Abstract
Diabetics have higher morbidity and mortality in cardiovascular disease (CVD). A variety of antidiabetic agents are available for clinical choice. Cardiovascular (CV) safety assessment of these agents is crucial in addition to hypoglycemic effect before clinical prescription. Adenosine 5′-monophosphate-activated protein kinase (AMPK) is an important cell energy sensor, which plays an important role in regulating myocardial energy metabolism, reducing ischemia and ischemia/reperfusion (I/R) injury, improving heart failure (HF) and ventricular remodeling, ameliorating vascular endothelial dysfunction, antichronic inflammation, anti-apoptosis, and regulating autophagy. In this review, we summarized the effects of antidiabetic agents to CVD according to basic and clinical research evidence and put emphasis on whether these agents can play roles in CV system through AMPK-dependent signaling pathways. Metformin has displayed definite CV benefits related to AMPK. Sodium-glucose cotransporter 2 inhibitors also demonstrate sufficient clinical evidence for CV protection, but the mechanisms need further exploration. Glucagon-likepeptide1 analogs, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors and thiazolidinediones also show some AMPK-dependent CV benefits. Sulfonylureas and meglitinides may be unfavorable to CV system. AMPK is becoming a promising target for the treatment of diabetes, metabolic syndrome and CVD. But there are still some questions to be answered.
Keywords: AMPK, coronary artery disease, Diabetes
Introduction
The prevalence of diabetes has been growing rapidly over the past 20 years. Number of diabetic patients of 20–79 years old worldwide is expected to increase to 439 million by 2030 [1]. Diabetes is considered as a significant risk factor for cardiovascular disease (CVD), the primary cause of mortality worldwide [2,3]. In 2012, about 1.5 million people died of diabetes in the world, of which about 80% were associated with myocardial infarction (MI) or stroke [4].
Diabetes treatment aims to control multiple risk factors, such as hyperglycemia, hyperlipidemia, and hypertension, etc., in order to decrease the incidence of CVD and other complications. A variety of antidiabetic agents are available clinically, for example: sulfonylureas and meglitinides, biguanides, α-glucosidase inhibitors (AGIs), thiazolidinediones (TZDs), glucagon-like peptide1 (GLP-1) analogs, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, insulin and insulin analogs, etc. Previously, cardiovascular (CV) safety was rarely assessed in large clinical trials before antidiabetic agents were approved for market, and the majority of data obtained originally from postmarketing clinical observations. In 2007, however, an amazing result of a meta-analysis from 42 randomized trials treated with rosiglitazone, one of TZDs, was reported by Nissen and Wolski [5], which showed a possible increased risk of MI and death from CVD when comparing to the control group. This result sparked long-standing debate, although no excess CVD risk was reported in the following studies [6]. Consequently, a guidance about requiring the evidence of CV safety of novel antidiabetic agents by the pharmaceutical industry before approval was released by the US Food and Drug Administration (US FDA) [7].
Diabetic CVD is caused by many pathophysiological processes, such as macroangiopathy, microangiopathy, metabolic abnormalities, chronic inflammation, and fibrosis [8,9]. Pathogenesis and protective molecular mechanisms of diabetic CVD have been the focus of research in recent years. Adenosine 5′-monophosphate-activated protein kinase (AMPK) is an important serine and threonine protein kinase with the structure of three subunits (α, β, γ), which plays crucial roles in cell energy metabolism [10]. Increasing the ratio between intracellular adenosine monophosphate (AMP) and adenosine triphosphate (ATP), such as during strenuous exercise, hypoxia or nutritional deficiency, could phosphorylate a threonine, the 172th amino acid of the α subunit, thereby activating AMPK [11]. In addition, liver kinase B1 (LKB1), calmodulin-dependent protein kinase kinase β (CaMKKβ) and AMPK kinase (AMPKK) can be employed as upstream molecules. After activation, AMPK shuts down pathways of ATP-consuming and switches on catabolic pathways of ATP-producing through downstream signaling and target molecules [12,13], regulates lipid and protein metabolism, fatty acid oxidation, glucose uptake, gluconeogenesis, and autophagy [12–15], etc. AMPK also plays an important role in reducing oxidative stress, regulating autophagy, and anti-apoptosis of cardiomyocytes [16,17].
Our research team and others have reported that AMPK played cardioprotective roles during ischemia by increasing glucose uptake and glucose transporter 4 (GLUT4) translocation [18], decreasing apoptosis, improving postischemic recovery and limiting MI [7,19]. Furthermore, our studies have also suggested that activated protein C could activate AMPK and protect the heart from ischemia/reperfusion (I/R) injury [20], and inhibit inflammatory responses during hypoxia/reoxygenation (H/R) by modulating a JNK-mediated nuclear factor κB (NF-κB) pathway [21].
Antidiabetic agents may affect the CV system through many molecular signaling pathways. In this review, we intend to summarize the literature and discuss whether commonly used antidiabetic agents can affect CVD through AMPK-related signaling and molecular pathways.
Sulfonylureas and meglitinides
Sulfonylureas act by binding to sulphonylurea receptor 1 (SUR1) of pancreatic β cells, and close the ATP-sensitive potassium channels (KATP), causing an augment of intracellular K+, triggering of membrane depolarization, opening of voltage-dependent Ca2+ channels, increasing intracellular Ca2+ influx, then inducing insulin secretion [22]. Glibenclamide, glipizide, gliclazide, and glimepiride are commonly used in clinical practice. Meglitinides, including repaglinide, nateglinide and mitiglinide, display a similar hypoglycemic mechanism (binding to SUR2) as sulfonylureas.
Concern exists regarding the CV safety of sulfonylureas [23]. In the UK Prospective Diabetes Study (UKPDS), CV mortality was similar in patients of chlorpropamide group and insulin group [24]. Glibenclamide has been associated with acute MI and mortality [25], as well as being associated with blocking the protective effects of postconditioning [26]. By contrast, gliclazide and repaglinide appear to be related a lower risk than other sulfonylureas [25, 27]. Multiple research has suggested that cardiotoxicity of sulfonylureas is associated to the closure of specific KATP channels expressed in the heart [28], which could worsen the myocardial injury. Some newer sulfonylureas may not inhibit myocardial protection. For example, gliclazide and glimepiride appear not to prevent the protective effect of ischemic preconditioning in animals [29] and humans [30].
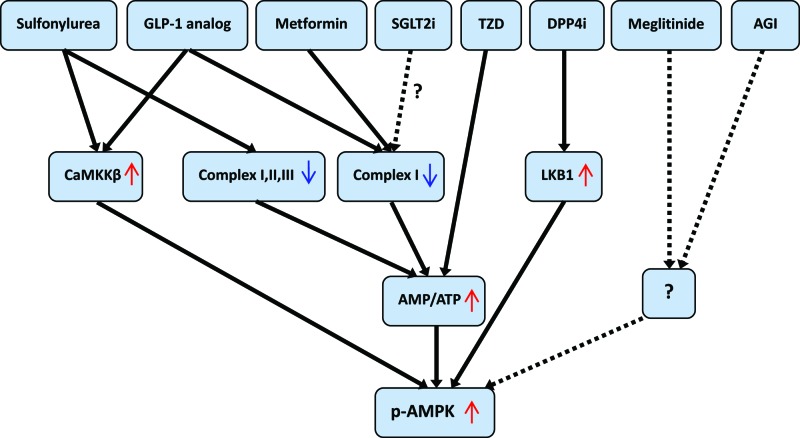
Very little research can be retrieved on the relationship between sulfonylureas and AMPK nor meglitinides and AMPK. Glibenclamide induced a dose-dependent increase of the AMP/ATP ratio by inhibiting complexes I, II, III [31], resulting in an increased AMPK phosphorylation in H9C2 cells. However, it profoundly changes cell metabolism in cardiomyocytes by impairing mitochondrial structure and function and induces irreversible damage beyond the benefits of AMPK activation. This may further explain the risk of CV events related to this drug. However, gliclazide can increase CaMKKβ and the phosphorylated AMPK levels in vascular smooth muscle cell (VSMC) and suppress platelet-derived growth factor (PDGF)-induced VSMC proliferation by the rising of intracellular Ca2+ concentration [32], which is beneficial for reducing CVD.
Biguanides
Biguanides were used for treatment of diabetes in humans in the 1920s [33] with several derivatives such as metformin, phenformin and buformin. Phenformin was withdrawn from the market in 1978 because of a rare but life-threatening side effect of lactic acidosis. Metformin is the most widely prescribed antidiabetic agent in individuals with type 2 diabetes (T2DM), which is the first-line oral therapy recommended by almost all guidelines, such as American Diabetes Association (ADA) [34], European Association of the Study of Diabetes (EASD) [35], and National Institute for Health and Care Excellence (NICE) [36], etc.
UKPDS [24] suggested that metformin reduced diabetes-related death by 42% and all-cause mortality by 36%. Similar results reported later from many clinical studies have shown CV protection and mortality reduction exerted by metformin appearing not to be dependent on its hypoglycemic effects [37–39]. Although the main antidiabetic effect of metformin was known as reducing hepatic glucose output and an increasing insulin-dependent peripheral glucose utilization [40,41], mainly by inhibiting gluconeogenesis [42], its molecular mechanism remained unclear until it was reported that it could activate AMPK in isolated hepatocytes [43]. Metformin inhibits complex I of the respiratory chain of the cell resulting in a decrease of the intracellular ATP concentration and an increase of the AMP/ATP ratio for the activation of AMPK [44–46], which is required for the CV protective effects of metformin [44,47]. Interestingly, there are also studies demonstrating that AMPK can be activated by metformin without changes in the AMP/ATP ratio [48,49] and metformin can also exert its beneficial metabolic effects on cardiomyocytes in an AMPK-independent manner [50].
Many studies suggest the pleiotropic effects of metformin mediated by activation of AMPK. We can summarize the effects of metformin on CVD through the AMPK pathways from the following aspects.
Energy metabolism of cardiomyocyte
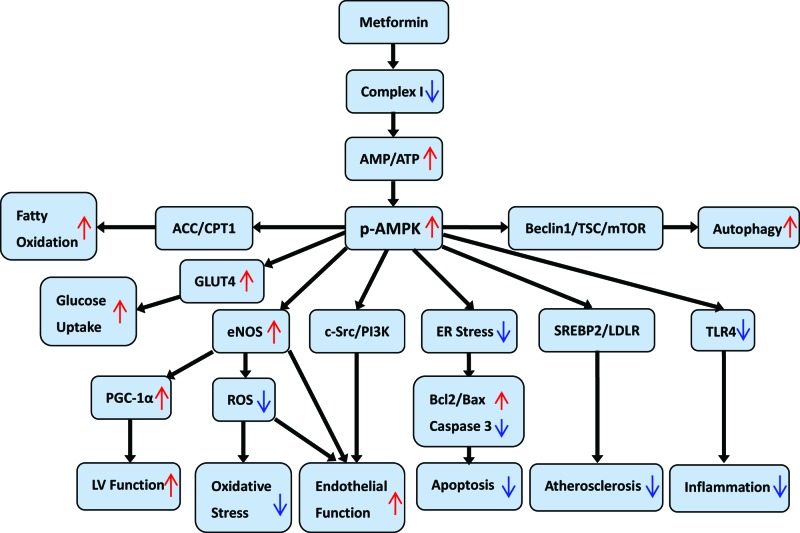
Impaired energy metabolism exists in many kinds of heart disease. After activation by metformin, AMPK can phosphorylate acetyl-coenzyme A carboxylase (ACC) and inhibit its function, which reduces the production of malonyl-CoA and the inhibitory effect of AMPK on carnitine palmitoyl transferase 1 (CPT1), promoting the oxidation of fatty acids [51]. In addition, activation of AMPK by metformin increases glucose uptake by inducing GLUT4 recruitment to the plasma membrane [52,53], prevents GLUT4 endocytosis and increases the residence time of GLUT4 in the plasma membrane thus increasing glucose transport and catabolism [54].
Vascular endothelium and oxidative stress
The dysfunction of endothelial cells plays a crucial role in the occurrence and development of CVD. Metformin exerts an inhibitory effect to mitochondrial reactive oxygen species (ROS) production by selectively blocking the reverse electron flow through complex I of respiratory chain [55]. Multiple studies indicated that activated AMPK is beneficial to endothelial function by suppressing oxidative stress in endothelial cells [56,57].
Endothelial nitric oxide synthase (eNOS) has a protective function in the CV system, which is attributed to NO production regulating the vascular tone. Administration of metformin in vivo increases AMPK phosphorylation in the aorta of mice, resulting in increased NO synthesis, and bioavailability [58]. Metformin can also increase mitochondria-derived peroxonitrite ONOO− to activate AMPK in c-Src/PI3K (phosphatidylinositol-3-kinases)-dependent manners in cultured bovine aortic endothelial cells [59]. A further study has demonstrated that AMPK activation by metformin increases the association between heat-shock protein 90 (Hsp90) and eNOS, which reduces eNOS-derived O2− [60]. In addition to antioxidant stress, metformin also regulates endothelial cell energy metabolism. For example, AMPK activation by metformin increases fatty acid oxidation, which can alleviate endothelial lipotoxicity and improve endothelial function [61].
AMPK is considered as an important target for endothelial dysfunction and atherosclerosis. As an AMPK activator, metformin has great potential for promoting endothelial function to resist atherosclerosis [62]. Metformin’s CV beneficial effects of atherosclerosis prevention are mediated in part through its ability of inhibiting the oxidative stress-mediated accumulation of cholesterol via AMPK-SREBP2 (sterol regulatory element-binding protein 2)-LDLR (low-density lipoprotein receptor) axis in vascular cells [63].
Heart failure and ventricular remodeling
Diabetes has a higher risk of developing heart failure (HF). Diabetic cardiomyopathy [64] is a common cause of HF in diabetics. It is characterized with reduced cardiomyocyte contractile function and apoptosis, mitochondrial pathology and dysfunction, and myocardial interstitial fibrosis [65].
Previously, metformin is considered contraindicated in patients with HF due to increase the risk of lactic acidosis. However, growing evidence indicates that this contraindication could be revised [66–68]. Accordingly, FDA removed the HF contraindication on the drug label for metformin in 2006, although congestive HF remains in the label’s warning section [69].
It has also been demonstrated that metformin has multiple beneficial AMPK-mediated effects in HF [70,71]. Several animal studies showed that metformin could delay the process of cardiac remodeling and the development of HF by a different pathway of AMPK activation [72]. Gundewar et al. [73] have carried out some experiments that metformin could significantly improve left ventricular (LV) function and survival by AMPK and its downstream mediators activation, peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and eNOS in a murine model of HF. Chronic administration of metformin to a dog model of cardiac pacing-induced HF attenuated the hemodynamic and structural changes by AMPK activation [74]. Moreover, chronic treatment with a low dose of metformin (100 mg/kg) exerts significant cardioprotection effect against HF of rat by activating the AMPK/eNOS pathway, as well as reducing circulating and myocardial levels of insulin, transforming growth factor beta 1 (TGF-β1), basic fibroblast growth factor (bFGF), and tumor necrosis factor α (TNFα) [75].
Myocardial ischemia and I/R injury
It has been tested that metformin and activated AMPK can play essential roles in the protection of myocardial ischemia and I/R injury by maintenance of the energy supply, and anti-oxidative stress [76]. Metformin (5 mM) in H9C2 cardiomyoblasts attenuated high glucose and H/R-induced cell injury, mitochondrial dysfunction, ROS over generation and inflammatory response through an AMPK/JNK-dependent signaling pathway [77]. A meta-analysis with 38 animals treated with metformin and 50 controls showed that the average infarct area at risk was reduced from 47.8 in the ischemia control group to 29.4 in the metformin group [78].In the study of isolated rat hearts, during the first 15 min of reperfusion metformin reduced infarct area with approximately 40–50% [79] by increased AMPK phosphorylation. Yin et al. [69] have also shown the reduction of the infarct size by metformin through AMPK phosphorylation in rats independent of systemic glucose levels. Metformin can also prevent acute death of cells in cardiac allografts by mainly suppressing intrinsic apoptosis due to I/R injury incurred from the transplantation procedure by AMPK activation [80].
Chronic myocardium inflammation
Recent studies have indicated that metformin has a direct anti-inflammatory action by inhibition of NF-κB via AMPK-dependent and independent pathways [81]. AMPK activation by short-term administration of metformin and the subsequent suppression of Toll-like receptor 4 (TLR4) expression and activity can suppress inflammatory responses and protect the infarcted heart [82]. However, Soraya et al. found that low dose pre-treatment of metformin chronically could suppress TLR4 signaling, inhibit the release of inflammatory mediators, and reverse LV contractile dysfunction in the setting of MI in an AMPK-independent manner [83].
Apoptosis
Cardiomyocyte apoptosis is common in CVD and diabetic cardiomyopathy. Experimental evidence suggested that metformin reduced the production of pro-apoptotic proteins, increased the anti-apoptotic proteins, and attenuated the percentage of apoptotic cardiomyocytes [84]. High-fat-induced cardiomyocyte apoptosis was partly blunted by metformin associated with increased AMPK phosphorylation [85]. Doxorubicin, a chemotherapy medication used to treat some cancer, can cause cardiotoxicity, and cardiomyocyte apoptosis. Metformin protected adult mouse cardiomyocytes (HL-1 cells) from doxorubicin-induced oxidative stress and apoptosis by modulating the expression of the adiponectin system via AMPK-mediated signaling [86]. Another research suggested that cardioprotective effects of metformin are mediated by AMPK activation, protein kinase A (PKA), Src, and platelet-derived growth factor receptor (PDGFR) [87].
Autophagy
Autophagy is a self-degradative process that is important for balancing sources of energy at critical times in development and in response to nutrient stress [88]. Declined AMPK activity and the following reduction in cardiac autophagy are central to the development of diabetic cardiomyopathy. Metformin significantly improved mitochondrial respiration and ATP synthesis of cardiomyocytes by an underlying mechanism requiring the AMPK activation and its downstream mediators eNOS and PGC-1α [73]. Xie et al. [72] found that AMPK activated by metformin stimulates autophagy activity in cardiomyocytes by modulating Beclin1 and the tuberous sclerosis complex (TSC) mammalian target of rapamycin (mTOR) pathway in OVE26 mice. Meanwhile, a study in a murine model demonstrated that activation of Pink1-AMPK signaling by metformin rescued against phosphatase and tensin homolog (PTEN) deletion-induced changes in myocardial geometry, function, and autophagy [89].
Downstream molecular signaling pathways of AMPK activated by metfromin for the protection of CV system are shown in Figure 1.
Figure 1. Potential downstream molecular signaling pathways of AMPK activated by metformin on cardioprotection.
Abbreviations: ER, endoplasmic reticulum; p-AMPK, phosphorylated AMPK.
α-Glucosidase inhibitors
AGIs are a type of widely used hypoglycemic agents with the mechanism of delaying the absorption of carbohydrates from the upper part of small intestine and producing a lowering effect to postprandial blood glucose. Acarbose, miglitol, and voglibose are involved.
Some clinical and basic studies have provided evidence of CV protection of AGIs. Acarbose reportedly reduced the morbidity of hypertension, CV events [90], and silent MI [91]. It also slowed progression of intima-media thickening of individuals with impaired glucose tolerance (IGT), improved carotid plaque echogenicity in patients with acute coronary syndrome (ACS) [92,93], and reduces the risk of MI in T2DM patients [94]. Acarbose could also stabilize carotid plaque within 1 month of therapy in patients with ACS and T2DM [93]. In the reports of basic research, acarbose reduced MI size in animals by opening mitochondrial KATP channels [95]. Some reports have suggested that the absorbed miglitol suppressed neointimal thickening of the arterial wall in animals [96,97]. Voglibose significantly decreased infarct size of nondiabetic rabbits during 30 min of ischemia and 48 h of reperfusion condition [98] by up-regulating GLP-1 levels and activating the GLP-1 receptors, with downstream activation of Akt, eNOS, and the mitochondrial KATP channels.
Research of AGIs on CVD through AMPK pathway is rare. Acarbose could improve vascular inflammation by enhancing NO expression to suppress cell cycle progression and inhibiting VSMC proliferation through AMPK activation and Ras inhibition, thus preventing or slowing the development of atherosclerosis [99]. Miglitol inhibits endothelial cell injury and protects against DNA damage under intensive oxidative stress, which may be involved in the activation of AMPK in endothelial cells, with the result of increasing NO production and reduced intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) mRNA expression [100]. Mulberry 1-deoxynojirimycin (DNJ) can be considered as an AGI analog with the effect of inhibiting α-glucosidase in the small intestine [101,102]. Chan’s group has indicated the mechanisms by which mulberry leaf DNJ effectively inhibit proliferation and migration of VSMCs, including AMPK/RhoB activation and down-regulation of FAK in vitro study [103].
Thiazolidinediones
TZDs increase insulin sensitivity through binding of the so-called peroxisome proliferator-activated receptor γ (PPAR-γ) to activate downstream genes that are involved in glucose and fatty acid metabolism. Of the members in this family, troglitazone has been withdrawed from the market, but rosiglitazone and pioglitazone remain in use.
In clinical studies, the effects of rosiglitazone and pioglitazone on CVD display diversity. Previous meta-analysis suggested that rosiglitazone use is associated with the risk of MI and death from CVD, and also increase risk of fluid retention which may exacerbate HF [5,104]. In a retrospective, observational trial, rosiglitazone was related with an increased risk of stroke, HF, and a composite outcomes of acute myocardial infarction (AMI) [105]. However, the prospective Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes (RECORD) trial found that there is no adverse effect of rosiglitazone on the composite outcome of CV death or hospitalization but report an increased risk of hospitalization for congestive heart failure (CHF) in patients using rosiglitazone versus active comparator [6]. Rosiglitazone has beneficial CV effects of nondiabetics in basic research [106]. Other clinical studies [107] and a meta-analysis [108] have demonstrated that pioglitazone reduces CV complications in individuals of T2DM. Despite the FDA’s release of rosiglitazone use and prescription restrictions in 2013 due to later clinical trial evidence [109,110], patients with HF are still very cautious to use.
TZDs are ligands for the nuclear hormone receptor family member PPAR-γ [111]. Both rosiglitazone and pioglitazone have been proved to activate AMPK in intact cells [112,113] by stimulating the release and expression of circulating adiponectin from adipose tissue [114–116], or indirectly by enhancing the cellular AMP/ATP ratio, possibly via a similar mechanism with biguanides [117]. Our group conducted a series of studies on the role of TZDs and AMPK in the heart. The result demonstrated that by using rosiglitazone acutely under I/R stress, MI is decreased and postischemic cardiac function is improved by modulating AMPK, Akt, and JNK signaling mechanisms in the nondiabetic mouse heart [118].
Many studies have suggested that TZDs can attenuate myocardial hypertrophy mediated by AMPK. It has been shown that adiponectin stimulates the phosphorylation of AMPK and suppresses agonist-stimulated extracellular regulated protein kinases (ERK1/2) activation and hypertrophic response in cultured cardiomyocytes through activating AMPK signaling [119]. Antihypertrophic effect of pioglitazone is attributed to reduced ERK1/2 activation that is involved in the adiponectin-AMPK regulatory axis [120]. Administration of pioglitazone with long term delayed the development of LV hypertrophy and fibrosis as well as inhibited phosphorylation of mTOR and p70S6 kinase in the heart, which are likely attributable, at least in part, not only to the AMPK activation through stimulation of adiponectin secretion but also to the Akt signaling inhibition in the heart [121].
TZDs also plays an essential role in adjusting energy metabolism. Troglitazone could significantly increase glucose uptake and activated both AMPK and eNOS signaling in isolated papillary muscles [122]. Adiponectin stimulated by TZDs can bind to adiponectin receptor 1 (AdipoR1) and activate AMPK/ACC/CPT-1 pathway to enhance fatty acid β-oxidation in the heart, a pathway that is also regulated by PGC-1α and PPARα [123]. However, rosiglitazone does not always increase glucose and fatty acid metabolism in adiponectin/AMPK pathway. An interesting research showed that activation of PPAR-γ in the late-gestation sheep fetus, rosiglitazone may decrease cardiac metabolism (glucose uptake, fatty acid β-oxidation) and cardiomyocyte size by down-regulating AdipoR1, phospho-AMPK, phospho-ACC, and PGC-1α [124].
GLP-1 analogs
GLP-1 is a type of incretin hormone that is secreted from L-cells of the small intestine in a glucose-dependent manner to stimulate insulin secretion, increase pancreatic β cell mass, and inhibits glucagon secretion and gastric emptying, thus reducing postprandial glycemia [125,126]. Due to rapid degradation by DPP-4, the half-life of endogenous GLP-1 is very short. Longer half-life synthetic analogs have been developed for clinical use as a new class of antidiabetic agents, such as: exenatide, liraglutide, lixisenatide, albiglutide, dulaglutide, and semaglutide, etc.
Several large clinical studies have revealed that GLP-1 analogs can reduce the risk of major adverse cardiovascular events (MACE), nonfatal MI, and CV death, etc., in T2DM [127–130]. GLP-1 analogs play CV protective roles mediated by GLP-1 receptor (GLP1R) in CV tissues, among which, activation of AMPK signaling pathway is still crucial. Considerable evidences demonstrate that GLP-1 protects the isolated mouse heart against I/R injury by AMPK pathway [131]. GLP-1 analogs have also been shown to exert direct cardioprotective effects of MI in murine models [132]. Liraglutide could increase AMPK phosphorylation in the hearts of obese mice with the similar effect to metformin [73]. A short-term treatment with a weight-neutral dose of liraglutide can reverse the molecular pathophysiology of obesity-induced heart disease in mice through a variety of putative mechanisms with a central role for AMPK [133]. GLP-1 analogs can also display the effect in balancing energy metabolism and maintaining heart function of diabetic models. Guo’s [134] study showed that exenatide treatment increased the level of phosphorylation of AMPK and the mRNA expression of GLUT4 in the diabetic heart of rats. The increased adiponectin may partially explain these change, which might contribute to the ameliorated heart function. After being treated with exenatide, the adiponectin and high-molecular-weight-adiponectin and the APPL1-AMPK-PPARα axis were increased, the NF-κB and the apoptosis were decreased, and the cardiac function of the diabetic rats was improved [135].
We have discussed the essentiality of autophagy for cell survival above. Liraglutide relieved myocardial damage by enhancing autophagy via AMPK-mTOR signaling pathway in Zucker diabetic fatty rat [136]. In adult rat cardiomyocytes, GLP-1 activates AMPK, then inhibits the hyperglycemia-induced NADPH oxidase 2 (NOX2) activation by limiting protein kinase C (PKC) phosphorylation and p47phox translocation to the caveolae; thereby, preventing glucotoxicity [137]. Abbas and Kabil [138] demonstrated that liraglutide treatment had been shown to relieve doxorubicin-induced cardiotoxicity, may be attributed to the antioxidant and anti-inflammatory effects as well as anti-apoptotic effects through the AMPK/Akt/GSK-3β (glycogen synthase kinase 3β) signaling pathway.
Meanwhile, administration of liraglutide protected against myocardial steatosis and oxidative stress by activation of the AMPK-Sirt1 (silent mating type information regulation 2 homolog) pathway [139] at least in part. GLP-1 analogs can also improve endothelial dysfunction. Liraglutide plays an anti-inflammatory role to primary human aortic endothelial cells (HAECs) by causing a subsequent increase in intracellular calcium, CaMKKβ activity and AMPK activation [140]. Exenatide significantly improves coronary artery endothelial function of individuals with newly diagnosed T2DM. The improvement effect may be mediated by activation of the AMPK/PI3K-Akt/eNOS pathway via a GLP-1R/cAMP-dependent mechanism [141].
DPP-4 inhibitors
DPP-4 inhibitors are a group of agents for treating T2DM [126]. They prevent the deactivation of the two endogenous incretine hormones, GLP-1 and glucose-dependent-insulinotropic-peptide (GIP), thus causing the accumulation of these hormones [142] to make these hormones play the role of antidiabetes. Sitagliptin, saxagliptin, vildagliptin, alogliptin, linagliptin, and trelagliptin are approved on market as DPP-4 inhibitors family members. Several large clinical studies have shown that DPP-4 inhibitors do not increase the risk of CVD in type 2 diabetics compared with placebos [143–145].
It has been reported that DPP-4 inhibitors can limit infarct size in the nondiabetic mice [146] and isolated rat hearts [147]. Sitagliptin has been reported to play a protective role in CVD and atherosclerosis [148,149]. Sitagliptin can decrease the atherosclerotic lesion area by activating AMPK-mediated Akt signaling pathway in ApoE−/− mice while attenuating the phosphorylation of p38 and ERK1/2 and mitogen-activated protein kinase (MAPK), therefore, inhibiting inflammatory responses in the aorta, such as the release of monocyte chemoattractant protein 1 (MCP-1) and interleukin 6 (IL-6), and the expression of the VCAM-1 and serum P-selectin [150]. One study indicated that sitagliptin inhibits endothelin-1 (ET-1) expression in the aortic endothelium by suppressing the NF-κB/IκBα system through the activation of the AMPK pathway in diabetic rats, which demonstrated some of the vasoprotective properties of DPP-4 inhibitors in vivo [151]. In other studies, sitagliptin prevented hyperglycemia induced apoptosis via activation of AMPK in HUVECs and also attenuated myocardial apoptosis by activating LKB-1/AMPK/Akt signaling pathway and suppressing the activity of GSK-3β and p38α/MAPK in diabetic cardiomyopathy of rat [152].
However, Lenski’s group found that [153] sitagliptin treatment reduced the increased phosphorylation of AMPK and ACC in db/db−/−mice, then reduced membrane translocation of GLUT4 in cardiomyocytes, thus prevented the metabolic alteration associated with the diabetes-obesity syndrome via AMPK and its downstream molecule in the myocardium.
SGLT2 inhibitors
SGLT2 inhibitors selectively inhibit SGLT2 of the renal proximal tubule, with a consequent decrease in renal tubular thresholds for glycosuria and increase in urinary excretion of glucose, reducing blood glucose independently of insulin. Canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin of this family have been approved by FDA.
As the request of FDA, some clinical trials for CV risk assessments were conducted before market. EMPA REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and Mortality in type 2 diabetes) demonstrated that empagliflozin exerts a 38% risk reduction in death from CV causes, 32% risk reduction death from any cause and 35% reduction on risk of hospitalization for HF [154]. CVD-REAL study has confirmed that the positive effects on HF of SGLT2 inhibitors can be considered a class effect, not only by empagliflozin [155]. CANVAS study for evaluating canagliflozin also showed similar CV protective effect [156]. FDA has approved a new indication for empagliflozin to reduce the risk of CVD death in adult patients with T2DM and CVD in December, 2016 [157].
After clinical analysis, the protective effect of SGLT2 inhibitors on heart may be related to lowering blood pressure, weight loss, decreasing serum uric acid level, osmotic diuresis, reducing volume load and hemodynamic changes, etc. Further molecular mechanism is still in the exploratory stage. At present, basic research are focusing on energy metabolism, inflammation, oxidative stress, myocardial fibrosis and electrolyte homeostasis [158]. SGLT2 inhibitors change the energy metabolism of the heart from glucose to fat [159–161] and slightly increase the ketone level [162], which is beneficial for cardiac energy supply during HF. Empagliflozin can significantly improve myocardial fibrosis in obese and diabetic mice [163,164], and also play the role of anti-oxidative stress and anti-apoptosis [165,166]. Meanwhile, empagliflozin can reduce infarct size after I/R [167], and improve diastolic function of the left ventricle in diabetic mice [168]. Dapagliflozin can delay the occurrence and progress of diabetic cardiomyopathy [169].
There are currently relatively few studies on SGLT2 inhibitors and AMPK. Canagliflozin activates AMPK human embryonic kidney (HEK-293) cells and hepatocytes by inhibiting complex I in the mitochondrial respiratory chain and increasing cellular AMP levels [170]. Clinically-relevant canagliflozin concentrations can directly inhibit endothelial pro-inflammatory chemokine/cytokine secretion by AMPK dependent and independent mechanisms without affecting early interleukin-1β (IL-1β) signaling [171]. Dapagliflozin decreases the activation of the NOD-like receptor family, pyrin domain containing 3/apoptosis-associated speck-like protein containing a CARD (NLRP3/ASC) inflammasome attenuated myocardial inflammation, fibrosis, apoptosis, and diabetic remodeling likely mediated through AMPK activation [164]. In another study, empagliflozin alleviated diabetic cardiac microvascular endothelial cell (CMEC) injury by inhibiting mitochondrial fission via the activation of AMPK-Drp1 (Dynamin-related protein 1) signaling pathways, preserved cardiac CMEC barrier function through suppressed mitochondrial ROS production and subsequently oxidative stress to inhibit CMEC senescence. So empagliflozin can be considered as a cardiac microvascular-protection agents to maintain cardiac circulatory function and structure upon hyperglycemic insult [172].
Our group is currently studying the molecular mechanisms underlying the cardioprotective effect of empagliflozin. Preliminary results show that a certain concentration of empagliflozin can enhance the contractility of isolated mice cardiomyocytes under the condition of intracellular hypoxia. At baseline, empagliflozin can phosphorylate AMPK in mice cardiomyocytes. In intracellular hypoxia state induced by sodium cyanide (NaCN), empagliflozin can prolong AMPK activation time of mice cardiomyocytes (unpublished data). The further molecular mechanisms of AMPK activation is still under exploration.
We summarized the upstream signal pathways of AMPK activated by antidiabetic agentsin CV system in Figure 2.
Figure 2. Upstream signaling pathways of AMPK activation by antidiabetic agents.
Abbreviations: DPP4i, DPP-4 inhibitors; p-AMPK, phosphorylated AMPK; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Pespectives
We enumerate the CV safety of commonly used antidiabetic agents in clinical practice and summarize whether these drugs can affect CVD through AMPK-related signaling pathways in this review to help clinicians for selection of antidiabetic agents. The mechanism of metformin on activating AMPK is relatively clear, but it is still obscure for other antidiabetic agents. Therefore, further research is needed to find answers from the intricate AMPK signal transduction network. Given the benefits of AMPK activation for diabetes and CVD, AMPK is becoming a promising target for the treatment of diabetes, metabolic syndrome, and CVD. There are still some problems to be solved. First, AMPK has many subtypes, and the expression of each subunit is different among species and tissues, making it more difficult to translate AMPK activators from pre-clinical animal experiment to clinical trial. Second, AMPK is expressed in many organs and tissues of the whole body. Whether systemic activation caused by nonspecific AMPK agonists has adverse effects on some organs is unknown. Therefore, it is necessary to develop organ-specific AMPK agonists. Third, the AMPK signaling network is very complicated, and there are many cross-talk with other pathways. The effect of activating AMPK on other pathways also needs a lot of research to confirm. At last, what is the activation duration and degree of AMPK? It is also unknown whether excessive or prolonged activation will bring adverse effects. Therefore, there is still a long way to go on progressing AMPK from basic research to clinical application.
Abbreviations
- ACC
coenzyme A carboxylase
- ACS
acute coronary syndrome
- AdipoR
adiponectin receptor
- AGI
α-glucosidase inhibitor
- AMPK
adenosine 5′-monophosphate-activated protein kinase
- CaMKK
calmodulin-dependent protein kinase kinase
- CMEC
cardiac microvascular endothelial cell
- CV
cardiovascular
- CVD
cardiovascular disease
- DNJ
deoxynojirimycin
- DPP
dipeptidyl peptidase
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular regulated protein kinase
- GLP
glucagon-like peptide
- GLUT
glucose transporter
- GSK
glycogen synthase kinase
- H/R
hypoxia/reoxygenation
- I/R
ischemia/reperfusion
- KATP
ATP-sensitive potassium channel
- LDLR
low-density lipoprotein receptor
- LKB
liver kinase B
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor κB
- PGC
peroxisome proliferator-activated receptor γ coactivator 1-α
- PI3K
phosphatidylinositol-3-kinase
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- SGLT
sodium-glucose cotransporter
- SREBP2
sterol regulatory element-binding protein
- T2DM
type 2 diabetes
- TLR
Toll-like receptor
- TZD
thiazolidinedione
- UKPDS
UK Prospective Diabetes Study
- VCAM
vascular cell adhesion molecule
- VSMC
vascular smooth muscle cell
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The present study was supported by American Diabetes Association [grant number 1-17-IBS-296]; [grant numbers NIH R01AG049835 R01GM124108, P01HL051971 and P20GM104357].
References
- 1.Shaw J.E., Sicree R.A. and Zimmet P.Z. (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Mazzone T. (2010) Intensive glucose lowering and cardiovascular disease prevention in diabetes: reconciling the recent clinical trial data. Circulation 122, 2201–2211 10.1161/CIRCULATIONAHA.109.913350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawshani A., Sattar N., Franzen S.. et al. (2018) Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392, 477–486 10.1016/S0140-6736(18)31506-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox C.S., Golden S.H., Anderson C.. et al. (2015) Update on prevention of cardiovascular disease in adults with type 2 Diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 38, 1777–1803 10.2337/dci15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissen S.E. and Wolski K. (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- 6.Home P.D., Pocock S.J., Beck-Nielsen H.. et al. (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373, 2125–2135 10.1016/S0140-6736(09)60953-3 [DOI] [PubMed] [Google Scholar]
- 7.Ma H., Wang J., Thomas D.P.. et al. (2010) Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation 122, 282–292 10.1161/CIRCULATIONAHA.110.953208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acar E., Ural D., Bildirici U., Sahin T. and Yilmaz I. (2011) Diabetic cardiomyopathy. Anadolu Kardiyol. Derg. 11, 732–737 [DOI] [PubMed] [Google Scholar]
- 9.Voulgari C., Papadogiannis D. and Tentolouris N. (2010) Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc. Health Risk Manag. 6, 883–903 10.2147/VHRM.S11681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie D.G. (2004) The AMP-activated protein kinase pathway–new players upstream and downstream. J. Cell Sci. 117, 5479–5487 10.1242/jcs.01540 [DOI] [PubMed] [Google Scholar]
- 11.Hardie D.G. and Carling D. (1997) The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 10.1111/j.1432-1033.1997.00259.x [DOI] [PubMed] [Google Scholar]
- 12.Ruderman N.B., Carling D., Prentki M. and Cacicedo J.M. (2013) AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 123, 2764–2772 10.1172/JCI67227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinberg G.R. and Kemp B.E. (2009) AMPK in health and disease. Physiol. Rev. 89, 1025–1078 10.1152/physrev.00011.2008 [DOI] [PubMed] [Google Scholar]
- 14.Woods A., Johnstone S.R., Dickerson K.. et al. (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004–2008 10.1016/j.cub.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 15.Hurley R.L., Anderson K.A., Franzone J.M., Kemp B.E., Means A.R. and Witters L.A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 10.1074/jbc.M503824200 [DOI] [PubMed] [Google Scholar]
- 16.Bertrand L., Ginion A., Beauloye C.. et al. (2006) AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am. J. Physiol. Heart Circ. Physiol. 291, H239–H250 10.1152/ajpheart.01269.2005 [DOI] [PubMed] [Google Scholar]
- 17.He C., Zhu H., Li H., Zou M.H. and Xie Z. (2013) Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 62, 1270–1281 10.2337/db12-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller E.J., Li J., Leng L.. et al. (2008) Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 451, 578–582 10.1038/nature06504 [DOI] [PubMed] [Google Scholar]
- 19.Russell R.R. 3rd, Li J., Coven D.L.. et al. (2004) AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 114, 495–503 10.1172/JCI19297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Yang L., Rezaie A.R. and Li J. (2011) Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J. Thromb. Haemost. 9, 1308–1317 10.1111/j.1538-7836.2011.04331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., Li X., Zhang W.. et al. (2018) Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-kappaB pathway. Metabolism 83, 256–270 10.1016/j.metabol.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi H., Shibasaki T., Park J.H.. et al. (2015) Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes 64, 1262–1272 10.2337/db14-0576 [DOI] [PubMed] [Google Scholar]
- 23.Klamann A., Sarfert P., Launhardt V., Schulte G., Schmiegel W.H. and Nauck M.A. (2000) Myocardial infarction in diabetic vs non-diabetic subjects. Survival and infarct size following therapy with sulfonylureas (glibenclamide). Eur. Heart J. 21, 220–229 [DOI] [PubMed] [Google Scholar]
- 24.(1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 25.Schramm T.K., Gislason G.H., Vaag A.. et al. (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur. Heart J. 32, 1900–1908 10.1093/eurheartj/ehr077 [DOI] [PubMed] [Google Scholar]
- 26.Yang X.M., Proctor J.B., Cui L., Krieg T., Downey J.M. and Cohen M.V. (2004) Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J. Am. Coll. Cardiol. 44, 1103–1110 10.1016/j.jacc.2004.05.060 [DOI] [PubMed] [Google Scholar]
- 27.Gribble F.M. and Ashcroft F.M. (2000) Sulfonylurea sensitivity of adenosine triphosphate-sensitive potassium channels from beta cells and extrapancreatic tissues. Metabolism 49, 3–6 10.1053/meta.2000.17822 [DOI] [PubMed] [Google Scholar]
- 28.Brown N.J. (2012) Cardiovascular effects of antidiabetic agents: focus on blood pressure effects of incretin-based therapies. J. Am. Soc. Hypertens. 6, 163–168 10.1016/j.jash.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddock H.L., Siedlecka S.M. and Yellon D.M. (2004) Myocardial protection from either ischaemic preconditioning or nicorandil is not blocked by gliclazide. Cardiovasc. Drugs Ther. 18, 113–119 10.1023/B:CARD.0000029028.75316.5e [DOI] [PubMed] [Google Scholar]
- 30.Lee T.M. and Chou T.F. (2003) Impairment of myocardial protection in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 88, 531–537 10.1210/jc.2002-020904 [DOI] [PubMed] [Google Scholar]
- 31.Salani B., Ravera S., Fabbi P.. et al. (2017) Glibenclamide mimics metabolic effects of metformin in H9c2 cells. Cell. Physiol. Biochem. 43, 879–890 10.1159/000481638 [DOI] [PubMed] [Google Scholar]
- 32.Lee K.Y., Kim J.R. and Choi H.C. (2018) Gliclazide, a KATP channel blocker, inhibits vascular smooth muscle cell proliferation through the CaMKKbeta-AMPK pathway. Vascul. Pharmacol. 102, 21–28 10.1016/j.vph.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 33.Hardie D.G., Ross F.A. and Hawley S.A. (2012) AMP-activated protein kinase: a target for drugs both ancient and modern. Chem. Biol. 19, 1222–1236 10.1016/j.chembiol.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes A (2018) 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care 41, S73–S85 [DOI] [PubMed] [Google Scholar]
- 35.Nathan D.M., Buse J.B., Davidson M.B.. et al. (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32, 193–203 10.2337/dc08-9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler A.I., Shaw E.J., Stokes T., Ruiz F. and Guideline Development G (2009) Newer agents for blood glucose control in type 2 diabetes: summary of NICE guidance. BMJ 338, b1668 10.1136/bmj.b1668 [DOI] [PubMed] [Google Scholar]
- 37.Masoudi F.A., Inzucchi S.E., Wang Y., Havranek E.P., Foody J.M. and Krumholz H.M. (2005) Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 111, 583–590 10.1161/01.CIR.0000154542.13412.B1 [DOI] [PubMed] [Google Scholar]
- 38.Johnson J.A., Majumdar S.R., Simpson S.H. and Toth E.L. (2002) Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 25, 2244–2248 10.2337/diacare.25.12.2244 [DOI] [PubMed] [Google Scholar]
- 39.Pantalone K.M., Kattan M.W., Yu C.. et al. (2010) The risk of overall mortality in patients with type 2 diabetes receiving glipizide, glyburide, or glimepiride monotherapy: a retrospective analysis. Diabetes Care 33, 1224–1229 10.2337/dc10-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirpichnikov D., McFarlane S.I. and Sowers J.R. (2002) Metformin: an update. Ann. Intern. Med. 137, 25–33 10.7326/0003-4819-137-1-200207020-00009 [DOI] [PubMed] [Google Scholar]
- 41.Cusi K., Consoli A. and DeFronzo R.A. (1996) Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 81, 4059–4067 [DOI] [PubMed] [Google Scholar]
- 42.Hundal R.S., Krssak M., Dufour S.. et al. (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 10.2337/diabetes.49.12.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natali A. and Ferrannini E. (2006) Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 49, 434–441 10.1007/s00125-006-0141-7 [DOI] [PubMed] [Google Scholar]
- 44.Zhou G., Myers R., Li Y.. et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen M.R., Doran E. and Halestrap A.P. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 607–614, 348 10.1042/bj3480607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Mir M.Y., Nogueira V., Fontaine E., Averet N., Rigoulet M. and Leverve X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 10.1074/jbc.275.1.223 [DOI] [PubMed] [Google Scholar]
- 47.Xie Z., He C. and Zou M.H. (2011) AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 7, 1254–1255 10.4161/auto.7.10.16740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawley S.A., Gadalla A.E., Olsen G.S. and Hardie D.G. (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51, 2420–2425 10.2337/diabetes.51.8.2420 [DOI] [PubMed] [Google Scholar]
- 49.Bergheim I., Guo L., Davis M.A.. et al. (2006) Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology 130, 2099–2112 10.1053/j.gastro.2006.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saeedi R., Parsons H.L., Wambolt R.B.. et al. (2008) Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am. J. Physiol. Heart Circ. Physiol. 294, H2497–H2506 10.1152/ajpheart.00873.2007 [DOI] [PubMed] [Google Scholar]
- 51.Ruderman N.B., Saha A.K. and Kraegen E.W. (2003) Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology 144, 5166–5171 10.1210/en.2003-0849 [DOI] [PubMed] [Google Scholar]
- 52.Kurth-Kraczek E.J., Hirshman M.F., Goodyear L.J. and Winder W.W. (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48, 1667–1671 10.2337/diabetes.48.8.1667 [DOI] [PubMed] [Google Scholar]
- 53.Yang J. and Holman G.D. (2006) Long-term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology 147, 2728–2736 10.1210/en.2005-1433 [DOI] [PubMed] [Google Scholar]
- 54.Zhang L., He H. and Balschi J.A. (2007) Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am. J. Physiol. Heart Circ. Physiol. 293, H457–H466 10.1152/ajpheart.00002.2007 [DOI] [PubMed] [Google Scholar]
- 55.Schafer H.J., Mainka L., Rathgeber G. and Zimmer G. (1983) Photoaffinity cross-linking of oligomycin-sensitive ATPase from beef heart mitochondria by 3′-arylazido-8-azido ATP. Biochem. Biophys. Res. Commun. 111, 732–739 10.1016/0006-291X(83)90366-2 [DOI] [PubMed] [Google Scholar]
- 56.Sambe T., Mason R.P., Dawoud H., Bhatt D.L. and Malinski T. (2018) Metformin treatment decreases nitroxidative stress, restores nitric oxide bioavailability and endothelial function beyond glucose control. Biomed. Pharmacother. 98, 149–156 10.1016/j.biopha.2017.12.023 [DOI] [PubMed] [Google Scholar]
- 57.Kukidome D., Nishikawa T., Sonoda K.. et al. (2006) Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55, 120–127 10.2337/diabetes.55.01.06.db05-0943 [DOI] [PubMed] [Google Scholar]
- 58.Calvert J.W., Gundewar S., Jha S.. et al. (2008) Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 57, 696–705 10.2337/db07-1098 [DOI] [PubMed] [Google Scholar]
- 59.Zou M.H., Kirkpatrick S.S., Davis B.J.. et al. (2004) Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J. Biol. Chem. 279, 43940–43951 [DOI] [PubMed] [Google Scholar]
- 60.Davis B.J., Xie Z., Viollet B. and Zou M.H. (2006) Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55, 496–505 10.2337/diabetes.55.02.06.db05-1064 [DOI] [PubMed] [Google Scholar]
- 61.McCarty M.F. (2005) AMPK activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med. Hypotheses 64, 1211–1215 10.1016/j.mehy.2004.01.042 [DOI] [PubMed] [Google Scholar]
- 62.Gao F., Chen J. and Zhu H. (2018) A potential strategy for treating atherosclerosis: improving endothelial function via AMP-activated protein kinase. Sci. China Life Sci. 61, 1024–1029 10.1007/s11427-017-9285-1 [DOI] [PubMed] [Google Scholar]
- 63.Gopoju R., Panangipalli S. and Kotamraju S. (2018) Metformin treatment prevents SREBP2-mediated cholesterol uptake and improves lipid homeostasis during oxidative stress-induced atherosclerosis. Free Radic. Biol. Med. 118, 85–97 10.1016/j.freeradbiomed.2018.02.031 [DOI] [PubMed] [Google Scholar]
- 64.Picano E. (2003) Diabetic cardiomyopathy. the importance of being earliest. J. Am. Coll. Cardiol. 42, 454–457 10.1016/S0735-1097(03)00647-8 [DOI] [PubMed] [Google Scholar]
- 65.Boudina S. and Abel E.D. (2007) Diabetic cardiomyopathy revisited. Circulation 115, 3213–3223 10.1161/CIRCULATIONAHA.106.679597 [DOI] [PubMed] [Google Scholar]
- 66.Khurana R. and Malik I.S. (2010) Metformin: safety in cardiac patients. Heart 96, 99–102 [DOI] [PubMed] [Google Scholar]
- 67.Inzucchi S.E., Masoudi F.A. and McGuire D.K. (2007) Metformin in heart failure. Diabetes Care 30, e129 10.2337/dc07-1686 [DOI] [PubMed] [Google Scholar]
- 68.Tahrani A.A., Varughese G.I., Scarpello J.H. and Hanna F.W. (2007) Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 335, 508–512 10.1136/bmj.39255.669444.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin M., van der Horst I.C., van Melle J.P.. et al. (2011) Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H459–H468 10.1152/ajpheart.00054.2011 [DOI] [PubMed] [Google Scholar]
- 70.Kim T.T. and Dyck J.R. (2015) Is AMPK the savior of the failing heart? Trends Endocrinol. Metab. 26, 40–48 10.1016/j.tem.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 71.Varjabedian L., Bourji M., Pourafkari L. and Nader N.D. (2018) Cardioprotection by metformin: beneficial effects beyond glucose reduction. Am. J. Cardiovasc. Drugs 18, 181–193 10.1007/s40256-018-0266-3 [DOI] [PubMed] [Google Scholar]
- 72.Xie Z., Lau K., Eby B.. et al. (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60, 1770–1778 10.2337/db10-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gundewar S., Calvert J.W., Jha S.. et al. (2009) Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 104, 403–411 10.1161/CIRCRESAHA.108.190918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasaki H., Asanuma H., Fujita M.. et al. (2009) Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 119, 2568–2577 10.1161/CIRCULATIONAHA.108.798561 [DOI] [PubMed] [Google Scholar]
- 75.Wang X.F., Zhang J.Y., Li L., Zhao X.Y., Tao H.L. and Zhang L. (2011) Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin. Exp. Pharmacol. Physiol. 38, 94–101 10.1111/j.1440-1681.2010.05470.x [DOI] [PubMed] [Google Scholar]
- 76.Ye Y., Perez-Polo J.R., Aguilar D. and Birnbaum Y. (2011) The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res. Cardiol. 106, 925–952 10.1007/s00395-011-0216-6 [DOI] [PubMed] [Google Scholar]
- 77.Hu M., Ye P., Liao H., Chen M. and Yang F. (2016) Metformin protects H9C2 cardiomyocytes from high-glucose and hypoxia/reoxygenation injury via inhibition of reactive oxygen species generation and inflammatory responses: role of AMPK and JNK. J. Diabetes Res. 2016, 2961954 10.1155/2016/2961954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paiva M., Riksen N.P., Davidson S.M.. et al. (2009) Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J. Cardiovasc. Pharmacol. 53, 373–378 10.1097/FJC.0b013e31819fd4e7 [DOI] [PubMed] [Google Scholar]
- 79.Paiva M.A., Goncalves L.M., Providencia L.A., Davidson S.M., Yellon D.M. and Mocanu M.M. (2010) Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc. Drugs Ther. 24, 25–32 10.1007/s10557-010-6222-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chin J.T., Troke J.J., Kimura N.. et al. (2011) A novel cardioprotective agent in cardiac transplantation: metformin activation of AMP-activated protein kinase decreases acute ischemia-reperfusion injury and chronic rejection. Yale J. Biol. Med. 84, 423–432 [PMC free article] [PubMed] [Google Scholar]
- 81.Saisho Y. (2015) Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr. Metab. Immune. Disord. Drug Targets 15, 196–205 10.2174/1871530315666150316124019 [DOI] [PubMed] [Google Scholar]
- 82.Soraya H., Farajnia S., Khani S.. et al. (2012) Short-term treatment with metformin suppresses toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat: are AMPK and TLRs connected? Int. Immunopharmacol. 14, 785–791 10.1016/j.intimp.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 83.Soraya H., Clanachan A.S., Rameshrad M., Maleki-Dizaji N., Ghazi-Khansari M. and Garjani A. (2014) Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur. J. Pharmacol. 737, 77–84 10.1016/j.ejphar.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 84.Yeh C.H., Chen T.P., Wang Y.C., Lin Y.M. and Fang S.W. (2010) AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediators Inflamm. 2010, 130636 10.1155/2010/130636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.An D., Kewalramani G., Chan J.K.. et al. (2006) Metformin influences cardiomyocyte cell death by pathways that are dependent and independent of caspase-3. Diabetologia 49, 2174–2184 10.1007/s00125-006-0338-9 [DOI] [PubMed] [Google Scholar]
- 86.Asensio-Lopez M.C., Lax A., Pascual-Figal D.A., Valdes M. and Sanchez-Mas J. (2011) Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic. Biol. Med. 51, 1861–1871 10.1016/j.freeradbiomed.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 87.Kobashigawa L.C., Xu Y.C., Padbury J.F., Tseng Y.T. and Yano N. (2014) Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: an in vitro study. PLoS ONE 9, e104888 10.1371/journal.pone.0104888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glick D., Barth S. and Macleod K.F. (2010) Autophagy: cellular and molecular mechanisms. J. Pathol. 221, 3–12 10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roe N.D., Xu X., Kandadi M.R.. et al. (2015) Targeted deletion of PTEN in cardiomyocytes renders cardiac contractile dysfunction through interruption of Pink1-AMPK signaling and autophagy. Biochim. Biophys. Acta 1852, 290–298 10.1016/j.bbadis.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papanas N. and Maltezos E. (2009) Oral antidiabetic agents: anti-atherosclerotic properties beyond glucose lowering? Curr. Pharm. Des. 15, 3179–3192 10.2174/138161209789057995 [DOI] [PubMed] [Google Scholar]
- 91.Zeymer U., Schwarzmaier-D’assie A., Petzinna D., Chiasson J.L. and Group S-NTR (2004) Effect of acarbose treatment on the risk of silent myocardial infarctions in patients with impaired glucose tolerance: results of the randomised STOP-NIDDM trial electrocardiography substudy. Eur. J. Cardiovasc. Prev. Rehabil. 11, 412–415 10.1097/00149831-200410000-00009 [DOI] [PubMed] [Google Scholar]
- 92.Hanefeld M., Chiasson J.L., Koehler C., Henkel E., Schaper F. and Temelkova-Kurktschiev T. (2004) Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke 35, 1073–1078 10.1161/01.STR.0000125864.01546.f2 [DOI] [PubMed] [Google Scholar]
- 93.Hirano M., Nakamura T., Obata J.E.. et al. (2012) Early improvement in carotid plaque echogenicity by acarbose in patients with acute coronary syndromes. Circ. J. 76, 1452–1460 10.1253/circj.CJ-11-1524 [DOI] [PubMed] [Google Scholar]
- 94.Hanefeld M., Cagatay M., Petrowitsch T., Neuser D., Petzinna D. and Rupp M. (2004) Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur. Heart J. 25, 10–16 10.1016/S0195-668X(03)00468-8 [DOI] [PubMed] [Google Scholar]
- 95.Minatoguchi S., Zhang Z., Bao N.. et al. (2009) Acarbose reduces myocardial infarct size by preventing postprandial hyperglycemia and hydroxyl radical production and opening mitochondrial KATP channels in rabbits. J. Cardiovasc. Pharmacol. 54, 25–30 10.1097/FJC.0b013e3181a98b53 [DOI] [PubMed] [Google Scholar]
- 96.Russell J.C., Graham S.E. and Dolphin P.J. (1999) Glucose tolerance and insulin resistance in the JCR:LA-corpulent rat: effect of miglitol (Bay m1099). Metabolism 48, 701–706 10.1016/S0026-0495(99)90168-3 [DOI] [PubMed] [Google Scholar]
- 97.Wang N., Minatoguchi S., Chen X.. et al. (2004) Antidiabetic drug miglitol inhibits myocardial apoptosis involving decreased hydroxyl radical production and Bax expression in an ischaemia/reperfusion rabbit heart. Br. J. Pharmacol. 142, 983–990 10.1038/sj.bjp.0705863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iwasa M., Kobayashi H., Yasuda S.. et al. (2010) Antidiabetic drug voglibose is protective against ischemia-reperfusion injury through glucagon-like peptide 1 receptors and the phosphoinositide 3-kinase-Akt-endothelial nitric oxide synthase pathway in rabbits. J. Cardiovasc. Pharmacol. 55, 625–634 10.1097/FJC.0b013e3181dcd240 [DOI] [PubMed] [Google Scholar]
- 99.Chan K.C., Yu M.H., Lin M.C.. et al. (2016) Pleiotropic effects of acarbose on atherosclerosis development in rabbits are mediated via upregulating AMPK signals. Sci. Rep. 6, 38642 10.1038/srep38642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aoki C., Suzuki K., Yanagi K., Satoh H., Niitani M. and Aso Y. (2012) Miglitol, an anti-diabetic drug, inhibits oxidative stress-induced apoptosis and mitochondrial ROS over-production in endothelial cells by enhancement of AMP-activated protein kinase. J. Pharmacol. Sci. 120, 121–128 10.1254/jphs.12108FP [DOI] [PubMed] [Google Scholar]
- 101.Kimura T., Nakagawa K., Kubota H.. et al. (2007) Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 55, 5869–5874 10.1021/jf062680g [DOI] [PubMed] [Google Scholar]
- 102.Kojima Y., Kimura T., Nakagawa K.. et al. (2010) Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. J. Clin. Biochem. Nutr. 47, 155–161 10.3164/jcbn.10-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chan K.C., Lin M.C., Huang C.N., Chang W.C. and Wang C.J. (2013) Mulberry 1-deoxynojirimycin pleiotropically inhibits glucose-stimulated vascular smooth muscle cell migration by activation of AMPK/RhoB and down-regulation of FAK. J. Agric. Food Chem. 61, 9867–9875 10.1021/jf403636z [DOI] [PubMed] [Google Scholar]
- 104.Lago R.M., Singh P.P. and Nesto R.W. (2007) Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 370, 1129–1136 10.1016/S0140-6736(07)61514-1 [DOI] [PubMed] [Google Scholar]
- 105.Graham D.J., Ouellet-Hellstrom R., MaCurdy T.E.. et al. (2010) Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304, 411–418 10.1001/jama.2010.920 [DOI] [PubMed] [Google Scholar]
- 106.Wang T.D., Chen W.J., Lin J.W., Chen M.F. and Lee Y.T. (2004) Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am. J. Cardiol. 93, 362–365 10.1016/j.amjcard.2003.10.022 [DOI] [PubMed] [Google Scholar]
- 107.Mazzone T., Meyer P.M., Feinstein S.B.. et al. (2006) Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296, 2572–2581 10.1001/jama.296.21.joc60158 [DOI] [PubMed] [Google Scholar]
- 108.Lincoff A.M., Wolski K., Nicholls S.J. and Nissen S.E. (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298, 1180–1188 10.1001/jama.298.10.1180 [DOI] [PubMed] [Google Scholar]
- 109.Duckworth W., Abraira C., Moritz T.. et al. (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 110.Hiatt W.R., Kaul S. and Smith R.J. (2013) The cardiovascular safety of diabetes drugs–insights from the rosiglitazone experience. N. Engl. J. Med. 369, 1285–1287 10.1056/NEJMp1309610 [DOI] [PubMed] [Google Scholar]
- 111.Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M. and Kliewer S.A. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 270, 12953–12956 10.1074/jbc.270.22.12953 [DOI] [PubMed] [Google Scholar]
- 112.Fryer L.G., Parbu-Patel A. and Carling D. (2002) The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 10.1074/jbc.M202489200 [DOI] [PubMed] [Google Scholar]
- 113.Schneider C.A., Ferrannini E., Defronzo R., Schernthaner G., Yates J. and Erdmann E. (2008) Effect of pioglitazone on cardiovascular outcome in diabetes and chronic kidney disease. J. Am. Soc. Nephrol. 19, 182–187 10.1681/ASN.2007060678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye J.M., Dzamko N., Hoy A.J., Iglesias M.A., Kemp B. and Kraegen E. (2006) Rosiglitazone treatment enhances acute AMP-activated protein kinase-mediated muscle and adipose tissue glucose uptake in high-fat-fed rats. Diabetes 55, 2797–2804 10.2337/db05-1315 [DOI] [PubMed] [Google Scholar]
- 115.Tomas E., Tsao T.S., Saha A.K.. et al. (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. U.S.A. 99, 16309–16313 10.1073/pnas.222657499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nawrocki A.R., Rajala M.W., Tomas E.. et al. (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 281, 2654–2660 10.1074/jbc.M505311200 [DOI] [PubMed] [Google Scholar]
- 117.Brunmair B., Staniek K., Gras F.. et al. (2004) Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions. Diabetes 53, 1052–1059 10.2337/diabetes.53.4.1052 [DOI] [PubMed] [Google Scholar]
- 118.Morrison A., Yan X., Tong C. and Li J. (2011) Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am. J. Physiol. Heart Circ. Physiol. 301, H895–H902 10.1152/ajpheart.00137.2011 [DOI] [PubMed] [Google Scholar]
- 119.Shibata R., Ouchi N., Ito M.. et al. (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 10, 1384–1389 10.1038/nm1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li P., Shibata R., Unno K.. et al. (2010) Evidence for the importance of adiponectin in the cardioprotective effects of pioglitazone. Hypertension 55, 69–75 10.1161/HYPERTENSIONAHA.109.141655 [DOI] [PubMed] [Google Scholar]
- 121.Kato M.F., Shibata R., Obata K.. et al. (2008) Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. J. Hypertens. 26, 1669–1676 10.1097/HJH.0b013e328302f0f7 [DOI] [PubMed] [Google Scholar]
- 122.Xiao X., Su G., Brown S.N., Chen L., Ren J. and Zhao P. (2010) Peroxisome proliferator-activated receptors gamma and alpha agonists stimulate cardiac glucose uptake via activation of AMP-activated protein kinase. J. Nutr. Biochem. 21, 621–626 10.1016/j.jnutbio.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 123.Vega R.B., Huss J.M. and Kelly D.P. (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20, 1868–1876 10.1128/MCB.20.5.1868-1876.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lie S., Hui M., McMillen I.C.. et al. (2014) Exposure to rosiglitazone, a PPAR-gamma agonist, in late gestation reduces the abundance of factors regulating cardiac metabolism and cardiomyocyte size in the sheep fetus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R429–R437 10.1152/ajpregu.00431.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holst J.J. (2007) The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 10.1152/physrev.00034.2006 [DOI] [PubMed] [Google Scholar]
- 126.Drucker D.J. and Nauck M.A. (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 127.Marso S.P., Daniels G.H., Brown-Frandsen K.. et al. (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sivertsen J., Rosenmeier J., Holst J.J. and Vilsboll T. (2012) The effect of glucagon-like peptide 1 on cardiovascular risk. Nat. Rev. Cardiol. 9, 209–222 10.1038/nrcardio.2011.211 [DOI] [PubMed] [Google Scholar]
- 129.Best J.H., Hoogwerf B.J., Herman W.H.. et al. (2011) Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care 34, 90–95 10.2337/dc10-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Horton E.S., Silberman C., Davis K.L. and Berria R. (2010) Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 33, 1759–1765 10.2337/dc09-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ban K., Noyan-Ashraf M.H., Hoefer J., Bolz S.S., Drucker D.J. and Husain M. (2008) Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 10.1161/CIRCULATIONAHA.107.739938 [DOI] [PubMed] [Google Scholar]
- 132.Noyan-Ashraf M.H., Momen M.A., Ban K.. et al. (2009) GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58, 975–983 10.2337/db08-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noyan-Ashraf M.H., Shikatani E.A., Schuiki I.. et al. (2013) A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127, 74–85 10.1161/CIRCULATIONAHA.112.091215 [DOI] [PubMed] [Google Scholar]
- 134.Guo Z., Qi W., Yu Y., Du S., Wu J. and Liu J. (2014) Effect of exenatide on the cardiac expression of adiponectin receptor 1 and NADPH oxidase subunits and heart function in streptozotocin-induced diabetic rats. Diabetol. Metab. Syndr. 6, 29 10.1186/1758-5996-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou Y., He X., Chen Y., Huang Y., Wu L. and He J. (2015) Exendin-4 attenuates cardiac hypertrophy via AMPK/mTOR signaling pathway activation. Biochem. Biophys. Res. Commun. 468, 394–399 10.1016/j.bbrc.2015.09.179 [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y., Ling Y., Yang L.. et al. (2017) Liraglutide relieves myocardial damage by promoting autophagy via AMPK-mTOR signaling pathway in zucker diabetic fatty rat. Mol. Cell. Endocrinol. 448, 98–107 10.1016/j.mce.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 137.Balteau M., Van Steenbergen A., Timmermans A.D.. et al. (2014) AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 307, H1120–1133 10.1152/ajpheart.00210.2014 [DOI] [PubMed] [Google Scholar]
- 138.Abbas N.A.T. and Kabil S.L. (2017) Liraglutide ameliorates cardiotoxicity induced by doxorubicin in rats through the Akt/GSK-3beta signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 390, 1145–1153 10.1007/s00210-017-1414-z [DOI] [PubMed] [Google Scholar]
- 139.Inoue T., Inoguchi T., Sonoda N.. et al. (2015) GLP-1 analog liraglutide protects against cardiac steatosis, oxidative stress and apoptosis in streptozotocin-induced diabetic rats. Atherosclerosis 240, 250–259 10.1016/j.atherosclerosis.2015.03.026 [DOI] [PubMed] [Google Scholar]
- 140.Krasner N.M., Ido Y., Ruderman N.B. and Cacicedo J.M. (2014) Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 9, e97554 10.1371/journal.pone.0097554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wei R., Ma S., Wang C.. et al. (2016) Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Endocrinol. Metab. 310, E947–E957 10.1152/ajpendo.00400.2015 [DOI] [PubMed] [Google Scholar]
- 142.Zannad F., Cannon C.P., Cushman W.C.. et al. (2015) Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 385, 2067–2076 10.1016/S0140-6736(14)62225-X [DOI] [PubMed] [Google Scholar]
- 143.Scirica B.M., Bhatt D.L., Braunwald E.. et al. (2013) Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 144.Green J.B., Bethel M.A., Armstrong P.W.. et al. (2015) Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 145.Ye Y., Keyes K.T., Zhang C., Perez-Polo J.R., Lin Y. and Birnbaum Y. (2010) The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am. J. Physiol. Heart Circ. Physiol. 298, H1454–H1465 10.1152/ajpheart.00867.2009 [DOI] [PubMed] [Google Scholar]
- 146.Huisamen B., Genis A., Marais E. and Lochner A. (2011) Pre-treatment with a DPP-4 inhibitor is infarct sparing in hearts from obese, pre-diabetic rats. Cardiovasc. Drugs Ther. 25, 13–20 10.1007/s10557-010-6271-7 [DOI] [PubMed] [Google Scholar]
- 147.Vittone F., Liberman A., Vasic D.. et al. (2012) Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in Apoe (-/-) mice. Diabetologia 55, 2267–2275 10.1007/s00125-012-2582-5 [DOI] [PubMed] [Google Scholar]
- 148.Shah Z., Kampfrath T., Deiuliis J.A.. et al. (2011) Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124, 2338–2349 10.1161/CIRCULATIONAHA.111.041418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeng Y., Li C., Guan M.. et al. (2014) The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc. Diabetol. 13, 32 10.1186/1475-2840-13-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang S.T., Su H., Zhang Q.. et al. (2016) Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-kappaB/IkappaBalpha system through the activation of AMP-activated protein kinase. Int. J. Mol. Med. 37, 1558–1566 10.3892/ijmm.2016.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu C., Hu S., Wang N. and Tian J. (2017) Dipeptidyl peptidase4 inhibitor sitagliptin prevents high glucoseinduced apoptosis via activation of AMPactivated protein kinase in endothelial cells. Mol. Med. Rep. 15, 4346–4351 10.3892/mmr.2017.6501 [DOI] [PubMed] [Google Scholar]
- 152.Al-Damry N.T., Attia H.A., Al-Rasheed N.M.. et al. (2018) Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3beta and p38alpha/MAPK in a rat model of diabetic cardiomyopathy. Biomed. Pharmacother. 107, 347–358 10.1016/j.biopha.2018.07.126 [DOI] [PubMed] [Google Scholar]
- 153.Lenski M., Kazakov A., Marx N., Bohm M. and Laufs U. (2011) Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J. Mol. Cell Cardiol. 51, 906–918 10.1016/j.yjmcc.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 154.Zinman B., Wanner C., Lachin J.M.. et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 155.Kosiborod M., Cavender M.A., Fu A.Z.. et al. (2017) Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 136, 249–259 10.1161/CIRCULATIONAHA.117.029190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neal B., Perkovic V., Mahaffey K.W.. et al. (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 157.Lopaschuk G.D., Belke D.D., Gamble J., Itoi T. and Schonekess B.O. (1994) Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim. Biophys. Acta 1213, 263–276 10.1016/0005-2760(94)00082-4 [DOI] [PubMed] [Google Scholar]
- 158.Bertero E., Prates Roma L., Ameri P. and Maack C. (2018) Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc. Res. 114, 12–18 10.1093/cvr/cvx149 [DOI] [PubMed] [Google Scholar]
- 159.Merovci A., Solis-Herrera C., Daniele G.. et al. (2014) Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 10.1172/JCI70704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ferrannini E., Mark M. and Mayoux E. (2016) CV Protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 39, 1108–1114 10.2337/dc16-0330 [DOI] [PubMed] [Google Scholar]
- 161.Ferrannini E., Baldi S., Frascerra S.. et al. (2016) Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 65, 1190–1195 10.2337/db15-1356 [DOI] [PubMed] [Google Scholar]
- 162.Taylor S.I., Blau J.E. and Rother K.I. (2015) SGLT2 Inhibitors May Predispose to Ketoacidosis. J. Clin. Endocrinol. Metab. 100, 2849–2852 10.1210/jc.2015-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee T.M., Chang N.C. and Lin S.Z. (2017) Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 104, 298–310 10.1016/j.freeradbiomed.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 164.Ye Y., Bajaj M., Yang H.C., Perez-Polo J.R. and Birnbaum Y. (2017) SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc. Drugs Ther. 31, 119–132 10.1007/s10557-017-6725-2 [DOI] [PubMed] [Google Scholar]
- 165.Lin B., Koibuchi N., Hasegawa Y.. et al. (2014) Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 13, 148 10.1186/s12933-014-0148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou Y. and Wu W. (2017) The sodium-glucose co-transporter 2 inhibitor, empagliflozin, protects against diabetic cardiomyopathy by inhibition of the endoplasmic reticulum stress pathway. Cell. Physiol. Biochem. 41, 2503–2512 10.1159/000475942 [DOI] [PubMed] [Google Scholar]
- 167.Andreadou I., Efentakis P., Balafas E.. et al. (2017) Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front. Physiol. 8, 1077 10.3389/fphys.2017.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hammoudi N., Jeong D., Singh R.. et al. (2017) Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes. Cardiovasc. Drugs Ther. 31, 233–246 10.1007/s10557-017-6734-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Joubert M., Jagu B., Montaigne D.. et al. (2017) The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes 66, 1030–1040 10.2337/db16-0733 [DOI] [PubMed] [Google Scholar]
- 170.Hawley S.A., Ford R.J., Smith B.K.. et al. (2016) The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 65, 2784–2794 10.2337/db16-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mancini S.J., Boyd D., Katwan O.J.. et al. (2018) Canagliflozin inhibits interleukin-1beta-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci. Rep. 8, 5276 10.1038/s41598-018-23420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou H., Wang S., Zhu P., Hu S., Chen Y. and Ren J. (2018) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox. Biol. 15, 335–346 10.1016/j.redox.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]