Abstract
The prevalence and incidence of atrial fibrillation/flutter (AF/AFL) in patients with human immunodeficiency virus type‐1 (HIV‐1) infection have been poorly investigated. We performed a systematic review using PubMed and Cochrane Database of Systematic Reviews, and screening of references, searching for clinical studies reporting on the association between HIV‐1 infection and AF/AFL. We also summarized the main interactions of antiretroviral agents with antithrombotic and antiarrhythmic drugs. We found a prevalence of AF/AFL ranging from 2.0% to 5.13% in patients with HIV‐1, with an incidence rate of 3.6/1000 person‐years. Low CD4+ count (<200–250 cells ml−1) and high viral load were predictors of AF/AFL. Regarding drugs interactions, nucleoside reverse transcriptase inhibitors, integrase inhibitor and maraviroc have the lowest interactions with oral anticoagulants. Among anticoagulants, dabigatran presents the most favourable profile. Most of antiarrhythmic drugs interact with protease inhibitors, with beta blockers and diltiazem having fewer interactions. The few studies available suggest a non‐negligible prevalence of AF/AFL in patients with HIV‐1 infection. Awareness of potential interactions with anticoagulation and antiarrhythmic drugs is needed to offer optimal management in this population.
Keywords: anticoagulants, antiretroviral, atrial fibrillation, atrial flutter, HIV‐1, stroke
Introduction: cardiovascular disease risk in HIV‐1 infection
The availability of highly effective antiretroviral therapy (ART) has resulted in markedly improved survival for people with human immunodeficiency virus type‐1 (HIV‐1) infection. While HIV‐1 related mortality is declining, the incidence of new cases of HIV‐1 infection remains stable, resulting in a growing number of older adults living with HIV‐1 infection 1. As a consequence of the increased life expectancy, the effects of ageing on HIV‐1 infected persons have begun to be evident. In particular, long‐term effects of HIV‐1 infection, ART use and traditional risk factors may be significant contributors to the increased risk of premature cardiovascular disease (CVD) described in this population.
A pathogenic relevant role in CVD risk development is played by the chronic immune activation and inflammation caused by HIV‐1 infection itself 2. Indeed, monocytes are an important source of inflammatory mediators that promote CVD, even in treated HIV‐1 patients 3. Moreover, low CD4+ T cell count and/or failure to restore normal CD4+ T cell counts during ART have been associated with the occurrence of CVD and increased risk of morbidity and mortality due to CV events 4.
In addition, several antiretroviral drugs, such as nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs), could result in an altered fat redistribution that characterizes the so‐called ‘lipodystrophy syndrome’ (subcutaneous fat tissue atrophy in the face, limbs and buttocks and/or lipo‐accumulation in different body districts, i.e., neck, trunk, abdomen and viscera) 5. These fat alterations were frequently associated with several metabolic and endocrine disorders similar to those of the metabolic syndrome (hypertriglyceridemia, low high‐density lipoprotein cholesterol and insulin resistance), which are well‐known risk factors for CVD 6. A dyslipidaemia induced by integrase inhibitors has been also described, which could be considered a risk factor for increased CVD 7.
Beside drugs, several pathophysiological alterations potentially contributing to the increased CVD risk have been reported in association with HIV‐1 infection. For instance, HIV‐1 infected patients are characterized by increased oxidative stress 8, endothelial/vascular dysfunction 9 and platelet activation 10, again all factors potentially contributing to the atherosclerotic burden in HIV‐1 patients 11.
Altogether these findings probably account for the increased risk of myocardial infarction (MI) with an incidence rate (IR) for type 1 MI of 2.57 per 1000 person‐years 12, and a higher ischaemic stroke risk (IR of 2.79 per 1000 person‐years) 13 described in HIV‐1+ patients. Nonetheless, the association between HIV‐1 and atrial arrhythmias, such as atrial fibrillation and atrial flutter (AF/AFL), has scarcely been investigated. HIV‐1 patients have been excluded from the randomized trials of stroke prevention, and thus, limited data are available on stroke risk and appropriate thromboprophylaxis in such patients.
The aim of this systematic review was to summarize the available evidence on the risk of AF/AFL during HIV‐1 infection. We also focused on the implications for the management of patients with concomitant AF/AFL and HIV‐1, considering several drug–drug interactions of ART with oral anticoagulants and antiarrhythmic drugs.
Systematic review of risk of AF/AFL in HIV‐1
Eligibility criteria for the systematic review
We included all original clinical research articles in English language with full text available. In particular, all observational studies (both prospective and retrospective) reporting data on the prevalence and/or incidence of AF/AFL in patients with HIV‐1 were selected. We did not include the following: (1) case reports, (2) editorials/comments/letters and (3) review articles.
Information sources and search strategy
We performed a systematic review of the literature searching MEDLINE via PubMed, and Cochrane Database of Systematic Reviews, for a combination of the following keywords ‘atrial fibrillation’, ‘HIV’ and ‘atrial flutter’. The research strategy had no time restriction and was performed according to PRISMA guidelines 14. The screening of reference lists of studies was also performed.
Study selection process
The study selection was performed in multiple phases. In the first phase, potentially relevant studies were obtained by combined searches of electronic databases using the selected above‐mentioned keywords. In the second phase, studies were reviewed and excluded by study typology; thus, letters, editorial, case reports and comments were excluded. Then we performed a detailed analysis of full‐text articles to assess whether they addressed the specific study question (Supporting Information Figure S1).
Data collection process
Two physicians (D.P. and I.M.) independently screened the titles and abstracts of manuscripts identified through the database searches to identify studies potentially eligible for further assessment. For each study we collected the following information: authors, year of publication, study design, number of patients included, duration of follow‐up, prevalence and/or incidence of AF/AFL.
Ethical review
Given the study type (review article), ethical approval was not necessary.
Study selection
We identified 127 results from the combined search (Supplementary Figure S1). Of these, 63 reports were excluded by study typology (i.e. non‐clinical studies). Detailed analysis of the remaining results showed that 60 studies did not address the study question. Of the remaining four studies, one was excluded, as it included only patients who already had concomitant HIV‐1 infection and AF 15. Thus, three studies were eligible and are reported in Table 1.
Table 1.
Clinical studies reporting prevalence/incidence of atrial fibrillation in HIV‐1 patients
| Study (Year) | Study design | Population | Male (%) | Age | FU (years) | Prevalence/incidence of AF | Other findings | NOS |
|---|---|---|---|---|---|---|---|---|
| Elnahar (2012) 17 | Retrospective | 780 HIV‐1 patients | 67.5a | 56.8 ± 9.4a | 3 | 40/780 (5.13%) developed AF. | 47% of HIV‐1 patients who developed AF had CD4+ T cell count <250 cells ml−1 vs. 20% of controls (P = 0.017) | 7 |
| Hsu (2013) 18 | Registry | 30 533 HIV‐1 infected veterans | 97.2 | 53.6 ± 11.4 | 6.8 | 780 incident cases (2.55%): 641 AF and 139 AFL. Incidence rate: 3.6 per 1000 person‐years (95% CI 3.4–3.9). | CD4+ T cell count (<200 vs. >350 cells ml−1; HR: 1.4; 95% CI: 1.1–1.8; P = 0.018) and viral load >100 000 vs. <500 copies ml−1; HR: 1.7; 95% CI: 1.2–2.4; P = 0.002) were associated to incident AF. | 7 |
| Sanders (2018) 19 | Retrospective | 5052 HIV‐1 patients | 82.5 | 48.2 ± 11.6 | 16 | 101 confirmed AF/AFL cases (2.00%) | OR 1.98, 95% CI 1.21–3.25 for nadir CD4+ T cell count <200 cells ml−1 for AF/AFL. No association between HIV viral load and AF/AFL (OR 1.03, 95% CI 0.86–1.24) | 7 |
Values refer to 40 patients developing AF
AF, atrial fibrillation; AFL, atrial flutter; CI, confidence interval; FU, follow‐up; HR, hazard ratio; NOS, Newcastle–Ottawa quality assessment scale; OR, odds ratio
The quality of the studies was assessed by the Newcastle–Ottawa quality assessment scale (NOS) for non‐randomized studies, including case–control and cohort studies 16. This scale evaluates observational study regarding (1) selection of patients (representativeness of the exposed and non‐exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at start of study); (2) comparability (comparability of cohorts on the basis of the design or analysis); (3) outcome (assessment of outcome; length of follow‐up ≥ 12 months; adequacy of follow‐up of cohorts) (Supplementary Table S1). The total score ranges from 0 to 9 points.
Study characteristics and results of individual studies
Only three clinical studies 17, 18, 19 investigated the risk of AF/AFL in patients with HIV‐1 (Table 1). The studies were of good quality, with a NOS score of 7 out of 9 (Table S1). The prevalence of AF/AFL ranged from 2.0% to 5.13%.
The first report on the association between HIV‐1 and AF was a retrospective study including 780 HIV‐1 patients, of whom 40 developed AF 17. In these patients, a low CD4+ cell count (<250 cells mm−3) was observed more frequently than matched control patients and independently associated with new‐onset AF (OR: 3.62, 95% CI 1.34–9.77) 17.
Similar findings were reported by Hsu et al. 18 who found in a national sample of HIV‐1 infected veterans that low CD4+ cell count (<200 cells mm−3, HR: 1.4; 95% CI 1.1–1.8, P = 0.018) and high viral load (>100 000 copies ml−1, HR: 1.7, 95% CI 1.2–2.4, P = 0.002) were risk factors for the development of AF 18, along with age ≥ 65 years (HR: 7.9, 95% CI 5.1–12.3, P < 0.001), coronary artery disease (HR: 2.4, 95% CI 2.0–2.9, P < 0.001), congestive heart failure (HR: 4.8, 95% CI 3.9–5.9, P < 0.001), alcoholism (HR: 1.4, 95% CI 1.1–1.7, P = 0.001), hypothyroidism (HR: 1.5, 95% CI 1.1–2.0, P = 0.010), severe renal impairment (HR: 1.7, 95% CI 1.2–2.4, P = 0.006) and proteinuria ≥2000 mg dl−1 (HR: 2.5, 95% CI 1.5–4.3, P = 0.001). Black ethnicity was instead associated with a lower incidence of AF (HR: 0.6, 95% CI 0.5–0.7, P < 0.001).
Finally, a study by Sanders et al. included 5052 HIV‐1 patients and found a prevalence of 2% of AF, which was marginally significant compared to 1.57% of 10 121 non‐HIV patients (P = 0.056) 19. In addition to low nadir CD4+ count (<200 cells mm−3), older age, diabetes, hypertension and COPD were risk factors for AF/AFL 19. Conversely, in this study, peak viral load was not associated with an increased risk of AF/AFL 19.
Of note, the majority of patients included in these studies were men, and thus risk factors for developing AF may be different in female HIV‐1 patients. This difference is arguable as a sex‐specific immune response to HIV‐1 has been described, including HIV‐1 viral load in women, partially due to a direct inhibition of viral transcription by oestrogen 20, 21. Furthermore, women have an average higher T‐cell activation for a given level of blood viraemia 21. As a consequence, the threshold of CD4+ T cells representing a risk factor for the development of AF/AFL may be different in women than men.
HIV‐1 and atrial abnormalities
HIV‐1 has been shown to possess a specific cardiac tropism. Specifically, some surface components, such as Nef, seem to be relevant for cardiac toxicity 22. In particular, Nef inhibits autophagy flux leading to cardio‐cytotoxicity and death of cardiomyocytes 22. Thus, a specific viral myocardial inflammation pattern has been described in HIV‐1 patients, directly related to HIV‐1 infection or to some mostly opportunistic microorganisms 23.
Atrial cardiomyopathies have been strongly associated with the development of atrial arrhythmias, such as AF/AFL 24. Some abnormalities in cardiac and specifically atrial structure and function have been described in patients with HIV‐1 infection. For example, cross‐sectional study of 95 HIV‐1 infected and 30 healthy subjects matched for characteristics and free from cardiovascular disease, showed that intramyocardial lipid levels, myocardial fibrosis and cardiac function (measured by strain) were both increased in patients with HIV‐1 as compared to controls 25. A large echocardiography study showed that 40% of 656 patients with HIV‐1 showed a left atrial enlargement (LAE) 26. Indeed, the latter has been associated to an increased risk of developing new AF 27 and AF recurrence 28, and to a high risk of ischaemic stroke 29, 30, 31 in patients with or without AF.
A study involving 42 HIV‐1 patients and 40 healthy subjects showed that the atrial electromechanical delay, assessed by tissue Doppler imaging, of both left and right atria was increased in patients compared to controls. This is a relevant finding as the atrial electromechanical delay reflects the electrical and structural morphology of the atria, and has been correlated with the development of new onset AF 32.
Managing drug–drug interactions in patients with HIV‐1 and AF/AFL
Anticoagulant treatment
The development of AF/AFL, which is per se associated with a five‐fold increased risk of ischaemic stroke 33, 34, may be an additional factor contributing to the increased thromboembolic risk in HIV‐1 infection. Apart from established stroke risk factors in AF/AFL, associated valvular heart disease would require consideration of oral anticoagulants 35.
A recent study from the Veterans Affairs HIV Clinical Case Registry including 914 patients with HIV‐1 and AF confirmed a high thromboembolic risk in this setting 15. Thus, the rate of events according to a CHA2DS2‐VASc score of 0, 1 and ≥2 was 5.4, 9.3 and 8.1 per 1000 person‐years, respectively 15. Furthermore, warfarin did not show a significant association with reduced rate of thromboembolic events raising concerns about the optimal thromboprophylaxis for HIV‐1 infected persons with AF.
Given the association with CVD risk factors and structural abnormalities on cardiac imaging, many patients with HIV‐1 and AF/AFL should be treated with oral anticoagulants, either vitamin K antagonists (VKAs) or non‐vitamin K direct oral anticoagulants (NOACs), according to the CHA2DS2‐VASc score. The choice of the most appropriate oral anticoagulant in patients with HIV‐1 infection is challenging given the several drug–drug interactions between ART and anticoagulants. Also, HIV‐1 infected subjects have been excluded from the randomized trials of stroke prevention in AF, thus robust trial data are lacking.
Most drugs used in combination ART for the treatment of HIV‐1 infection have significant effects on liver cytochromes, such as CYP2C9 and CYP3A4 (Table 2), which are responsible for the metabolism of many drugs, including oral anticoagulants (CYP3A4: R‐Warfarin, R‐Acenocumarol, Apixaban, Rivaroxaban, Edoxaban; CYP2C9: S‐Warfarin, S/R‐Acenocumarol) 36. For this reason, interactions between VKAs and ART are likely, especially with PIs or non‐nucleoside reverse transcriptase inhibitors (NNRTIs) 37. Conversely, NRTIs are renally excreted, thus presenting no interactions with drugs metabolized at liver site. Dolutegravir and maraviroc are CYP3A4 substrate and are influenced by drugs modulating this enzyme.
Table 2.
Effect of antiretroviral drugs on liver cytochromes CYP2C9 and CYP3A4
| CYP2C9 | CYP3A4 | |
|---|---|---|
| Protease inhibitors | ||
| Saquinavir | Inhibition | Inhibition |
| Tipranavir | Induction | Inhibition |
| Ritonavir a | Modest Inductiona | Strong Inhibitiona |
| Atazanavir | Inhibition | Modest Inhibition |
| Darunavir | Induction | Modest Inhibition |
| Lopinavir | Induction | Strong Inhibition |
| Nelfinavir | Induction | Induction/inhibition |
| Indinavir | No significant effect | Inhibition |
| Non‐nucleoside reverse‐transcriptase inhibitors | ||
| Delavirdine | Inhibition | Inhibition |
| Efavirenz | Modest Inhibition | Modest Induction |
| Nevirapine | Induction | Strong Induction |
| Etravirine | Inhibition | Modest Induction |
| Rilpivirine | No significant effect | Induction |
| Doravirine | – | Substrate |
| CCR5 inhibitor | ||
| Maraviroc | – | Substrate |
| Integrase inhibitors | ||
| Raltegravir | – | – |
| Dolutegravir | – | Substrate |
| Elvitegravir | Modest Induction | Inhibition |
| Cobicistat b | – | Strong Inhibition |
| Bictegravir | – | Substrate |
Ritonavir is used only as low ‘boosting’ dose (100–200 mg) in association with other PIs to enhance their pharmacokinetic properties by inhibiting CYP3A4
Not an integrase inhibitor and available in combination with elvitegravir, atazanavir and darunavir
By the same mechanism, NOACs also have interaction with ART drugs 38, 39. A case of decreased rivaroxaban concentration by concomitant nevirapine administration has been described, as the result of putative CYP3A induction and accelerated drug clearance 40.
Dabigatran is the only NOAC with non‐enzymatic liver metabolism and predominant renal excretion, thus presenting fewer interactions with ART drugs. Table 3 reports expected interactions between NOACs and antiretroviral drugs 41, 42.
Table 3.
Expected interactions between non‐vitamin K oral anticoagulants and antiretroviral drugs
| Antiretroviral drugs | Apixaban | Dabigatran | Edoxaban | Rivaroxaban |
|---|---|---|---|---|
| Protease inhibitors |
Increase of Apixaban expected with protease inhibitor/cobicistat, protease inhibitor/ritonavir. Use of strong inhibitors of both CYP3A4 and P‐gp contraindicated in SPC. Coadministration not recommended; if necessary, reduce apixaban dose by 50% and monitor for apixaban toxicity 52. |
Limited data but no significant interaction expected. |
Increase of edoxaban expected. Coadministration is not recommended 52. |
Increase of rivaroxaban concentration expected. Coadministration is not recommended 52. Use of strong inhibitors of both CYP3A4 and P‐gp contraindicated in SPC. |
| Non‐nucleoside reverse‐transcriptase inhibitors | Decrease of apixaban possible. | No drug interaction expected. Increase with etravirine possible. | No drug interaction expected. Increase with etravirine possible. | Decrease of rivaroxaban possible. |
| Nucleoside reverse transcriptase inhibitors | No drug interaction expected | |||
| Integrase inhibitors Raltegravir Dolutegravir Bictegravir | No drug interaction expected | |||
| Elvitegravir (always administered with cobicistat) | Drugs concentration increase expected. Coadministration is not recommended 52 | |||
| Cobicistat | Increase of apixaban expected. | Increase in dabigatran concentration by 110% to 127%. Coadministration is not recommended 52. | Increase of edoxaban expected. | Increase of rivaroxaban expected. |
| CCR5 inhibitor | No drug interaction expected | |||
SPC, Summary of product characteristics
Despite results indicating a differential effect of NOACs with men being more protected from ischaemic stroke/systemic embolism and women more protected from major bleeding events, the application of these findings to the HIV‐1 population remains uncertain 43.
Antiarrhythmic drugs
Similarly to antithrombotic drugs, significant drug–drug interaction may be detected also with antiarrhythmic treatments commonly used for the management of AF/AFL. A list of the most important interactions is reported in Table 4.
Table 4.
Interactions between antiarrhythmic and antiretroviral drugs
| Antiarrhythmic drug | Expected interaction |
|---|---|
| Beta blockers | Possible increase of drug levels with PIs. Adjust dose based on clinical response (see text). |
| Digoxin | Use with caution in patients with PIs, titrating the initial dose. Expected increase in digoxin concentration with DRV/r, RTV (200 mg), and COBI. |
| Amiodarone | Use with caution with PIs. Contraindicated with TPV/r. |
| Dronedarone | Increased drug levels with PIs and ATV. Do not co‐administer with ATV. Contraindicated with PIs. |
| Flecainide | Increased drug levels with PIs. Do not co‐administer with PIs (contraindicated with TPV/r). |
| Propafenone | Possible increased drug levels with PIs. Do not co‐administer with PIs. Contraindicated with TPV/r. Possible interaction with ATV (unboosted). |
| Dofetilide | Possible increased drug levels with PIs. Do not co‐administer with PIs and with dolutegravir. Possible interaction with ATV (unboosted). |
| Diltiazem | In patients treated with ATV(c/r), decrease the dose of diltiazem by 50%. Use with caution with DRV c/r, LPV/r, TPVr. |
| Verapamil | Possible increased drug levels with PIs. Titrate the initial dose and adjust based on clinical response. |
ATV, atazanavir; COBI or c, cobicistat; DRV, darunavir; LPV, lopinavir; NNRTIs, non‐nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; RTV or r, ritonavir; TPV, tipranavir
Drug serum concentration of antiarrhythmic medications can be significantly increased by an antiretroviral therapy containing a PI with or without booster (ritonavir/cobicistat). For instance, two case reports showed a significant increase of serum digoxin concentration (SDC) in patients receiving ritonavir as part of ART regimen, possibly mediated by the inhibition of P‐glycoprotein (P‐gp) 44, 45. These results were confirmed in healthy subjects where the co‐administration of ritonavir 300 mg b.i.d. increased SDC up to 86% 46. Currently, it is suggested to use ritonavir only as boosting dose of 100–200 mg.
The indication is to not co‐administer flecainide, propafenone or dronedarone (contraindicated) in patients taking PIs. A significant increase of serum amiodarone concentrations has been described in a patient taking indinavir, which is, however, no longer widely used 47. Another case report described a progressive accumulation of amiodarone 200 mg daily after the initiation of atazanavir 48, reinforcing the evidence that co‐administration of amiodarone with PIs should be closely monitored.
In these patients, beta blockers, especially those not metabolized by CYP450 such as atenolol, labetalol, nadolol and sotalol, may be used, as well as diltiazem. Also, verapamil, digoxin and amiodarone could be used, but with caution as an increase of drug levels is possible.
The class of NNRTIs does not appear to have a clinically relevant interaction with antiarrhythmic therapy requiring clinical or laboratory monitoring. No significant interactions have been reported so far between integrase inhibitors and cardiac medications, with the exception of dolutegravir, which increases the serum concentration of dofetilide.
Conclusions
Effective management of HIV‐1 infection may help lower the risk of AF/AFL, as the HIV‐1 infection itself and its systemic effects such as high viral load and low CD4+ T cells count have been shown to be additive risk factors for ischaemic stroke 49, 50.
Until more data are available, thromboembolic risk stratification for patients with HIV‐1 and AF/AFL should be performed according to the current guidelines developed for AF/AFL non‐HIV patients 51. Whether HIV‐1 infection should probably be regarded to as an additional stroke risk factor beyond the CHA2DS2‐VASc score is uncertain, although such patients are clearly at high risk. In one study involving 914 HIV‐1 patients with AF (free from events at baseline) the event rate of thromboembolic events (including pulmonary embolism, peripheral embolism and ischaemic stroke) ranged from 5.4% per 1000 person‐years for CHA2DS2‐VASc score of 0, to 9.3% and 8.1% for score of 1 and ≥2, respectively 15. Of note, none of the single components of the CHA2DS2VASC score was associated to thromboembolic risk in this cohort and most patients scored 0 for ‘age’ due to the low mean age of the study cohort. Furthermore, the therapy with warfarin showed no significant effect in preventing thromboembolic events, even if no data on the quality of anticoagulation were reported. All these findings raise concerns about the most appropriate thromboprophylaxis strategy for patients with HIV‐1 and AF/AFL. Some additional HIV‐specific factors, including the type of ART, CD4+ T cells count and viral load, may play a substantial role in this setting.
In summary, HIV‐1 infection may increase the risk of stroke through systemic effects (i.e. systemic low‐grade inflammation and increased oxidative stress), or with a direct mechanism of cardiac toxicity, which may favour AF onset, and in turn the risk of ischaemic stroke (Figure 1).
Figure 1.
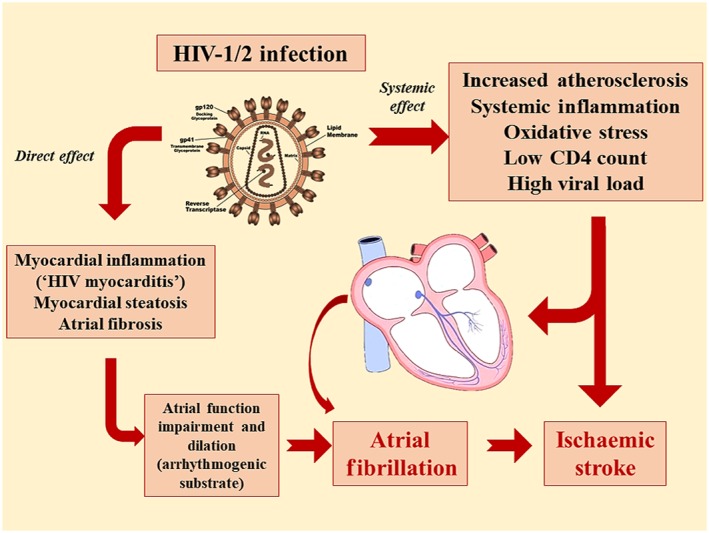
Mechanisms of HIV‐1 related ischaemic stroke. HIV‐1 infection may increase the risk of atrial fibrillation and ischaemic stroke through a direct mechanism of cardiac toxicity, or by increasing systemic inflammation and oxidative stress
The choice of an ART with the lowest drug–drug interaction and with lower impact on the cardiovascular system, and the use of NOACs (particularly dabigatran) may help reduce the risk of stroke in this high‐risk subgroup of patients.
However, the current evidence on the association between HIV‐1 infection and AF/AFL stems from very few studies with retrospective designs. Prospective ‘real‐world’ studies are needed to establish the real contribution of HIV‐1 infection to the risk of developing cardiac arrhythmias and thromboembolic complications.
Competing Interests
There are no competing interests to declare.
Supporting information
Table S1 Newcastle–Ottawa quality assessment scale for cohort studies (point assigned only to criteria marked with star)
Figure S1 Study selection process
Pastori, D. , Mezzaroma, I. , Pignatelli, P. , Violi, F. , and Lip, G. Y. H. (2019) Atrial fibrillation and human immunodeficiency virus type‐1 infection: a systematic review. Implications for anticoagulant and antiarrhythmic therapy. Br J Clin Pharmacol, 85: 508–515. 10.1111/bcp.13837.
References
- 1. Antiretroviral Therapy Cohort Collaboration . Survival of HIV‐positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4: e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al Arterial inflammation in patients with HIV. JAMA 2012; 308: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV‐related comorbidities during cART. J Immunol Res 2014; 2014: 569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al Normalisation of CD4 counts in patients with HIV‐1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet 2007; 370: 407–413. [DOI] [PubMed] [Google Scholar]
- 5. Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS 1999; 13: 2493–2505. [DOI] [PubMed] [Google Scholar]
- 6. Worm SW, Lundgren JD. The metabolic syndrome in HIV. Best Pract Res Clin Endocrinol Metab 2011; 25: 479–486. [DOI] [PubMed] [Google Scholar]
- 7. Lennox JL. The use of HIV‐1 integrase inhibitors in antiretroviral naive patients. Curr Opin HIV AIDS 2012; 7: 409–414. [DOI] [PubMed] [Google Scholar]
- 8. Thangavel S, Mulet CT, Atluri VSR, Agudelo M, Rosenberg R, Devieux JG, et al Oxidative stress in HIV infection and alcohol use: role of redox signals in modulation of lipid rafts and ATP‐binding cassette transporters. Antioxid Redox Signal 2018; 28: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iantorno M, Schar M, Soleimanifard S, Brown TT, Moore R, Barditch‐Crovo P, et al Coronary artery endothelial dysfunction is present in HIV‐positive individuals without significant coronary artery disease. AIDS 2017; 31: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pastori D, Esposito A, Carnevale R, Bartimoccia S, Novo M, Fantauzzi A, et al HIV‐1 induces in vivo platelet activation by enhancing platelet NOX2 activity. J Infect 2015; 70: 651–658. [DOI] [PubMed] [Google Scholar]
- 11. Kearns A, Gordon J, Burdo TH, Qin X. HIV‐1‐associated atherosclerosis: unraveling the missing link. J Am Coll Cardiol 2017; 69: 3084–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, et al Increased risk of myocardial infarction in HIV‐infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr 2017; 75: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sico JJ, Chang CC, So‐Armah K, Justice AC, Hylek E, Skanderson M, et al HIV status and the risk of ischemic stroke among men. Neurology 2015; 84: 1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chau KH, Scherzer R, Grunfeld C, Hsue PY, Shlipak MG. CHA2DS2‐VASc score, warfarin use, and risk for thromboembolic events among HIV‐infected persons with atrial fibrillation. J Acquir Immune Defic Syndr 2017; 76: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Ottawa: Ottawa Hospital Research Institute, 2014. [Google Scholar]
- 17. Elnahar Y, Daoko J, Al‐Dehneh A, Gupta N, DeBari VA, Shamoon F, et al Risk factors for the development of atrial fibrillation in HIV infected patients. J Atr Fibrillation 2012; 4: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu JC, Li Y, Marcus GM, Hsue PY, Scherzer R, Grunfeld C, et al Atrial fibrillation and atrial flutter in human immunodeficiency virus‐infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol 2013; 61: 2288–2295. [DOI] [PubMed] [Google Scholar]
- 19. Sanders JM, Steverson AB, Pawlowski AE, Schneider D, Achenbach CJ, Lloyd‐Jones DM, et al Atrial arrhythmia prevalence and characteristics for human immunodeficiency virus‐infected persons and matched uninfected controls. PloS One 2018; 13: e0194754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV‐1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344: 720–725. [DOI] [PubMed] [Google Scholar]
- 21. Scully EP, Gandhi M, Johnston R, Hoh R, Lockhart A, Dobrowolski C, et al Sex‐based differences in HIV‐1 reservoir activity and residual immune activation. J Infect Dis 2018; 10.1093/infdis/jiy617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta MK, Kaminski R, Mullen B, Gordon J, Burdo TH, Cheung JY, et al HIV‐1 Nef‐induced cardiotoxicity through dysregulation of autophagy. Sci Rep 2017; 7: 8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ntusi NAB . HIV and myocarditis. Curr Opin HIV AIDS 2017; 12: 561–565. [DOI] [PubMed] [Google Scholar]
- 24. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016; 18: 1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV‐infected adults. J Infect Dis 2015; 212: 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al High prevalence of echocardiographic abnormalities among HIV‐infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis 2011; 52: 378–386. [DOI] [PubMed] [Google Scholar]
- 27. Conen D, Glynn RJ, Sandhu RK, Tedrow UB, Albert CM. Risk factors for incident atrial fibrillation with and without left atrial enlargement in women. Int J Cardiol 2013; 168: 1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang RB, Yan XL, Dong JZ, Kalifa J, Long DY, Yu RH, et al Predictors of recurrence after a repeat ablation procedure for paroxysmal atrial fibrillation: role of left atrial enlargement. Europace 2014; 16: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 29. Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, et al Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non‐valvular atrial fibrillation. Sci Rep 2016; 6: 31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogata T, Matsuo R, Kiyuna F, Hata J, Ago T, Tsuboi Y, et al Left atrial size and long‐term risk of recurrent stroke after acute ischemic stroke in patients with nonvalvular atrial fibrillation. J Am Heart Assoc 2017; 6: e006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Overvad TF, Nielsen PB, Larsen TB, Søgaard P. Left atrial size and risk of stroke in patients in sinus rhythm. Thromb Haemost 2016; 116: 206–219. [DOI] [PubMed] [Google Scholar]
- 32. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002; 54: 230–246. [DOI] [PubMed] [Google Scholar]
- 33. Violi F, Pastori D, Pignatelli P. Mechanisms and management of thrombo‐embolism in atrial fibrillation. J Atr Fibrillation 2014; 7: 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision‐making. Thromb Haemost 2017; 117: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 35. Lip GYH, Collet JP, de Caterina R, Fauchier L, Lane DA, Larsen TB, et al Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: Executive Summary of a Joint Consensus Document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, Endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Thromb Haemost 2017; 117: 2215–2236. [DOI] [PubMed] [Google Scholar]
- 36. Rathbun RC, Liedtke MD. Antiretroviral drug interactions: overview of interactions involving new and investigational agents and the role of therapeutic drug monitoring for management. Pharmaceutics 2011; 3: 745–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liedtke MD, Rathbun RC. Drug interactions with antiretrovirals and warfarin. Expert Opin Drug Saf 2010; 9: 215–223. [DOI] [PubMed] [Google Scholar]
- 38. Egan G, Hughes CA, Ackman ML. Drug interactions between antiplatelet or novel oral anticoagulant medications and antiretroviral medications. Ann Pharmacother 2014; 48: 734–740. [DOI] [PubMed] [Google Scholar]
- 39. West TA, Perram J, Holloway CJ. Use of direct oral anticoagulants for treatment of atrial fibrillation in patients with HIV: a review. Curr Opin HIV AIDS 2017; 12: 554–560. [DOI] [PubMed] [Google Scholar]
- 40. Bates D, Dalton B, Gilmour J, Kapler J. Venous thromboembolism due to suspected interaction between rivaroxaban and nevirapine. Can J Hosp Pharm 2013; 66: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al Updated European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace 2015; 17: 1467–1507. [DOI] [PubMed] [Google Scholar]
- 42. The Canadian HIV/AIDS Pharmacists Network (CHAP) . A management tool for HIV drug–drug interactions [online]. Available at https://hivclinic.ca/downloads/DDI%20tool_English_final.pdf (last accessed 28 December 2018).
- 43. Proietti M, Cheli P, Basili S, Mazurek M, Lip GYH. Balancing thromboembolic and bleeding risk with non‐vitamin K antagonist oral anticoagulants (NOACs): a systematic review and meta‐analysis on gender differences. Pharmacol Res 2017; 117: 274–282. [DOI] [PubMed] [Google Scholar]
- 44. Phillips EJ, Rachlis AR, Ito S. Digoxin toxicity and ritonavir: a drug interaction mediated through p‐glycoprotein? AIDS 2003; 17: 1577–1578. [DOI] [PubMed] [Google Scholar]
- 45. Yoganathan K, Roberts B, Heatley MK. Life‐threatening digoxin toxicity due to drug–drug interactions in an HIV‐positive man. Int J STD AIDS 2017; 28: 297–301. [DOI] [PubMed] [Google Scholar]
- 46. Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, et al Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther 2004; 76: 73–84. [DOI] [PubMed] [Google Scholar]
- 47. Lohman JJ, Reichert LJ, Degen LP. Antiretroviral therapy increases serum concentrations of amiodarone. Ann Pharmacother 1999; 33: 645–646. [DOI] [PubMed] [Google Scholar]
- 48. Naccarato M, Yoong D, la Porte C, Fong I. Amiodarone and concurrent antiretroviral therapy: a case report and review of the literature. Antivir Ther 2014; 19: 329–339. [DOI] [PubMed] [Google Scholar]
- 49. D'Ascenzo F, Quadri G, Cerrato E, Calcagno A, Omedè P, Grosso W, et al A meta‐analysis investigating incidence and features of stroke in HIV‐infected patients in the highly active antiretroviral therapy era. J Cardiovasc Med 2015; 16: 839–843. [DOI] [PubMed] [Google Scholar]
- 50. Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS 2014; 28: 2573–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. European Heart Rhythm Association , European Association for Cardio‐Thoracic Surgery , Camm AJ, Kirchhof P, Lip GY, Schotten U, et al Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 52. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) . Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents [online]. Available at https://aidsinfo.nih.gov/guidelines (last accessed 28 December 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Newcastle–Ottawa quality assessment scale for cohort studies (point assigned only to criteria marked with star)
Figure S1 Study selection process


