, Wheeler CM, Perez G, Koutsky LA, et al Efficacy of a quadrivalent prophylactic human papillomavirus (types 6,11,16 and 18) L1 virus‐like particles vaccine against high grade vulval and vaginal lesions. Lancet 2007; 369: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 15. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli‐Martins CM, et al Sustained efficacy up to 4·5 years of a bivalent L1 virus‐like particle vaccine against human papillomavirus types 16 and 18: follow‐up from a randomised control trial. Lancet 2006; 367: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 16. Karafillakis E, Larson HJ, ADVANCE consortium . The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine 2017; 35: 4840–4850. [DOI] [PubMed] [Google Scholar]
- 17. Nordic Cochrane Centre . Complaint to the European Medicines Agency (EMA) over maladministration at the EMA. Available at https://nordic.cochrane.org/sites/nordic.cochrane.org/files/public/uploads/ResearchHighlights/Complaint‐to‐EMA‐over‐EMA.pdf (last accessed 5 October 2018).
- 18. European Medicine Agency . EMA to further clarify safety profile of human papillomavirus (HPV) vaccines. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vaccines_20/Procedure_started/WC500189476.pdf (last accessed 5 October 2018).
- 19. European Medicine Agency . Scientific conclusions. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vaccines_20/European_Commission_final_decision/WC500200002.pdf (last accessed 5 October 2018).
- 20. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine 2015; 33: 4398–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999; 20: 109–117. [DOI] [PubMed] [Google Scholar]
- 22. Food and Drug Administration . 21 CFR Part 600.80. Postmarketing reporting of adverse experiences, vol. 62. Federal Register; 1997. p. 52252–3.
- 23. Centers for Disease Control (CDC)/Food and Drug Administration (FDA) . United States Department of Health and Human Services (DHHS), Public Health Service (PHS), Vaccine Adverse Event Reporting System (VAERS) 1990 ‐ last month, CDC WONDER On‐line Database. Available at http://wonder.cdc.gov/vaers.html (last accessed 5 October 2018).
- 24. Google Trends. Available at www.google.com/trends (last accessed 5 October 2018).
- 25. Health Resources & Services Administration . National Vaccine Injury Compensation Program Data Report, Update May 1 2018. Available at https://www.hrsa.gov/sites/default/files/hrsa/vaccine‐compensation/data/monthly‐stats‐may‐2018.pdf (last accessed 5 October 2018).
- 26. Food and Drug Administration (FDA). Vaccines, Blood & Biologics, Gardasil. Available at https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094042.htm (last accessed 5 October 2018).
- 27. Food and Drug Administration (FDA), Vaccines, Blood & Biologics, Cervarix. Available at https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186957.htm (last accessed 5 October 2018).
- 28. Food and Drug Administration (FDA), Vaccines, Blood & Biologics, Gardasil9. Available at https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426445.htm (last accessed 5 October 2018).
- 29. Suragh TA, Lewis P, Arana J, Mba‐Jonas A, Li R, Stewart B, et al Safety of bivalent human papillomavirus vaccine in the US vaccine adverse event reporting system (VAERS), 2009‐2017. Br J Clin Pharmacol 2018; 84: 2928–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arana JE, Harrington T, Cano M, Lewis P, Mba‐Jonas A, Rongxia L, et al Post‐licensure safety monitoring of quadrivalent human papillomavirus vaccine in the vaccine adverse event reporting system (VAERS), 2009–2015. Vaccine 2018; 36: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 31. Chandler RE, Juhlin K, Fransson J, Caster O, Edwards IR, Norén GN. Current safety concerns with human papillomavirus vaccine: a cluster analysis of reports in VigiBase. Drug Saf 2017; 40: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angelo MG, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post‐licensure safety surveillance for human papillomavirus‐16/18‐AS04‐adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf 2014; 23: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention . Syncope After Vaccination ‐ United States, January 2005 – July 2007, Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5717a2.htm (last accessed 5 October 2018).
- 34. Slade B, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302: 750–757. [DOI] [PubMed] [Google Scholar]
- 35. Arnheim‐Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A. Autoimmune, neurological and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ 2013; 347: f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Costa APF, Cobucci RNO, da Silva JM, da Costa Lima PH, Giraldo PC, Gonçalves AK. Safety of human papillomavirus 9‐valent vaccine: a meta‐analysis of randomized trials. J Immunol Res 2017; 2017: 3736201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wise RP, Kiminyo KP, Salive ME. Hair loss after routine immunizations. JAMA 1997; 278: 1176–1178. [PubMed] [Google Scholar]
- 38. Tuccori M, Pisani C, Bachini L, Pardini M, Mantarro S, Antonioli L, et al Telogen effluvium following bivalent human papillomavirus vaccine administration: a report of two cases. Dermatology 2012; 224: 212–214. [DOI] [PubMed] [Google Scholar]
- 39. Hartnell NR, Wilson JP. Replication of the weber effect using Postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004; 24: 743–749. [DOI] [PubMed] [Google Scholar]
- 40. The Pew Internet & American Life Project. The online health care revolution: how the Web helps Americans take better care of themselves. Available at http://www.pewinternet.org/2000/11/26/the‐online‐health‐care‐revolution/ (last accessed 5 October 2018).
- 41. Kata A. Anti‐vaccine activists, web 2.0, and the postmodern paradigm: an overview of tactics and tropes used online by the anti‐vaccination movement. Vaccine 2012; 30: 3778–3789. [DOI] [PubMed] [Google Scholar]
- 42. Eberth JM, Kline KN, Moskowitz DA, Montealegre JR, Scheurer ME. The role of media and the internet on vaccine adverse event reporting: a case study of human papillomavirus vaccination. J Adolesc Health 2014; 54: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]


