Abstract
Aims
Tacrolimus has been associated with notable extrarenal adverse effects (AEs), which are unpredictable and impact patient morbidity. The association between model‐predicted tacrolimus exposure metrics and standardized extrarenal AEs in stable renal transplant recipients was investigated and a limited sampling strategy (LSS) was developed to predict steady‐state tacrolimus area under the curve over a 12‐h dosing period (AUCss,0–12h).
Methods
All recipients receiving tacrolimus and mycophenolic acid ≥6 months completed a 12‐h cross‐sectional observational pharmacokinetic–pharmacodynamic study. Patients were evaluated for the presence of individual and composite gastrointestinal, neurological, and aesthetic AEs during the study visit. The associations between AEs and tacrolimus exposure metrics generated from a published population pharmacokinetic model were investigated using a logistic regression analysis in NONMEM 7.3. An LSS was determined using a Bayesian estimation method with the same patients.
Results
Dose‐normalized tacrolimus AUCss,0–12h and apparent clearance were independently associated with diarrhoea, dyspepsia, insomnia and neurological AE ratio. Dose‐normalized tacrolimus maximum concentration was significantly correlated with skin changes and acne. No AE associations were found with trough concentrations. Using limited sampling at 0, 2h; 0, 1, 4h; and 0, 1, 2, 4h provided a precise and unbiased prediction of tacrolimus AUC (root mean squared prediction error < 10%), which was not well characterized using trough concentrations only (root mean squared prediction error >15%).
Conclusions
Several AEs (i.e. diarrhoea, dyspepsia, insomnia and neurological AE ratio) were associated with tacrolimus dose normalized AUCss,0–12h and clearance. Skin changes and acne were associated with dose‐normalized maximum concentrations. To facilitate clinical implementation, a LSS was developed to predict AUCss,0–12h values using sparse patient data to efficiently assess projected immunosuppressive exposure and potentially minimize AE manifestations.
Keywords: adverse effects, area under the curve, AUC, limited sampling, tacrolimus; pharmacokinetics
What is Already Known about this Subject
Adverse effects (AEs) from tacrolimus immunosuppression are common and significantly contribute to morbidity in renal transplant patients. In addition, minimal investigations have reported use of standardized extrarenal AE criteria in stable transplant recipients.
The association between AEs and tacrolimus exposure is poorly understood in relation to trough blood concentrations, the most commonly measured exposure metric used for routine therapeutic drug monitoring.
Innovative therapeutic drug monitoring strategies are needed to predict and prevent tacrolimus AEs in the clinical arena.
What this Study Adds
Several extrarenal AEs were associated with dose‐normalized tacrolimus area under the curve and maximum concentration as well as apparent clearance. These extrarenal AEs were not associated with tacrolimus trough concentrations.
The developed limited sampling methods enabled accurate tacrolimus exposure predictions, represented by area under the curve, and may improve individualized therapeutic drug monitoring. Poor predictive performance was found when using trough concentrations only.
Introduction
Tacrolimus and mycophenolic acid have become the immunosuppressant regimen of choice to prevent allograft rejection in renal transplant recipients 1, 2, 3. Tacrolimus has a narrow therapeutic range from 4 to 15 ng ml–1 4. This drug exhibits notable inter‐ and intraindividual variability in pharmacokinetics and clinical response 4, 5, 6, 7. Therapeutic drug monitoring of trough concentrations is the standard of care to help minimize individual treatment inefficacy, adverse effects, and allograft rejection 8. Tacrolimus pharmacokinetic variability has been well characterized in numerous population‐based pharmacokinetic analyses, which aim to describe interpatient variability as well as identify the covariate contribution 9. However, there is lack of pharmacodynamic modelling endeavours directed at tacrolimus efficacy and adverse effects as clinical endpoints 9.
Tacrolimus treatment is associated with various adverse effects (AEs) including acute and chronic nephrotoxicities as well as extrarenal manifestations including neurotoxicities, hypertension, post‐transplant diabetes mellitus, gastrointestinal (GI) manifestations, aesthetic alterations and hyperlipidaemia 10, 11, 12. Whereas acute nephrotoxicity is a limiting factor in the clinical use of tacrolimus and may be associated with trough concentrations, extrarenal AEs must also be carefully monitored to prevent medication noncompliance and avoid long term consequences such as increased cardiovascular risks that impact long‐term allograft survival 13, 14, 15, 16. Identifying pharmacokinetic factors contributing to AE manifestations is critical to guide tacrolimus dosing adjustment, improve treatment tolerance and reduce risk potential among these patients. A validated and standardized immunosuppressive extrarenal AE scoring system that includes physical findings, laboratory results and concomitant medications was aimed to minimize reporting bias of these manifestations 17, 18. Previous associations between extrarenal AEs, sex and ABCB1 haplotypes have been reported, but the relationship between tacrolimus pharmacokinetics and these validated adverse events has not been investigated 18. Several studies reported that tacrolimus‐related AEs were more frequent or severe at higher tacrolimus exposures 12, 19, 20. By reducing the targeted trough concentration ranges as recommended with tacrolimus minimization post‐transplant 14, the frequency of specific AE events, such as neurotoxicities, has improved 19. A recent study reported increased donor specific antibody formation with allograft failure as tacrolimus troughs decrease below the target range. These findings create a therapeutic dilemma requiring verification of tacrolimus drug exposure as dose minimization protocols are prescribed 6, 21.
The area under the tacrolimus concentration–time curve (AUC) is considered an essential objective marker for drug exposure and predictive of pharmacological response 19. However, therapeutic drug monitoring of tacrolimus trough concentrations at steady‐state is the current clinical practice, and this surrogate marker is not consistently correlated with dose or AUC 5, 7. The European Consensus recommends a tacrolimus AUC range targeted at 120 and 200 ng h ml–1 to achieve efficacy based upon prior studies using Bayesian analysis 19, 22. As a result, 12‐h intensive sampling over dosing intervals is the ideal means for accurately determining tacrolimus AUC 19. However, collecting multiple time‐points over a dosing interval is inconvenient for patients, costly, and time‐consuming for clinicians. To overcome these issues, limited sampling strategies (LSS) have been developed to predict tacrolimus AUC using the minimal number of concentrations at sampling times in the early periods after drug administration 23. These analyses can be conducted using a maximum a posteriori (MAP) Bayesian approach, underpinned by a population pharmacokinetic model 24. LSS may offer an efficient and accurate surrogate tool to quantify tacrolimus AUC in clinical practice rather than using a single trough concentration to monitor drug efficacy and adverse effects. Ideally, specific correlations between tacrolimus AUC and AE manifestations would support the use of this drug exposure metric. However, minimal investigations have been conducted with tacrolimus AUC and associated AEs post‐transplant 9.
The first aim of this study is to investigate whether standardized extrarenal AEs are associated with tacrolimus exposure metrics, generated using a previously published population pharmacokinetic model 25, in stable renal transplant recipients. The second aim is to develop a LSS to enable therapeutic drug monitoring strategies in the clinic using model‐predicted tacrolimus exposure metrics.
Methods
Ethics Statement
This study was approved by the University at Buffalo Health Sciences Institutional Review Board (IRB# PHP0599703–4). All patients provided informed consent with adherence to the Declaration of Helsinki data, which was reported according to the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Study design
Sixty‐seven stable African American and Caucasian renal transplant recipients, receiving oral tacrolimus (Prograf) and enteric‐coated mycophenolate sodium (ECMPS; Myfortic) for ≥6 months, were included in a cross‐sectional, open‐label, 12‐h clinical pharmacokinetic‐pharmacodynamic study. The study was conducted at the UB Renal Research Unit at the Erie County Medical Center. Study design and methods have been previously described 18, 25. Clinical stability was based on physical examination, comprehensive metabolic panel, fasting lipid panel and complete blood count at enrolment and on study morning. Tacrolimus doses were administered twice daily and adjusted to 4–9 ng ml–1 trough concentrations using a minimization dosing protocol, time post‐transplant and clinical response. ECMPS dose was adjusted based upon clinical response. Medication adherence was verified at enrolment and prior to the study by research personnel. The inclusion criteria were: (i) ≥ 6 months post‐renal transplant; (ii) age 25–70 years; (iii) first or second time deceased‐donor or living allograft recipient; (iv) stabilized on same dose of immunosuppressive drugs for ≥7 days prior to study; (v) serum creatinine ≤3.25 mg dl–1 with no change in serum creatinine >0.25 mg dl–1 during prior 2 visits; and (vi) leucocyte count ≥3000 cells μl–1 and haemoglobin ≥8.0 g dl–1. Exclusion criteria were: (i) infection within 2 weeks; (ii) acute rejection within 2 weeks; (iii) concomitant drugs interfering with tacrolimus or MPA absorption; (iv) concomitant cytochrome P450 3A4/3A5 or P‐gp inhibitors or inducers within 4 weeks; and (v) significant cardiovascular, GI, haematological, psychiatric, and neurological or oncological diseases that would limit participation. Patients were enrolled only if they had received the same dose of tacrolimus and ECMPS for ≥7 days prior to study. This was assumed to be sufficient to approach steady‐state concentrations. Patients took immunosuppressives between 5:30 and 6:30 PM followed by a 12‐h fast prior to study. At 6:00 AM of study day, patients were admitted, vital signs were documented and an intravenous angiocatheter inserted. Serial blood samples were collected at times 0 (predose) and 1, 2, 3, 4, 6, 8, 10 and 12 h after drug administration. Whole blood samples were aliquoted immediately and stored at –70°C until analysis.
Adverse effect assessment
Patients were evaluated during the pharmacokinetic study for the severity and/or frequency of extrarenal adverse effects using a validated rating system summarized in the Supplemental Data: Table S1 17, 18. The nephrologists assessed each patient for all AEs during the morning of the pharmacokinetic study as a change from pre‐transplant status. Each AE was scored as 0 (absence), +1, +2 and + 3 for severity and frequency. Individual AEs were included to determine a composite system AE including: GI (vomiting, diarrhoea, dyspepsia and acid suppressive therapy); neurological (headache, tremor and insomnia); and aesthetic (acne, skin changes, hirsutism and gingival hyperplasia) 18. Myopathy, post‐transplant diabetes and fasting lipids (total cholesterol, high‐density lipoprotein, low‐density lipoprotein and triglycerides) were also evaluated. A ratio of the sum of individual AE scores divided by the maximum score was generated as the adverse effect ratio for interpatient comparisons 18. A cumulative AE ratio represented the summation of GI, neurological and aesthetic AE ratios plus myopathy and post‐transplant diabetes 18. No AEs were re‐evaluated or immunosuppressive dosage adjustments were made in these stable patients during this study.
Bioanalysis
Tacrolimus concentrations were measured using the ARCHITECT chemiluminescent microparticle immunoassay system (Abbott, Abbott Park, IL). The lower limit of detection was 0.5 ng ml–1 and intraday assay variability was <7%. The calibration standard curve ranged from 1 to 30 ng ml–1 and quality controls were 3.0, 12.0, 25 ng ml–1 (Bio‐Rad, Hercules, CA, USA). The interday coefficient of variation for each quality control was <4% and intraday coefficient of variation was <5%.
Blood was also collected in Cell Preparation Tubes (CPT‐ BD Vacationer) with sodium citrate predose for separation of peripheral blood mononuclear cells according to processing protocol at 25°C. These samples were all viable and analysed in a genomics laboratory (University of New England's Genomics Analytical Core). The genotypes were determined using TaqMan allelic discrimination assays (Applied Biosystems, Foster City, CA, USA) with a CFX96 Real‐Time Polymerase Chain Reaction Detection System (Bio‐Rad). Personnel de‐identified to patient demographics assayed for ABCB1: 1236C > T (rs1128503), 2677G > T/A (rs2032582), 3435C > T (rs1045642) and CYP3A5 *3 (rs776746), CYP3A5*6 (rs10264272), and CYP3A5*7 (rs41303343). Allele frequencies were confirmed in Hardy–Weinberg equilibrium when adjusted for race. A metabolic composite CYP3A5*3*6*7 was generated based upon the combined allelic status for each single‐nucleotide polymorphism (SNP). Patients were classified as extensive, intermediate and poor CYP3A5*3*6*7 metabolizers 25, 26.
Population pharmacokinetic model
A population pharmacokinetic analysis for tacrolimus post renal transplant has been published 25. Briefly, a two‐compartment model was developed with first‐order absorption and elimination with a lag‐time for the absorption process to describe tacrolimus concentration–time profiles using NONMEM 7.3.0 (ICON Development Solutions, Ellicott City, MD, USA). The volume of distribution was allometrically scaled to total body weight. The CYP3A5*3*6*7 metabolic composite was a significant covariate for tacrolimus apparent clearance (CL/F). The mean CL/F was 19.7 l h–1 for poor, 28.6 l h–1 for intermediate and 44.3 l h–1 for extensive metabolizers. In this study, the published population pharmacokinetic model 25 was used to estimate for each patient, the maximum tacrolimus concentrations (CMAX) and tacrolimus AUC between 0 and 12 h, at steady‐state (AUCss,0–12h), calculated based upon the individual study dose and CL/F as follows:
| (1) |
Exposure–response modelling
The AE events (composite ratio or individual AE) were treated as ordered categorical data, and the AE events were modelled using a logistic regression approach 27. The analysis was conducted with NONMEM 7.3.0 using the first‐order conditional estimation with interaction method to estimate the parameters. The logit transform of the probability of an adverse effect event occurring in an individual (p i) was defined as:
| (2) |
with β as an underlying baseline parameter, and X represents one or several covariates associated with a scale factor (θ). For binary adverse effects (absence or presence), the probabilities were calculated as follows: the probability of an adverse effect occurring equalled p i (1), and the probability of an adverse effect not occurring was defined as 1 – p i (1). For adverse effects with several possible outcomes and levels of severity (e.g. 0, absence; 1, low; 2, high), the logit was re‐defined for ordered categorical variables:
| (3) |
with Y i as the observation from the ith individual, m represents the number of severity grade categories, and βk is the underlying baseline probability for each category. Cumulative probabilities of categories not greater or lower than a certain value were calculated as follows:
Probability of an adverse effect grade 2 occurring: p i (2)
Probability of an adverse effect grade 1 occurring: p i (1) – p i (2)
Probability of an adverse effect grade not occurring (grade 0): 1 – p i (1)
The AE events were evaluated one time per patient, and between‐subject variability could not be estimated. Individual drug exposure metrics obtained from the population pharmacokinetic model were investigated as potential continuous covariates, such as: tacrolimus exposure at steady‐state including absolute and dose‐normalized AUCss0–12h and AUCss0–4 h, CMAX with trough concentrations, and CL/F. Dose‐normalized AUC and CMAX were derived from the ratio of the AUC0–12h (ng h ml–1) or CMAX (ng ml–1) and the tacrolimus dose (mg), and the units reported for these metrics were ng h ml–1 mg–1 and ng ml–1 mg–1. Categorical patient variables that were investigated included: sex, race, CYP3A5*3*6*7 and ABCB1 polymorphisms. Covariates were tested by forward inclusion and were selected if a significant decrease of at least 3.84 (χ2‐test, P < 0.05) in the objective function from the base model without any covariate was demonstrated 28. To visually assess the impact of covariates on the adverse effects, bar plots were produced. For continuous covariates such as tacrolimus exposure metrics, probabilities of each event were calculated for the 5th, 50th and 95th percentiles of the variable observed in the population. For categorical covariates, probabilities of each event were calculated for each value of the variable. The precision of the parameter estimates was evaluated using a nonparametric bootstrap resampling method 29. Two hundred replicates of the analysis dataset were generated by bootstrapping and run with the final model to obtain the median and 95% confidence interval of all parameter estimates, which were compared to those of the final model.
LSS
The LSS determined the optimal limited combination of sampling times to calculate an accurate tacrolimus AUCss0–12h using a Bayesian forecasting approach 24. The published population pharmacokinetic model 25 was developed originally from intensive serial samples and determined individual tacrolimus AUCss,0–12h values, which were used as the reference AUCint. In the absence of an external validation group, data from these patients were used for the LSS. New datasets were built with the same patient characteristics (dose, weight and genotype) and feedback concentrations were used for different combinations of sampling times. Final estimates of typical pharmacokinetic parameters and between patient variabilities from the population pharmacokinetic model were used as prior information and computed with the new datasets in NONMEM without the estimation step (MAXEVAL = 0 approach). With this approach, the model used the available concentration–time profiles at different sampling times with the fixed population pharmacokinetic parameters and their between‐subject variabilities to obtain individualized pharmacokinetic parameter estimates or so‐called MAP Bayesian estimates. The individual estimates were then used to calculate the projected AUCss,0–12h or AUClss (Eq.(1)), which were compared to the reference AUCint in terms of precision and bias 30. For precision, the relative root mean squared error (RMSE) and the mean absolute prediction error (MAPE) were calculated:
| (4) |
| (5) |
For bias, the mean prediction error (MPE) and the mean percentage prediction error (MPPE) were calculated:
| (6) |
| (7) |
The acceptable criteria for RMSE, MAPE and MPPE are <15% 31. With a narrow therapeutic index drug such as tacrolimus, stricter criteria for predictive performance have been reported for MAPE as below 10% and MPPE between –5 and 5% 23, 32. The coefficient of determination (r 2) assessed the correlation between the projected AUClss and reference AUCint. The percentage of AUClss estimated within 15% of the reference AUCint was also reported. To visually evaluate the overall predictive performance, correlation plots, Bland–Altman plots, and scatter plots of the absolute mean prediction error vs. reference AUCint were produced. Combinations of sampling times were selected based upon the intensive specimen schedule. The LSS was restricted to the first 6 h after drug administration with inclusion of one to four time points with troughs incorporated to reflect the required parameter for tacrolimus therapeutic drug monitoring.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 33, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 34, 35.
Results
Patient characteristics and extrarenal adverse effects
Sixty‐seven patients participated in the 12‐h clinical pharmacology study. Demographics, laboratory parameters, and SNPs are summarized in Table 1. The distribution of CYP3A5*3*6*7 metabolic composite was correlated with race: 91% of Caucasians were poor metabolizers, and 89% of African Americans were intermediate or extensive metabolizers. Table 2 summarizes the frequency and percentage of the composite and individual AEs with fasting lipids using the validated criteria summarized in Table S1 17, 18. Approximately 76% of recipients exhibited low to high frequency and/or severity for composite GI or neurological AEs. Lipid profiles were generally well‐controlled attributed to statin therapy 36.
Table 1.
Patient characteristics
| Parameters | |
|---|---|
| Tacrolimus 12‐h study dose (mg) | 3.0 (1.0–8.0) |
| Tacrolimus trough (ng ml –1 ) | 7.2 (3.3–14) |
| MPA daily dose (mg) | 1260 (360–2160) |
| MPA trough (ng ml –1 ) | 4.0 (0.3–10) |
| Race | |
| African‐American, n (%) | 35 (52.2%) |
| Caucasian, n (%) | 32 (47.8%) |
| Sex | |
| Male, n (%) | 38 (56.7%) |
| Female, n (%) | 29 (43.3%) |
| Age (years) | 46 (24–76) |
| Total body weight (kg) | 86 (52–125) |
| Body mass index (kg m –2 ) | 30.1 (18.7–44.4) |
| Albumin (g dl –1 ) | 4.2 (3.4–4.7) |
| Triglycerides (mg dl –1 ) | 117 (45–950) |
| Total cholesterol (mg dl –1 ) | 154 (82–388) |
| HDL (mg dl –1 ) | 45 (24–122) |
| LDL (mg dl –1 ) | 73 (15–172) |
| eGFR (ml min –1 1.73 m –2 ) | 54 (22–98) |
| Serum creatinine (mg dl –1 ) | 1.4 (0.8–3.1) |
| Haemoglobin (g dl –1 ) | 12 (10–16) |
| Haematocrit (%) | 37 (31–53) |
| Platelet (cells mm –3 ) | 195 (93–428) |
| Leukocytes (cells mm –3 ) | 4.9 (2.3–13) |
| ABCB1 1236C > T | 29/25/13 |
| CC/CT/TT, n (%) | (43%/37%/20%) |
| ABCB1 2677G > T/A | 35/25/6 |
| GG/GT/TT, n (%) | (53%/38%/9%) |
| ABCB1 3435C > T | 30/28/9 |
| CC/CT/TT, n (%) | (45%/42%/13%) |
| CYP3A5*3*6*7 metabolic composite | 35/24/8 |
| poor/intermediate/extensive n (%) | (52%/36%/12%) |
| Time post‐transplant (years) | 2.2 (0.6–14) |
| Diabetes presence, n (%) | 25 (36) |
| Prednisone use, n (%) | 14 (20.9) |
| Statin use, n (%) | 23 (34.3) |
Data represented as median (range) or frequency (percentage)
MPA, mycophenolic acid; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein;
eGFR estimated glomerular filtration rate
Allele frequencies were confirmed to be in Hardy–Weinberg equilibrium when adjusted for race
Table 2.
Frequency/(%) of patients exhibiting severity scores for tacrolimus adverse effects 18
| Adverse effects ratio | Absence | Low | Moderate | High |
|---|---|---|---|---|
| Cumulative | 5/7.5 | 18/26.9 | 36/53.7 | 8/11.9 |
| Range of ratio score (0–0.34) | (0) | (0.03–0.09) | (0.125–0.22) | (0.25–0.34) |
| Gastro‐intestinal | 16/23.9 | 13/19.4 | 31/46.3 | 7/10.4 |
| Range of ratio score (0–0.7) | (0) | (0.1–0.2) | (0.1–0.4) | (0.5–0.7) |
| Neurological | 15/22.4 | 20/30 | 21/31.3 | 11/16.4 |
| Range of ratio score (0–0.57) | (0) | (0.14) | (0.28) | (0.43–0.57) |
| Cosmetic | 38/56.7 | 21/31.3 | 4/6.0 | 4/6.0 |
| Range of ratio score (0–0.5) | (0) | (0.08) | (0.16) | (0.25–0.5) |
| Adverse effects | 0 | 1+ | 2+ | 3+ |
|---|---|---|---|---|
| Vomiting | 60/89.6 | 7/10.4 | 0/0.0 | NA |
| Diarrhoea | 44/65.7 | 15/22.4 | 8/11.9 | NA |
| Dyspepsia | 25/37.3 | 3/4.5 | 34/50.7 | 5/7.5 |
| Acid suppressive therapy | 28/41.8 | 35/52.2 | 4/6.0 | NA |
| Tremor | 33/49.3 | 31/46.3 | 3/4.5 | 0/0.0 |
| Headache | 55/82.1 | 12/17.9 | NA | NA |
| Insomnia | 34/50.7 | 16/23.9 | 16/23.9 | 1/1.5 |
| Acne a | 56/83.6 | 9/13.4 | 2/3.0 | 0/0.0 |
| Skin changes | 54/80.6 | 11/16.4 | 1/1.5 | 1/1.5 |
| Hirsutism | 58/86.6 | 8/11.9 | 0/0.0 | 1/1.5 |
| Gingival hyperplasia | 62/92.5 | 5/7.5 | 0/0.0 | NA |
| Myopathy | 54/80.6 | 12/17.9 | 1/1.5 | 0/0.0 |
| Post‐transplant diabetes mellitus | 64/95.5 | 3/4.5 | NA | NA |
| Lipids | Normal values | High values b | ||
|---|---|---|---|---|
| Total cholesterol (mg dl –1 ) | < 200 | 64/95.5 | > 200 | 3/4.5 |
| LDL (mg dl –1 ) | <100 | 52/77.6 | >100 | 15/22.4 |
| HDL (mg dl –1 ) | >60 | 16/23.9 | <60 | 51/76.1 |
| Triglycerides (mg dl –1 ) | <200 | 57/85.1 | >200 | 10/14.9 |
NA, not applicable since no rating
HDL, high‐density lipoprotein; LDL, low‐density lipoprotein
No patients exhibited a 4+ for acne
Lipids were categorized based upon the values considered as potential risk: > 200 mg/dl from total cholesterol and triglycerides, > 100 mg dl–1 for LDL and < 60 mg dl–1 for HDL 36
Permission granted for reproduction of Table 1 from Venuto R, Meaney C, Chang S, Leca N, Consiglio J, Wilding G, et al. Association of extrarenal adverse effects of posttransplant immunosuppression with sex and ABCB1 haplotypes. Medicine 2015; 94: e1315
Exposure–response modelling
Logistic regression identified tacrolimus dose‐normalized AUCss0–12h and dose‐normalized CMAX with apparent clearance (CL/F) to be significant predictors for adverse events. Dose‐normalized AUCss0–12h and CMAX distributions across all patients and CYP3A5 variants are represented in Figure 1. Diarrhoea, dyspepsia, insomnia and composite neurological ratio were associated with dose‐normalized AUCss0–12h depicted in Figures 2A‐D. Acne and skin changes were related to dose‐normalized CMAX (Figures 2E, F). For diarrhoea and acne, the scores of 0, 1 and 2 were treated as ordered categorical data. Due to a low incidence of dyspepsia, insomnia and skin changes, each AE was treated as a binary category, which included absence (0) and presence (1, 2 or 3). For composite neurological ratio, data were pooled in three categories: 0 for absence, 1 for low ratio (0.14) and 2 for high ratios (0.28, 0.42 or 0.57). All parameters were estimated with acceptable precision and summarized in the logit scale in Tables 3 and 4. The drug exposure metrics showed the most impact on the composite neurological ratio (P < 0.005) and the skin changes (P < 0.02). The greater the dose‐normalized AUCss0–12h or CMAX, the more frequent or severe diarrhoea, insomnia, composite neurological ratio, acne, and skin changes is predicted. Dyspepsia was inversely correlated with dose‐normalized AUCss0–12h. The 5th, 50th and 95th percentiles for dose‐normalized tacrolimus AUCss0–12h were 19, 42 and 88 ng h ml–1 mg–1, whereas dose‐normalized CMAX were 2.4, 6.4 and 12.8 ng ml–1 mg–1, respectively. These percentiles were defined as low, median and high, and used with the exposure–response model parameter estimates to predict the occurrence of each AE (Figure 2A–F). For example, to compare AE in patients with a low dose‐normalized AUCss0–12h, the probability of exhibiting insomnia (score 1, 2 or 3) is 31.1% compared to 71.7% at high dose‐normalized AUCss0–12h (Figure 2C). This dose normalization enables comparison between different stable regimens. No significant trends were identified with individual and/or composite AEs or fasting lipids with tacrolimus trough concentrations (absolute or dose‐normalized). Sex was found as a notable predictor for the composite AE ratios and fasting lipids (Supporting Information Table S2). Females exhibited more frequent and/or severe composite GI, neurological and aesthetic AE ratio, whereas males showed greater probabilities of exhibiting low high‐density lipoprotein and high triglycerides. CYP3A5 and ABCB1 polymorphisms and demographics were not significant covariates on AEs.
Figure 1.
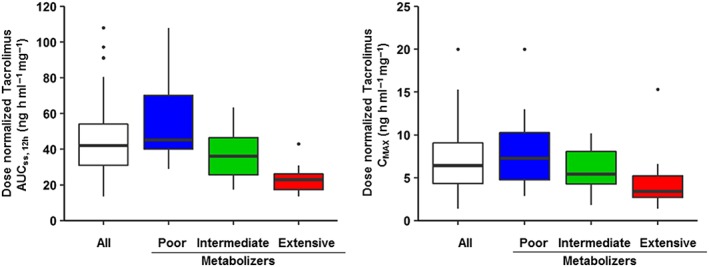
Dose‐normalized tacrolimus exposure (left) and maximum concentration (right) in the study population. Tacrolimus exposure is represented as the dose normalized area under the concentration curve at steady‐state between 0 and 12 h (AUCss0–12h), and on the right as the dose normalized maximum concentration (CMAX). Both exposure metrics were obtained from the population pharmacokinetic model previously developed 25. In each graph, the distribution of the exposure metrics is shown for all patients (white) and stratified by CYP3A5*3*6*7 metabolic composite as poor (blue), intermediate (green) and extensive (red) metabolizers. Extensive metabolizers, who are African Americans only, exhibit less interpatient variability in dose normalized tacrolimus AUCss0–12h and CMAX with a reduction in tacrolimus exposure of approximately 50% compared to poor metabolizers who are primarily Caucasians
Figure 2.
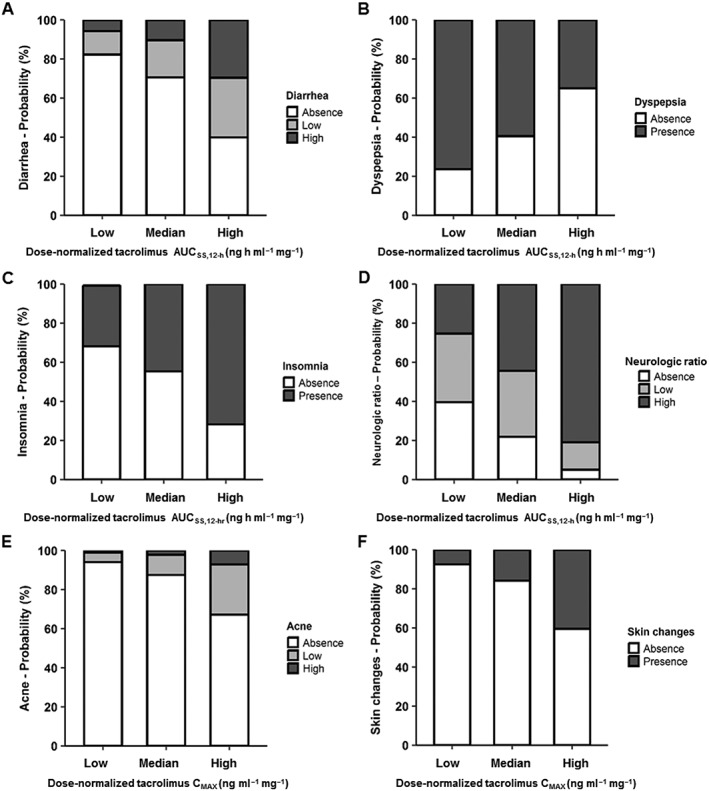
Probabilities of the occurrence of adverse effects after tacrolimus administration in renal transplant recipients according to dose‐normalized tacrolimus exposure AUCss0–12h and maximum concentration (CMAX). Tacrolimus exposure is represented as the area under the concentration curve at steady‐state between 0 and 12 h (AUCss0–12h; A–D). The 5th, 50th, and 95th percentiles of the distribution of dose‐normalized tacrolimus exposure and maximum concentration obtained from the population pharmacokinetic model for all patients were used to calculate the probabilities of adverse effect occurrence. For dose‐normalized tacrolimus AUCss0–12h, the 5th, 50th and 95th percentiles were 18 (low), 42 (median), and 88 (high) ng h ml–1 mg–1. Note that at high dose‐normalized AUCss0–12h, the severity and/or frequency of the AE including diarrhoea, insomnia and neurological ratio are greater. For dose‐normalized tacrolimus CMAX, the 5th, 50th, and 95th percentiles were 2.4 (Low), 6.4 (Median) and 12.8 (High) ng ml–1 mg–1. The frequency or severity of AEs is greater at higher dose‐normalized CMAX (E, F)
Table 3.
Summary of association of tacrolimus exposure metrics with extrarenal adverse effects: model parameter estimates
| Adverse effect: categories | PK predictor | Parameter estimates (95% CI) | OR | P‐value† | |||
|---|---|---|---|---|---|---|---|
| Gastrointestinal | |||||||
| Diarrhoea | 0 | Absence | Dose‐normalized AUCss0–12h (ng h ml–1 mg–1) | β 1 = –2 | (–2.97, –0.86) | 1.03 | <0.05 |
| 1 | Low | α 2 = –1.28 | (–1.92, –0.68) | ||||
| 2 | High | θ AUCN = 0.027 | (0.003, 0.05) | ||||
| Dyspepsia | 0 | Absence | Dose‐normalized AUCss0–12h (ng h ml–1 mg–1) | β 1 = 1.73 | (0.69, 2.77) | 0.96 | < 0.05 |
| 1/2/3 | Presence | θ AUCN = –0.032 | (–0.063, –0.005) | ||||
| Neurological | |||||||
| Insomnia | 0 | Absence | Dose‐normalized AUCss0–12h (ng h ml–1 mg –1) | β 1 = –1.21 | (–1.92, –0.52) | 1.02 | < 0.05 |
| 1/2/3 | Presence | θ AUCN = 0.024 | (0.004, 0.04) | ||||
| Neurological ratios | 0 | Absence | Dose‐normalized AUCss0–12h (ng h·ml–1 mg–1) | β 1 = –0.18 | (–0.97, –0.04) | 1.03 | < 0.005 |
| 0.14 | Low | α 2 = –1.5 | (–1.96, –0.97) | ||||
| >0.28 | High | θ AUCN = 0.027 | (0.02, 0.05) | ||||
| Cosmetic | |||||||
| Skin changes | 0 | Absence | Dose‐normalized CMAX (ng ml–1 mg–1) | β 1 = –3 | (–3.94, –1.76) | 1.23 | < 0.02 |
| 1/2/3 | Presence | θ CmaxN = 0.208 | (0.02, 0.32) | ||||
| Acne | 0 | Absence | Dose‐normalized CMAX (ng ml–1 mg–1) | β 1 = –3.24 | (–3.92, –1.78) | 1.22 | < 0.05 |
| 1 | Low | α 2 = –1.86 | (–2.93, –1.15) | ||||
| 2 | High | θ CmaxN = 0.201 | (0.03, 0.3) | ||||
PK, pharmacokinetic; AUCss0–12h, tacrolimus area under the concentration curve at steady‐state between 0 and 12 h; CL/F, tacrolimus apparent oral clearance; θ CL, slope for tacrolimus CL/F effect; OR, odds ratio
θ AUCN slope for AUCss0‐12 h dose‐normalized effect; CMAX maximum tacrolimus concentration;
θ CmaxN slope for dose‐normalized CMAX effect
The probability pi of an adverse effect event is defined as follows:
For binary adverse effect events (dyspepsia, skin changes, insomnia), β 1 represents the underlying baseline for p(1), the probability of an adverse effect occurring. The probability of an adverse effect not occurring is defined as 1 – p(1)
For ordered categorical variables (diarrhoea, acne, neurological ratios), β 1 represents the underlying baseline for p(1), the sum of β 1 and α 2 represents the underlying baseline p(2). The probability of an adverse effect grade 2 occurring is p(2). The probability of an adverse effect grade 1 occurring is p(1) – p(2). The probability of an adverse effect not occurring (grade 0) is 1 – p(1)
Neurological adverse effect ratio represents a composite of individual rated adverse effects: tremors, headaches, insomnia 17, 18
The odds of interest increase by eθAUCN or eθCmaxN fold for every one unit increase in dose‐normalized AUC or maximum concentration. †The P‐value indicates the statistical significance of the PK predictor on the adverse effect model developed in NONMEM. The PK predictor was considered as significant if it showed a decrease of at least 3.84 points (χ2‐test, P < 0.05) in the objective function value from the initial logistic model
Table 4.
Tacrolimus apparent clearance CL/F association with extrarenal adverse effects: model parameter estimates
| Adverse effect: categories | PK predictor | Parameter estimates (95% CI) | OR | P‐valuea | |||
|---|---|---|---|---|---|---|---|
| Gastrointestinal | |||||||
| Diarrhea | 0 | Absence | Tacrolimus CL/F (l h–1) | β 1 = 0.745 | (0.019, 0.99) | 0.94 | <0.02 |
| α 2 = 1.29 | (–2.57, –0.74) | ||||||
| 1 | Low | θ CL = –0.059 | (–0.093, –0.018) | ||||
| 2 | High | ||||||
| Dyspepsia | 0 | Absence | Tacrolimus CL/F (l h–1) | β 1 = 0.208 | (0.020, 0.69) | 1.03 | <0.05 |
| 1/2/3 | Presence | θ CL = 0.027 | (0.0008, 0.065) | ||||
| Neurological | |||||||
| Insomnia | 0 | Absence | Tacrolimus CL/F (l h–1) | β 1 = –0.947 | (–1.84, –0.18) | 0.95 | <0.05 |
| 1/2/3 | Presence | θ CL = –0.053 | (–0.10, –0.019) | ||||
| Neurological ratios | 0 | Absence | Tacrolimus CL/F (l h–1) | β 1 = 0.391 | (0.109, 1.53) | 2.74 | <0.05 |
| >0.14 | Presence | θ CL = 1.01 | (0.25, 1.91) | ||||
PK, pharmacokinetic; CL/F, tacrolimus apparent oral clearance; θ CL, slope for tacrolimus CL/F effect; OR, odds ratio
The probability pi of an adverse effect event is defined as follows:
For binary adverse effect events (dyspepsia, insomnia, neurological ratios), β 1 represents the underlying baseline for p(1), the probability of an adverse effect occurring. The probability of an adverse effect not occurring is defined as 1 – p(1)
For ordered categorical variables (diarrhoea), β 1 represents the underlying baseline for p(1), the sum of β 1 and α 2 represents the underlying baseline p(2). The probability of an adverse effect grade 2 occurring is p(2). The probability of an adverse effect grade 1 occurring is p(1) – p(2). The probability of an adverse effect not occurring (grade 0) is 1 – p(1)
Neurological adverse effect ratio represents a composite of individual rated adverse effects: tremors, headaches, insomnia 17, 18
The P‐value indicated the statistical significance of the exposure metric on the adverse effect model developed in NONMEM. The exposure metric was considered as significant if it showed a decrease of at least 3.84 points (χ2‐test, P < 0.05) in the objective function value from the initial logistic model
The odds of interest increases by eθCL fold for every one unit increase in tacrolimus CL/F
LSS
Twenty‐six different combinations of sampling times were evaluated, which included the maximum of four timed concentrations collected from 0 (trough) to 6 h after drug administration to generate tacrolimus AUCss,0–12h (AUClss) using a Bayesian forecasting approach. The optimal predictive performance of these combinations using one, two, three or four timed concentrations from 0 to 4 h of the dosing interval is summarized in Table 5. The timed combinations provided low bias of <5%. The combination of four timed samples at 0, 1, 2 and 4 h exhibited the best predictive performance of RMSE 5.1% and MAPE 4.2% followed by the three timed sample combination at 0, 1 and 4 h. The coefficient of determination greater than 0.95 was achieved for both these timed concentration combinations (Table 5). For clinical feasibility in predicting AUC, two timed collections at 0 and 2 h provide good estimates with r2 = 0.89. The tacrolimus reference AUCint compared to AUClss predicted by LSS were well below the clinically accepted criteria 23, 32. For comparison, the predictive performances for all combinations are summarized in Table S3. When troughs only were used for LSS, the projected AUClss values did not achieve acceptable precision (i.e. RMSE 19.5% and MAPE 20.4%) compared to reference AUCint with a coefficient of determination of 0.61. The correlation, Bland–Altman and scatter plots of the absolute mean prediction error vs. reference AUCint for selected timed concentration combinations are presented in Supporting Information Figures S1–S3.
Table 5.
Predictive performance of most accurate selected limited sampling strategies predicting tacrolimus exposure in adult kidney transplant recipients
| Sampling times | Precision | Bias | % AUClss within 15% AUCint | r2 | ||
|---|---|---|---|---|---|---|
| RMSE (%) | MAPE (%) | MPE (ng h ml–1) | MPPE (%) | |||
| 0 h | 19.5 | 20.4 | –0.9 | 0.5 | 59.7 | 0.61 |
| 0, 2 h | 9.2 | 7.3 | –3.2 | –2.4 | 92.5 | 0.89 |
| 0, 1, 4 h | 6.0 | 5.2 | –2.3 | –1.5 | 100.0 | 0.95 |
| 0, 1, 2, 4 h | 5.1 | 4.2 | –2.5 | –1.8 | 100.0 | 0.96 |
RMSE, relative root mean squared prediction error; MAPE, mean absolute percentage prediction error; MPE, mean prediction error; MPPE, mean percentage prediction error; AUClss, tacrolimus area under the concentration curve predicted by Bayesian forecasting; AUCint, tacrolimus area under the concentration curve calculated from the final pharmacokinetic model with intensive serial sampling; r2, coefficient of determination between AUClss and AUCint
Discussion
This is the first report linking tacrolimus exposure metrics (i.e. clearance and dose normalized AUC) to extrarenal adverse effects in renal transplant patients using an exposure–response logistic regression approach. This method can be used to analyse one or several occurrences of adverse effects, and a similar approach using total AUC has been frequently utilized for safety assessment of antineoplastic agents 27, 37, 38. This method could also be used to account for past events when predicting future AE occurrences by including a transition (Markov) model 39, 40, 41. The model and limited sampling analysis in the present study describes important clinical links using tacrolimus exposure metrics, such as dose‐normalized AUCss0–12h and CMAX, as well as apparent CL/F, as predictors of standardized extrarenal AEs and may provide a method to minimize these manifestations. This predictive relationship of tacrolimus exposure metrics to adverse effects offers well‐timed therapeutic alternatives to empirical dose minimization of this immunosuppressive that often results in sub‐therapeutic trough concentrations and donor specific antibody formation compounding the renal allograft rejection process 21. Once validated using a longitudinal assessment, this report will enable the prospective evaluation of dose‐normalized AUC as a tool for minimizing adverse effects. Using dose‐normalized AUC or apparent clearance to compare different dosing regimens may offer an improved clinical approach compared to the therapeutic drug monitoring of trough concentrations 19.
The regimen of tacrolimus and mycophenolic acid has been found to be associated with notable GI AEs 18, 42. These GI AEs may reduce medication adherence, patient tolerability, and therapeutic drug exposure impacting allograft survival. This report utilized a tacrolimus population pharmacokinetic model to predict exposure metrics and establish standardized GI AEs relationships, which are poorly defined 19, 42. Dose‐normalized tacrolimus AUCss0–12h and CL/F were associated with diarrhoea and dyspepsia. The more frequent or severe diarrhoea was observed at higher dose‐normalized AUCss0–12h. In clinical trials, diarrhoea was reported in 22–72% of recipients receiving tacrolimus with no objective AE quantification provided 42. In contrast, the more frequent or severe dyspepsia was observed at the lower dose‐normalized AUCss0–12h with an unclear mechanism. However, the combined inter‐relationship of the pharmacokinetics of MPA, MPA glucuronide and tacrolimus contribute to the overall GI AE observed in these patients 43, 44. Due to the limited patient number, joint contributions of both immunosuppressive drugs were not evaluated.
Dose‐normalized tacrolimus AUCss0–12h and CL/F were positively associated with insomnia and the composite neurological AE ratio consisting of tremor, headache and insomnia 17, 18. Insomnia was reported in 24–64% in major clinical trials 42. Our analysis revealed that more frequent or severe insomnia was observed at the higher dose‐normalized AUCss0–12h. Previous reports of subjective associations of headaches and tremor with tacrolimus dose conflict with the absence of these AEs 45, 46. The pathophysiology of tacrolimus‐induced neurotoxicity could be attributed to notable drug accumulation in the central nervous system due to high lipophilicity 47, 48. P‐glycoprotein may also play an important role in modulating tacrolimus metabolism and cellular distribution at the blood brain barrier 49, 50. Variations in ABCB1 genotypes, which encodes for this transporter, can result in loss of function of P‐glycoprotein and could lead to drug‐brain accumulation. However, no clinically meaningful associations between these genotypes and tacrolimus‐induced neurotoxicity have been identified 47, 48.
Interestingly, dose‐normalized tacrolimus CMAX was associated with two aesthetic AEs of acne and skin changes. More frequent or severe aesthetic AEs were reported at higher tacrolimus CMAX, but remained globally rare since <20% of patients exhibited either manifestation. These side effects are generally less reported adverse reactions 42, with reduced frequency in tacrolimus regimens compared to other immunosuppressive drugs 51.
The prior population pharmacokinetic model highlighted the significant impact of the metabolic CYP3A5*3*6*7 composite on tacrolimus CL/F 25. As shown in Figure 1, subsequent distributions in the dose‐normalized AUCss0–12h and CMAX stratified by CYP3A5 genotype were observed. This suggests that patients with poor, intermediate and extensive metabolism would have different probabilities in the occurrence of extrarenal AEs. However, the CYP3A5*3*6*7 genotypes were not identified as a significant predictor for AEs in our analysis. Although genotype explains a significant amount of the variability in CL/F, it is not the only factor contributing to this variability. Therefore, the entire population model may provide a better tool for identifying individual CL/F values than using genotypes alone. Another investigation explored the impact of CYP3A5*3 genotypes only on tacrolimus renal and extrarenal AEs across time using a Markov chain model and concluded that the probability of an adverse event occurrence in the first 3 months in the CYP3A5 nonexpressers is 2‐fold greater compared to the CYP3A5 expressers 52. Another important genomic contribution to AE manifestations are the ABCB1 SNPs, 1236C > T (rs1128503), 2677G > T/A (rs2032582), and 3435C > T (rs1045642) variants that encode for P‐glycoprotein and modulates cellular distribution of tacrolimus. This model found no association of these individual ABCB1 genotypes as an AE predictor. This finding conflicts with our previous report of notable associations between extrarenal AEs and ABCB1 haplotypes (i.e. TTT) determined by Theasis computation 18. Future models should include ABCB1 haplotypes with CYP3A5*3*6*7 genotypes that may provide more comprehensive mechanistic insights into the inter‐relationship between CYP3A5 enzymes and P‐glycoprotein in transplant subpopulations that include sex and race. 47, 48, 53.
To apply these results in clinical practice, tacrolimus AUCss0–12h must be generated for each individual. An LSS was developed using the population pharmacokinetic model and a Bayesian forecasting method to predict tacrolimus AUCss0–12h, and different combinations of minimum timed samples were evaluated. Using one to four samples including troughs within the first 6 h after drug administration, the most accurate and convenient LSS strategies selected were within the first 4 h after tacrolimus administration and included 0–2 h, 0–1‐4 h and 0–1–2‐4 h, which provided good precision and low bias. This outcome was expected since the inclusion of more timed concentrations provided greater reliance on the measured timed concentrations than on the population model predictive power 22, 31. Our results showed no major differences between the selected strategies using three or four timed concentrations with RMSE<6% and MAPE<5%, compared to the limited strategy including only two timed concentrations (Table 5). Based on these results, the LSS with three time‐points at 0, 1 and 4 h might be considered an optimal estimate for use in clinical practice to estimate an accurate and reliable tacrolimus AUCss0–12h. In addition, for clinical feasibility and cost, the use of two timed specimens at trough and 2 h postdrug administration provides a reasonable AUCss0–12h.
Our LSS is consistent with a previous review of different tacrolimus analyses conducted during early and late post‐transplant periods using a Bayesian estimator 9. The majority of the studies concluded that a combination of a trough concentration with at least one timed concentration 2 h postdose is required to predict an accurate and reliable tacrolimus AUCss0–12h using RMSE and MAPE 31. The selected LSS across these studies were 0–1‐3 h, 0–1‐4 h, 0–2‐3 h and 0–2‐4 h 22, 54, 55, 56, 57. Instead of testing all possible combinations of timed concentrations collected during the 12‐h intensive sampling schedule, several studies were based on the D‐optimality criterion for selection of the optimal sampling design 58. This approach resulted in the following LSS including: 0.3–2‐4 h, 1–3‐6 h, 1–3‐8 h, 0–1.5‐4 h and 0–1‐3 h and is consistent with the other analyses 59, 60, 61, 62. The inclusion of at least one timed concentration after 2 h post‐dose is in concordance with the absorption process of tacrolimus, in which CMAX is generally achieved within the first 3 h post‐dose 3, 8, 12. Numerous tacrolimus LSS were developed using a multiple linear regression method 31. Although this approach is easily applied, it does not allow for any flexibility. Unlike with the Bayesian forecasting approach, exact sampling times must be collected to assure the predictive performance of the equation developed, which may not be feasible in clinical practice 63. In our study, using only a trough concentration to predict tacrolimus AUC resulted in low precision with RMSE = 19.5% and MAPE = 20.4%, compared to the acceptable criteria of RMSE<15% and MAPE<10%. A low coefficient of correlation (r2 = 0.61) in spite of low bias (0.5%) also resulted. These results are consistent with previous studies that reported unacceptable precision and bias and poor correlation between tacrolimus troughs and AUC (range r2: 0.27–0.79), which is concerning with a narrow therapeutic range drug 22, 31, 57, 64, 65, 66. The interpatient variability in the relationship between tacrolimus trough concentration and AUC has been described and remains controversial 19. Therapeutic drug monitoring based on trough measurements is justified in the clinical setting due to patient ease and is economical but does not consistently predict an accurate AUC. As a result, the LSS represents an easy and clinically applicable approach to predict a reliable estimate of drug exposure to elicit the desired pharmacological response for tacrolimus. It is important to emphasize that a qualified population pharmacokinetic model is a prior requirement to use this method 67.
A few limitations do exist with this report. One limitation is the cross‐sectional study design that incorporates simultaneous evaluation of drug exposure and adverse effects in stable recipients. No longitudinal follow‐up studies were conducted to serve as comparators for adverse effects manifested as tacrolimus dosing regimens were adjusted. Ideally, the LSS should be validated using an external patient group separate from the cohort used to build the pharmacokinetic model 67. In our analysis, due to a limited number of stable patients included, no randomization into an index and validation group was possible. This limitation may explain the consistent low bias observed in the different combinations of timed concentrations evaluated. However, employing this configuration, our analysis showed a poor prediction when using troughs only to project tacrolimus exposure accurately. This finding may raise concerns with relying only on monitoring trough concentrations. The drug exposure associations to chronic AE in stable patients are preliminary findings and require confirmation with repeated studies. Another study limitation that may be addressed more comprehensively in a larger patient population are the associations of CYP3A5*3*6*7 metabolic composite and ABCB1 haplotypes to tacrolimus exposure and AE manifestations.
Conclusions
An exposure–response logistic regression approach was used to identify the impact of dose‐normalized tacrolimus AUCss0–12h and CMAX or with CL/F for several extrarenal AEs such as diarrhoea, dyspepsia, insomnia, acne, skin changes, and the composite neurological ratio. A Bayesian estimator based on a population pharmacokinetic model and LSS was developed to provide an efficient tool for clinicians to predict tacrolimus exposure with accuracy and maintain patient convenience to facilitate individualized monitoring and minimize adverse effects. Our study identified a notable correlation of tacrolimus dose‐normalized AUCss0–12h and CL/F with extrarenal AEs. In addition, the LSS analysis confirmed precise estimation of AUC using multiple timed concentrations collected from 0 to 4 h, which was an improvement over the use of trough concentrations. Future research may be aimed at confirming these results in larger study cohorts with collection of serial AEs.
Competing Interests
There are no competing interests to declare.
The assistance of the following individuals is greatly appreciated: Louise Cooper RPh, Vanessa Gray RN, Jean Meyers and Joseph Kabacinski, MBA from Erie County Medical Center and UB‐MD Renal Division and UB School of Pharmacy and Pharmaceutical Sciences.
This study was supported by grants from National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) ARRA R21: DK077325‐01A1 (KMT‐PI) and an Investigator Initiated Research Grant (KMT‐PI) from Astellas Pharma Global Development, Inc. The authors of this manuscript have no conflicts of interest or financial relationships to disclose during the time this study was ongoing.
Supporting information
Table S1 Immunosuppressive adverse effects scoring system [18]
Table S2 Sex association with composite adverse effect ratio: model parameter estimates
Table S3 Predictive performance of all tested limited sampling strategies predicting tacrolimus exposure in adult kidney transplant recipients
Figure S1 Correlation plots A–D between the reference AUCint and the projected AUClss for the selected limited sampling strategies. AUCint is the tacrolimus area under the curve between 0 and 12 h estimated with the full intensive sampling strategy. AUClss is the projected area under the curve obtained from the different combinations of sampling times using a Bayesian forecasting approach. Continuous lines represent the linear regression lines. Dashed lines represent the 95% confidence interval for each linear regression line. The limited sampling strategies including three and four time‐point (panels C and D) exhibited the best correlation between reference AUCint and projected AUClss with r2 of 0.947 and 0.961, respectively. Patients were categorized as poor (blue dots), intermediate (green dots) or extensive (red dots) metabolizer based on a CYP3A5*3*6*7 metabolic composite
Figure S2 Scatter plots A–D of the absolute percentage prediction error vs. the reference AUCint for the selected limited sampling strategies. The AUCint represents the tacrolimus area under the curve between 0 and 12 h estimated using the full intensive sampling strategy. The absolute percentage prediction error was calculated between AUCint and AUClss, which is the projected area under the curve obtained from the different combinations of sampling times using a Bayesian forecasting approach, as following:
Accuracy within 15%, which is the clinical acceptable level criteria, is represented by the dashed line for each combination of sampling times. 100% of the projected AUClss obtained with the limited sampling strategies including three time‐points (C, D) fell within 15% of reference AUCint, as opposed to 59.7% for the limited sampling strategy including tacrolimus trough concentration (A). Patients were categorized as poor (blue dots), intermediate (green dots), or extensive (red dots) metabolizers based on a CYP3A5*3*6*7 metabolic composite
Figure S3 Bland–Altman graphs (A–D) comparing the difference between individual AUCint and AUClss (y‐axis) and the average of individual AUCint and AUClss (x‐axis) for the selected limited sampling strategies. Dashed lines represent the 5th, 50th and 95th percentiles. Reference AUCint is the tacrolimus area under the curve between 0 and 12 h estimated using the 12‐h intensive sampling strategy. AUClss is the projected area under the curve obtained from the different combinations of sampling times using a Bayesian forecasting approach. The limited sampling strategies including three or four timed concentrations (C, D) showed the best precision with the narrowest agreement interval between the 5th and 95th percentiles. No systematic bias was depicted in the four graphs with no consistent pattern to the direction of bias, with AUClss both under‐ and overestimating AUCint. Patients were categorized as poor, intermediate or extensive metabolizers based on a CYP3A5*3*6*7 metabolic composite, and are represented by blue, green and red dots, respectively
Campagne, O. , Mager, D. E. , Brazeau, D. , Venuto, R. C. , and Tornatore, K. M. (2019) The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br J Clin Pharmacol, 85: 516–529. 10.1111/bcp.13811.
References
- 1. Matas A, Smith J, Skeans M, Thompson B, Gustafson S, Stewart D, et al OPTN/SRTR 2013 Annual Data Report: Kidney. Am J Transplant 2015; 15: 1–34. [DOI] [PubMed] [Google Scholar]
- 2. Hart A, Smith J, Skeans M, Gustafson S, Stewart D, Cherikh W, et al OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant 2017; 17: 21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowman L, Brennan D. The role of tacrolimus in renal transplantation. Expert Opin Pharmacother 2008; 9: 635–643. [DOI] [PubMed] [Google Scholar]
- 4. Shuker N, Bouamar R, van Schaik R, Clahsen‐van Groningen M, Damman J, Baan C, et al A Randomized controlled trial comparing the efficacy of Cyp3a5 genotype‐based with body‐weight‐based tacrolimus dosing after living donor kidney transplantation. Am J Transplant 2016; 16: 2085–2096. [DOI] [PubMed] [Google Scholar]
- 5. Vanhove T, Annaert P, Kuypers D. Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev 2016; 48: 88–112. [DOI] [PubMed] [Google Scholar]
- 6. de Jonge H, Naesens M, Kuypers D. New insights into the pharmacokinetics and pharmacodynamics of the calcineurin inhibitors and mycophenolic acid: possible consequences for therapeutic drug monitoring in solid organ transplantation. Ther Drug Monit 2009; 31: 416–435. [DOI] [PubMed] [Google Scholar]
- 7. Knops N, Levtchenko E, van den Heuvel B, Kuypers D. From gut to kidney: transporting and metabolizing calcineurin‐inhibitors in solid organ transplantation. Int J Pharm 2013; 452: 14–35. [DOI] [PubMed] [Google Scholar]
- 8. Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2007; 2: 374–384. [DOI] [PubMed] [Google Scholar]
- 9. Brooks E, Tett S, Isbel N, Staatz C. Population pharmacokinetic modelling and bayesian estimation of tacrolimus exposure: is this clinically useful for dosage prediction yet? Clin Pharmacokinet 2016; 55: 1295–1335. [DOI] [PubMed] [Google Scholar]
- 10. Jose M. Calcineurin inhibitors in renal transplantation: adverse effects. Nephrol Ther 2007; 12: S66–S74. [DOI] [PubMed] [Google Scholar]
- 11. Miller L. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2002; 2: 807–818. [DOI] [PubMed] [Google Scholar]
- 12. Staatz C, Tett S. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004; 43: 623–653. [DOI] [PubMed] [Google Scholar]
- 13. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDGIO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2016; 9 (Suppl. 3): S1–S157. [DOI] [PubMed] [Google Scholar]
- 14. Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562–2575. [DOI] [PubMed] [Google Scholar]
- 15. Boots J, Christiaans M, van Hooff J. Effect of immunosuppressive agents on long‐term survival of renal transplant recipients: focus on the cardiovascular risk. Drugs 2004; 64: 2047–2073. [DOI] [PubMed] [Google Scholar]
- 16. Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009; 69: 2227–2243. [DOI] [PubMed] [Google Scholar]
- 17. Meaney C, Arabi Z, Venuto R, Consiglio J, Wilding G, Tornatore K. Validity and reliability of a novel immunosuppressive adverse effects scoring system in renal transplant recipients. BMC Nephrol 2014; 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venuto R, Meaney C, Chang S, Leca N, Consiglio J, Wilding G, et al Association of extrarenal adverse effects of posttransplant immunosuppression with sex and ABCB1 haplotypes. Medicine 2015; 94: e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallemacq P, Armstrong V, Brunet M, Haufroid V, Holt D, Johnston A, et al Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit 2009; 31: 139–152. [DOI] [PubMed] [Google Scholar]
- 20. Shuker N, Shuker L, van Rosmalen J, Roodnat J, Borra L, Weimar W, et al A high intrapatient variability in tacrolimus exposure is associated with poor long‐term outcome of kidney transplantation. Transpl Int 2016; 29: 1158–1167. [DOI] [PubMed] [Google Scholar]
- 21. Davis S, Gralla J, Klem P, Tong S, Wedermyer G, Freed B, et al Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor‐specific antibodies in the first year of kidney transplantation. Am J Transplant 2017; 18: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scholten E, Cremers S, Schoemaker R, Rowshani A, van Kan E, den Hartigh J, et al AUC‐guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int 2005; 67: 2440–2447. [DOI] [PubMed] [Google Scholar]
- 23. van Boekel G, Donders A, Hoogtanders K, Havenith T, Hilbrands L, Aarnoutse R. Limited sampling strategy for prolonged‐release tacrolimus in renal transplant patients by use of the dried blood spot technique. Eur J Clin Pharmacol 2015; 71: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheiner L, Beal B. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non‐Bayesian methods. J Pharm Sci 1982; 71: 1344–1318. [DOI] [PubMed] [Google Scholar]
- 25. Campagne O, Mager D, Brazeau D, Venuto R, Tornatore K. Tacrolimus population pharmacokinetics and multiple CYP3A5 genotypes in black and white renal transplant recipients. J Clin Pharmacol 2018; 58: 1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birdwell K, Decker B, Barbarino J, Peterson J, Stein C, Sadee W, et al Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther 2015; 98: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mould D, Walz A, Lave T, Gibbs J, Frame B. Developing exposure/response models for anticancer drug treatment: special considerations. CPT Pharmacometrics Syst Pharmacol. 2015; 4: e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boeckmann AJ, Sheiner LB, Beal SL. NONMEM users guide, Part V, Introductory guide. San Fransisco (CA): University of California: NONMEM Project Group, 1994. [Google Scholar]
- 29. Parke J, Holford N, Charles B. A procedure for generating bootstrap samples for the validation of nonlinear mixed‐effects population models. Comput Methods Programs Biomed 1999; 59: 19–29. [DOI] [PubMed] [Google Scholar]
- 30. Sheiner L, Beal S. Some suggestions for measuring predictive performance. J Pharmacokinetics Biopharmaceutics 1981; 9: 503–512. [DOI] [PubMed] [Google Scholar]
- 31. Barraclough K, Isbel N, Kirkpatrick C, Lee K, Taylor P, Johnson D, et al Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol 2011; 71: 207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niioka T, Miura M, Kagaya H, Saito M, Numakura K, Habuchi T, et al A limited sampling strategy to estimate the area under the concentration–time curve of tacrolimus modified‐release once‐daily preparation in renal transplant recipients. Ther Drug Monit 2013; 35: 228–232. [DOI] [PubMed] [Google Scholar]
- 33. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174 (Suppl. 1): S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 2017; 174 (Suppl. 1): S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129 (25 Suppl. 2): S1–S45. [DOI] [PubMed] [Google Scholar]
- 37. Mould DR, Holford NH, Schellens JH, Beijnen JH, Hutson PR, Rosing H, et al Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther 2002; 71: 334–348. [DOI] [PubMed] [Google Scholar]
- 38. Mould DR, Sweeney K, Duffull SB, Neylon E, Hamlin P, Horwitz S, et al A population pharmacokinetic and pharmacodynamic evaluation of pralatrexate in patients with relapsed or refractory non‐Hodgkin's or Hodgkin's lymphoma. Clin Pharmacol Ther 2009; 86: 190–196. [DOI] [PubMed] [Google Scholar]
- 39. Ito K, Hutmacher M, Liu J, Qiu R, Frame B, Miller R. Exposure‐response analysis for spontaneously reported dizziness in pregabalin‐treated patient with generalized anxiety disorder. Clin Pharmacol Ther 2008; 84: 127–135. [DOI] [PubMed] [Google Scholar]
- 40. Zingmark P, Kagedal M, Karlsson M. Modelling a spontaneously reported side effect by use of a Markov mixed‐effects model. J Pharmacokinet Pharmacodyn 2005; 32: 261–281. [DOI] [PubMed] [Google Scholar]
- 41. Hansson E, Ma G, Amantea M, French J, Milligan P, Friberg L, et al PKPD modeling of predictors for adverse effects and overall survival in sunitinib‐treated patients with GIST. CPT Pharmacometrics Syst Pharmacol 2013; 2: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tacrolimus ‐ FDA prescribing information, side effects and uses 2017. Available at https://www.drugs.com/pro/tacrolimus.html (last accessed 12 November 2017).
- 43. Staatz C, Tett S. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol 2014; 88: 1351–1389. [DOI] [PubMed] [Google Scholar]
- 44. Tornatore KM, Meaney CJ, Consiglio JD, Wilding G, Chang SS, Venuto RC. Sex and race influences on tacrolimus pharmacokinetics in renal transplant recipients: American Society of Nephrology annual meeting 2015. JASN 2015; 26; (Suppl): 748A. [Google Scholar]
- 45. Pirsch J, Miller J, Deierhoi M, Vincenti F, Filo R. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 1997; 63: 977–983. [DOI] [PubMed] [Google Scholar]
- 46. Mayer A, Dmitrewski J, Squifflet J, Besse T, Grabensee B, Klein B, et al Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 1997; 64: 434–443. [DOI] [PubMed] [Google Scholar]
- 47. Tang J, Andrews L, van Gelder T, Shi Y, van Schaik R, Wang L, et al Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol 2016; 12: 555–565. [DOI] [PubMed] [Google Scholar]
- 48. Yanagimachi M, Naruto T, Tanoshima R, Kato H, Yokosuka T, Kajiwara R, et al Influence of CYP3A5 and ABCB1 gene polymorphisms on calcineurin inhibitor‐related neurotoxicity after hematopoietic stem cell transplantation. Clin Transplant 2010; 24: 855–861. [DOI] [PubMed] [Google Scholar]
- 49. Mizutani T, Masuda M, Nakai E, Furumiya K, Togawa H, Nakamura Y, et al Genuine functions of P‐glycoprotein (ABCB1). Curr Drug Metab 2008; 9: 167–174. [DOI] [PubMed] [Google Scholar]
- 50. Lin JH, Yamazaki M. Role of P‐glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet 2002; 42: 59–98. [DOI] [PubMed] [Google Scholar]
- 51. Kramer B, Montagnino G, del Castillo D, Margreiter R, Sperschneider H, Olbricht C, et al Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow‐up results. Nephrol Dial Transplant 2005; 20: 968–973. [DOI] [PubMed] [Google Scholar]
- 52. Sy S, Heuberger J, Shilbayeh S, Conrado D, Derendorf H. A Markov chain model to evaluate the effect of CYP3A5 and ABCB1 polymorphisms on adverse events associated with tacrolimus in pediatric renal transplantation. AAPS J 2013; 15: 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamauchi A, Ieiri I, Kataoka Y, Tanabe M, Nishizaki T, Oishi R, et al Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation 2002; 74: 571–572. [DOI] [PubMed] [Google Scholar]
- 54. Kassir N, Labbé L, Delaloye J, Mouksassi M, Lapeyraque A, Alvarez F, et al Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in paediatric liver transplant recipients. Br J Clin Pharmacol 2014; 77: 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschenes G, et al Population pharmacokinetics and pharmacogenetics of once daily prolonged‐release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol 2013; 69: 189–195. [DOI] [PubMed] [Google Scholar]
- 56. Saint‐Marcoux F, Debord J, Undre N, Rousseau A, Marquet P. Pharmacokinetic modeling and development of Bayesian estimators in kidney transplant patients receiving the tacrolimus once‐daily formulation. Ther Drug Monit 2010; 32: 129–135. [DOI] [PubMed] [Google Scholar]
- 57. Benkali K, Prémaud A, Picard N, Rérolle J, Toupance O, Hoizey G, et al Tacrolimus population pharmacokinetic‐pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet 2009; 48: 805–816. [DOI] [PubMed] [Google Scholar]
- 58. Van der Meer A, Marcus M, Touw D, Proost J, Neef C. Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther Drug Monit 2011; 33: 133–146. [DOI] [PubMed] [Google Scholar]
- 59. Monchaud C, de Winter B, Knoop C, Estenne M, Reynaud‐Gaubert M, Pison C, et al Population pharmacokinetic modelling and design of a Bayesian estimator for therapeutic drug monitoring of tacrolimus in lung transplantation. Clin Pharmacokinet 2012; 51: 175–186. [DOI] [PubMed] [Google Scholar]
- 60. Benkali K, Rostaing L, Premaud A, Woillard J, Saint‐Marcoux F, Urien S, et al Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in renal transplant recipients on a new once‐daily formulation. Clin Pharmacokinet 2010; 49: 683–692. [DOI] [PubMed] [Google Scholar]
- 61. Saint‐Marcoux F, Knoop C, Debord J, Thiry P, Rousseau A, Estenne M, et al Pharmacokinetic study of tacrolimus in cystic fibrosis and non‐cystic fibrosis lung transplant patients and design of Bayesian estimators using limited sampling strategies. Clin Pharmacokinet 2005; 44: 1317–1328. [DOI] [PubMed] [Google Scholar]
- 62. Woillard J, de Winter B, Kamar N, Marquet P, Rostaing L, Rousseau A. Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations‐‐twice daily Prograf and once daily Advagraf. Br J Clin Pharmacol 2011; 71: 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rousseau A, Marquet P. Application of pharmacokinetic modelling to the routine therapeutic drug monitoring of anticancer drugs. Fundam Clin Pharmacol 2002; 16: 253–262. [DOI] [PubMed] [Google Scholar]
- 64. Macchi‐Andanson M, Charpiat B, Jelliffe R, Ducerf C, Fourcade N, Baulieux J. Failure of traditional trough levels to predict tacrolimus concentrations. Ther Drug Monit 2001; 23: 129–133. [DOI] [PubMed] [Google Scholar]
- 65. Zhao W, Fakhoury M, Baudouin V, Maisin A, Deschênes G, Jacqz‐Aigrain E. Limited sampling strategy for estimating individual exposure of tacrolimus in pediatric kidney transplant patients. Ther Drug Monit 2011; 33: 681–687. [DOI] [PubMed] [Google Scholar]
- 66. Willis C, Staatz C, Tett S. Bayesian forecasting and prediction of tacrolimus concentrations in pediatric liver and adult renal transplant recipients. Ther Drug Monit 2003; 25: 158–166. [DOI] [PubMed] [Google Scholar]
- 67. Ting L, Villeneuve E, Ensom M. Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit 2006; 28: 419–430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Immunosuppressive adverse effects scoring system [18]
Table S2 Sex association with composite adverse effect ratio: model parameter estimates
Table S3 Predictive performance of all tested limited sampling strategies predicting tacrolimus exposure in adult kidney transplant recipients
Figure S1 Correlation plots A–D between the reference AUCint and the projected AUClss for the selected limited sampling strategies. AUCint is the tacrolimus area under the curve between 0 and 12 h estimated with the full intensive sampling strategy. AUClss is the projected area under the curve obtained from the different combinations of sampling times using a Bayesian forecasting approach. Continuous lines represent the linear regression lines. Dashed lines represent the 95% confidence interval for each linear regression line. The limited sampling strategies including three and four time‐point (panels C and D) exhibited the best correlation between reference AUCint and projected AUClss with r2 of 0.947 and 0.961, respectively. Patients were categorized as poor (blue dots), intermediate (green dots) or extensive (red dots) metabolizer based on a CYP3A5*3*6*7 metabolic composite
Figure S2 Scatter plots A–D of the absolute percentage prediction error vs. the reference AUCint for the selected limited sampling strategies. The AUCint represents the tacrolimus area under the curve between 0 and 12 h estimated using the full intensive sampling strategy. The absolute percentage prediction error was calculated between AUCint and AUClss, which is the projected area under the curve obtained from the different combinations of sampling times using a Bayesian forecasting approach, as following:
Accuracy within 15%, which is the clinical acceptable level criteria, is represented by the dashed line for each combination of sampling times. 100% of the projected AUClss obtained with the limited sampling strategies including three time‐points (C, D) fell within 15% of reference AUCint, as opposed to 59.7% for the limited sampling strategy including tacrolimus trough concentration (A). Patients were categorized as poor (blue dots), intermediate (green dots), or extensive (red dots) metabolizers based on a CYP3A5*3*6*7 metabolic composite
Figure S3 Bland–Altman graphs (A–D) comparing the difference between individual AUCint and AUClss (y‐axis) and the average of individual AUCint and AUClss (x‐axis) for the selected limited sampling strategies. Dashed lines represent the 5th, 50th and 95th percentiles. Reference AUCint is the tacrolimus area under the curve between 0 and 12 h estimated using the 12‐h intensive sampling strategy. AUClss is the projected area under the curve obtained from the different combinations of sampling times using a Bayesian forecasting approach. The limited sampling strategies including three or four timed concentrations (C, D) showed the best precision with the narrowest agreement interval between the 5th and 95th percentiles. No systematic bias was depicted in the four graphs with no consistent pattern to the direction of bias, with AUClss both under‐ and overestimating AUCint. Patients were categorized as poor, intermediate or extensive metabolizers based on a CYP3A5*3*6*7 metabolic composite, and are represented by blue, green and red dots, respectively


