Abstract
As indigenous malaria has decreased over recent decades, the increasing number of imported malaria cases has provided a new challenge for China. The proportion of imported cases due to Plasmodium ovale has increased during this time, and the difference between P. ovale curtisi and P. ovale wallikeri is of importance. To better understand P. ovale epidemiology and the differences between the two subspecies, information on imported malaria in Henan Province was collected during 2010–2017. We carried out a descriptive study to analyze the prevalence, proportion, distribution, and origin of P. o. curtisi and P. o. wallikeri. It showed that imported P. ovale spp. accounts for a large proportion of total malaria cases in Henan Province, even more than that of P. vivax. This suggests that the proportion of P. ovale cases is underestimated in Africa. Among these cases, the latency period of P. o. curtisi was significantly longer than that of P. o. wallikeri. More attention should be paid to imported ovale malaria to avoid the reintroduction of these two subspecies into China.
Introduction
Malaria, one of the most important global public health problems, is still a serious cause of mortality, especially in Africa. The World Health Organization (WHO) states that about 216 million cases of malaria occurred worldwide in 2016 compared with 211 million cases in 2015, and estimated that 445,000 deaths occurred from malaria globally in 2016 compared to 446,000 in 20151,2. An estimated 90% of malaria cases and 92% of all malaria deaths in 2016 were in Africa1,2. Plasmodium falciparum, P. vivax, P. ovale, and P. malariae are the four species that normally cause illness in humans. Mixed infections occur in areas where more than one species is epidemic3. P. knowlesi is considered as a fifth pathogenic species, which is mainly a simian malaria occurring in Southeast Asia, and humans can be infected occasionally4–6. According to the World Malaria Report 20172, P. falciparum is the most prevalent malarial species in Africa, accounting for 99% of estimated malaria cases in 2016. Due to this, almost all of the attention is paid to P. falciparum, while the other Plasmodium species are typically neglected.
The National Malaria Elimination Action Plan for 2010–2020 was issued in China in 2010, with the goal of achieving complete elimination by 20207. There have been no local malaria cases reported in Henan Province since 20128, and zero indigenous malaria cases were reported in China in 20179. However, the number of imported malaria cases from overseas increased gradually, presenting a new challenge for China10,11. Among the imported malaria cases, all of the human malarial species have been detected, with P. falciparum being the main species. The proportion of P. ovale reported has increased and overtaken that of P. vivax11. Occasionally, malaria cases caused by P. knowlesi are reported in China12.
As one of the four main human malarial species, P. ovale was the last one to be reported in 192213. Due to its low parasitemia and low prevalence in limited areas14–16, and its similar morphology with P. vivax and mixed infections with other Plasmodium species, P. ovale has been given relatively little attention compared with the other species, and its prevalence has apparently been underestimated17–20. However, by using molecular assays, it has been found that P. ovale occurs in most of Africa, India, and Southeast Asia21–24, and its prevalence has reached as high as 15% in Papua New Guinea25 and rural Nigeria26. Furthermore, according to sequence analysis, P. ovale is considered to be comprised of two different subspecies21–24,27, which were named classic and variant P. ovale22 and later formally named P. ovale curtisi and P. ovale wallikeri23. So far, only a few clinical, epidemiological, and therapeutic studies have reported specific data for P. ovale subspecies. However, the geographical distribution of P. ovale seems larger than previously thought based on molecular analysis.
Currently, detailed information about the incidence and distribution of malaria caused by the four Plasmodium species in the world is limited. Especially in epidemic regions of Africa, social and economic factors, medical facilities, and personnel capabilities might affect the identification of Plasmodium species, so it is more difficult to discriminate P. o. curtisi and P. o. wallikeri. Current knowledge of P. ovale malaria is mostly based on small trials with minor impact, case reports, and clinical observations, and there is a lack of systematic research. To date, information about the differences between P. o. curtisi and P. o. wallikeri is relatively scant, and more distinguishing features of the two subspecies should be uncovered. Presently, P. falciparum as the main imported Plasmodium species has attracted much more attention in China and the rest of the world. Thus, there have been relatively few studies about imported P. ovale cases in China. In the present study, based on imported malaria cases in Henan Province during 2010–2017, information was collected and the differences between P. o. curtisi and P. o. wallikeri were described and analyzed.
Results
Prevalence of imported malaria in Henan Province, 2010–2017
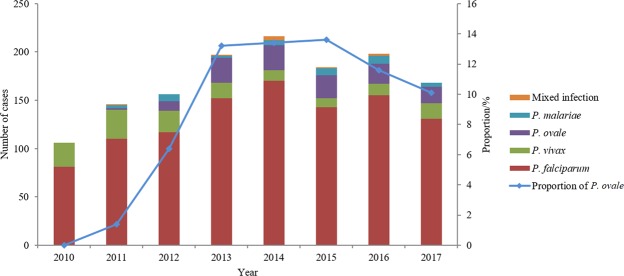
A total of 1,372 confirmed imported malaria cases were reported in Henan Province during 2010 to 2017, and no indigenous malaria case has been reported since 2012. The number of imported malaria cases increased yearly and reached a peak in 2014 (n = 216). Among all of the cases, 77.2% were infected with P. falciparum (n = 1059), followed by P. vivax (n = 142), P. ovale (n = 126), and P. malariae (n = 36), accounting for 10.3%, 9.2%, and 2.6%, respectively. One patient was infected with P. knowlesi imported from Indonesia in 2017. Nine patients with mixed infections accounted for 0.7% of cases, mostly with the two species P. falciparum and P. ovale. There were four cases of P. falciparum coinfected with P. ovale, two of P. malariae with P. ovale, two of P. falciparum with P. vivax, and one of P. falciparum with P. malariae. Since 2013, the number of ovale malaria cases was more than that of P. vivax each year, and P. falciparum and P. ovale were the two main species of imported malaria in Henan Province (Fig. 1).
Figure 1.
Imorted malaria cases in Henan Province, 2010–1017.
Confirmation of ovale malaria and identification of subspecies
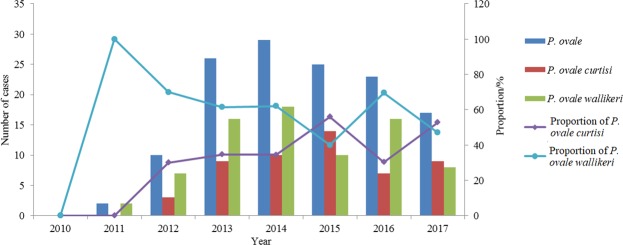
Including the 6 mixed infection cases of P. ovale with other species, there were a total of 132 ovale malaria cases. Three patients were diagnosed by microscopy without obtaining blood samples, and the other cases were all diagnosed by microscopy and nested PCR. No case of P. ovale was found in 2010, but after that, the number of P. ovale cases increased yearly and reached a peak in 2014 (n = 29). Fifty-two (52/132, 39.4%) cases were confirmed as P. o. curtisi and 77 were P. o. wallikeri (77/132, 58.3%); the other 3 cases could not be identified and are considered as P. ovale spp. Except in 2015 and 2017, the number of P. o. wallikeri cases was much more than that of P. o. curtisi (Fig. 2). As for the 6 mixed infection cases, 5 cases were P. o. wallikeri while the other one was P. o. curtisi.
Figure 2.
Imported malaria cases of P. ovale in Henan Province, 2010–2017.
Origin of the imported ovale malaria
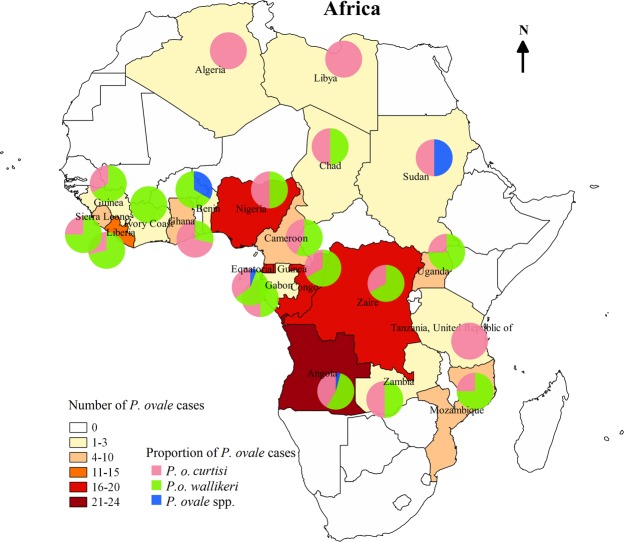
All 132 of the patients infected with P. ovale had the parasite imported from 20 countries in Africa, with the five top 5 countries being Angola, Congo, Equatorial Guinea, Nigeria, and Liberia, which accounted for 18.2%, 13.6%, 12.9%, 12.1%, and 10.6% of the cases, respectively. The 52 cases of P. o. curtisi were from 17 countries in Africa, with the top 5 countries being Angola (19.2%), Nigeria (15.4%), Congo (11.5%), Equatorial Guinea (11.5%), and Ghana (9.6%). The 77 cases of P. o. wallikeri were from 17 different countries in Africa, with the top 5 countries being Angola (16.9%), Congo (15.6%), Equatorial Guinea (13.0%), Liberia (13.0%), and Nigeria (10.4%). Except for the 5 countries with one case of P. ovale, the case numbers of P. o. curtisi and P. o. wallikeri were the same in Nigeria, Gabon, Chad, and Zambia, and the number of P. o. curtisi cases was more than that of P. o. wallikeri only in Ghana; in contrast, the number of P. o. curtisi cases was less than that of P. o. wallikeri in the other 10 countries. For the 6 mixed infection cases of P. ovale, the 4 cases coinfected with P. falciparum were from Angola (n = 2), Equatorial Guinea (n = 1), and Cameroon (n = 1), and two cases coinfected with P. malariae were from Congo and Liberia (Fig. 3).
Figure 3.
Geographic distribution of the origin countries of the imported P. ovale cases in Africa. The proportion of the P. ovale subspecies in the origin country was showed with the pie charts.
Proportion of imported malaria in the country of origin
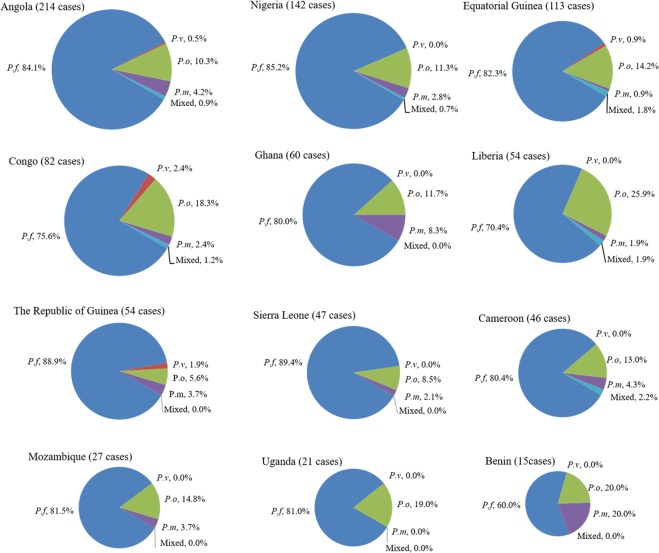
For the origin countries, the total number of imported malaria cases was different, so more cases of P. ovale malaria did not mean a higher proportion among the four Plasmodium species. To obtain the proportion of the 4 Plasmodium species in the origin countries, the top 12 countries with the most P. ovale malaria cases were analyzed, and among them Liberia had the highest proportion of P. ovale malaria, and the Republic of Guinea had the lowest. For all 12 of the countries, except for mixed infection, the proportion of P. falciparum malaria cases was largest, followed by P. ovale and P. malariae, and that of P. vivax was smallest or equal to P. malariae. Except for Uganda, imported P. malariae existed in the other 11 countries, but imported P. vivax was just found in Angola, Equatorial Guinea, Congo, and Guinea (Fig. 4).
Figure 4.
Proportion of the Plasmodium species among the imported malaria cases in the origin countries. The top 12 countries with most P. ovale cases were analyzed. P. f: Plasmodium falciparum, P. v: Plasmodium vivax, P. o: Plasmodium ovale, P. m: Plasmodium malariae, mixed: mixed infection.
Temporal distribution of ovale malaria
There were ovale malaria cases reported every month, but the peaks appeared in April and October for all of the cases, accounting for 12.9% (n = 17) and 11.4% (n = 15), respectively. Compared with the other months, cases in January, July, and November were fewer. For P. o. curtisi, two peaks appeared in March and October, and cases in February, July, and November were fewer compared with the other months. For P. o. wallikeri, most cases were concentrated in February to October without an obvious peak, accounting for 85.7% (66/77) of the total (Fig. 5).
Figure 5.
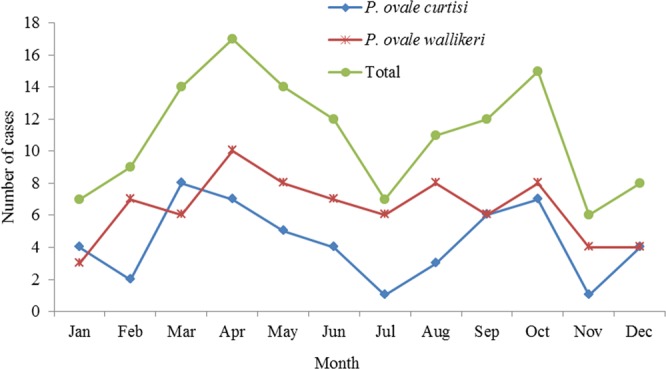
Monthly distribution of reported P. ovale cases, 2010–2017.
Interval between arrival in China and illness onset
Except for the 6 mixed infection cases of P. ovale with other species, among the 126 imported ovale malaria cases, 120 (95.2%) had complete information about the date of arrival in China and illness onset, from which the interval days could be calculated. For the interval between arrival and disease onset, the shortest was 23 days before returning to China, the longest was 1265 days after arrival in China, the median was 59 days, and the interquartile range was (14–160) days. Three (2.5%) patients had symptoms onset before arrival in China. After arriving in China, 45 (37.5%) patients had symptoms onset within one month. The median and interquartile range of P. o. curtisi was 97.5 days (7.5–188.5), and that of P. o. wallikeri was 31 days (14–99). The difference between P. o. curtisi and P. o. wallikeri for the interval of arrival and disease onset was statistically significant (Z = 10.159, p = 0.001). More patients infected with P. o. wallikeri (33/67, 49.3%) had illness onset within one month after arrival in China compared with people infected with P. o. curtisi (13/50, 26.0%), and this difference was statistically significant (Pearson χ2 = 5.013, p = 0.010). In contrast, fewer patients infected with P. o. wallikeri (18/67, 26.7%) had symptoms onset after returning three months later compared with those with P. o. curtisi (26/50, 52.0%), and the difference was statistically significant (Pearson χ2 = 7.709, p = 0.005) (Fig. 6).
Figure 6.
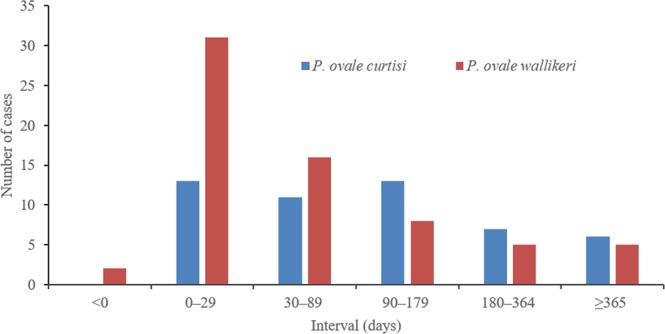
The interval between date of arrival in China and illness onset.
Interval between disease onset and diagnosis
Except for the 6 mixed infection cases of P. ovale with other species, information about the date of symptom onset and diagnosis was collected from the 124 imported ovale malaria cases, and this allowed for calculating the interval days. For the interval between disease onset and diagnosis, the shortest was 1 day, the longest was 59 days, the mode was 1 day, and the median and interquartile range was 4 days (2–7). In addition, 49.2% (61/124) of the patients were diagnosed within 3 days after disease onset, and 78.2% (97/124) were diagnosed within one week. However, there were a few patients diagnosed 15 days later, or even 1 month after disease onset, accounting for 6.5% (8/124) and 2.4% (3/124) of cases, respectively. The differences between P. o. curtisi and P. o. wallikeri for the interval of disease onset and diagnosis were not statistically significant for any groups.
Discussion
After the launch of the National Malaria Elimination Action Plan for 2010–2020 in China in 20107, local malaria cases have been reduced yearly until no indigenous malaria case has been reported in Henan Province since 20128. However, reported malaria cases have increased every year and reached a peak in 2014, but decreased by about 15% in 2015, which might be affected by reducing travel due to the transmission of Ebola in western Africa during 2014–2015; however, the proportion of P. ovale cases was increased in 2015. At present, the few studies are available about imported malaria in China that shows Africa is the top area of origin, and Angola, Congo, Equatorial Guinea, Ghana, and Nigeria are the most common countries of origin10,11,28,29. However, there are relatively few studies about imported malaria that describe the proportion of the four human Plasmodium species in Chinese patients following African travel. Imported P. falciparum cases accounted for 83.4% of the total in Jiangsu Province during 2011–2014, although the proportion of P. ovale cases increased yearly to 3.7%, 9.6%, 8.8%, and 13.0%, respectively28. In the present study, the proportion of imported P. falciparum cases was 77.2% during 2010–2011 in Henan Province, which remained about 75.0% in these years, while imported P. ovale cases accounted for 0.0%, 1.4%, 6.4%, 13.2%, 12.0%, 13.0%, 10.6%, and 10.1%, respectively, which was similar to the results in Jiangsu Province28.
In this study, we found that P. ovale cases were imported from 20 countries in Africa, with Angola, Congo, Equatorial Guinea, Nigeria, and Liberia being the 5 top origin countries for the 132 imported P. ovale cases. P. ovale is widespread in Africa and fairly common. However, studies about the prevalence of P. ovale in Africa are relatively few. Until recently, species-level diagnosis of malaria has been difficult in some African countries, so the prevalence of ovale malaria may be underestimated. Also, differences in prevalence may be due to different characteristics of Chinese travelers compared to locals in African countries. In addition, factors, such as length of exposure, access to care, past treatment, usage of prophylaxis, and clinical presentation, could explain these differences, which need more study. There are some limitations to this study: the results were obtained without carefully planned sampling, so the largest number of imported malaria cases just showed which origin country was the most common in Henan Province, and the true prevalence of Plasmodium species in the origin country cannot be estimated from this proportion. The proportion of P. ovale malaria in cases travelling to Liberia was highest (25.9%), while that of Angola was just 10.3%. However, there were only 20 African countries analyzed in this study, which were the most common sites of origin with P. ovale in Henan Province, so more surveys about the prevalence of P. ovale in Africa should be performed.
Since P. ovale was classified into two different subspecies, P. o. curtisi and P. o. wallikeri23, more attention has been paid to the detection and genotype of the two subspecies30–35, and studies focusing on the prevalence and distribution of P. ovale spp. have been relatively few. Among 98 imported cases of P. ovale in Jiangsu Province, China, the proportions of P. o. curtisi and P. o. wallikeri were 48% and 52%, which are comparable12. In this study, P. o. curtisi and P. o. wallikeri accounted for 39.4% and 58.3%, respectively, with the number of P. o. curtisi cases being less than that of P. o. wallikeri in most countries. The proportion of the two subspecies is similar to that in Ethiopia and Senegal. In northwest Ethiopia, out of 9 P. ovale cases, 7 patients were infected with P. o. wallikeri while the other 2 were infected with P. o. curtisi36. In Senegal, out of 235 malaria cases, 4 patients were infected with P. ovale, 3 with P. o. wallikeri, and 1 with P. o. curtisi37.
For indigenous malaria, the peak has appeared around August and September in China. However, there is no obvious peak for imported malaria8. In this study, the peaks of total P. ovale and P. o. curtisi were similar and appeared in March and October, which might be related with the Chinese festivals, the Spring Festival in February, and National Day in October. Migrant workers prefer to return to China during these festivals. However, no obvious peak was observed for P. o. wallikeri. The differences in temporal pattern between P. o. curtisi and P. o. walliker deserve more study.
It was difficult to obtain the exact date of malaria infection in this epidemiological investigation. However, imported malaria cases are suitable for observing the latency period of malaria. The date of arrival in China was used as the date of malaria infection, and the intervals between the date of arrival and illness onset were calculated. Although imprecise, this can be used as a proxy to estimate the latency period, for which P. ovale malaria is mostly observed through imported cases28,38. Nolder et al. observed that the latency period of P. o. curtisi (85.7 days) was much longer than that of P. o. wallikeri (40.6 days)38, whereas the latency period of the two subspecies showed no difference in the study of Cao et al.28. In the present study, the latency period of P. o. curtisi (97.5 days) was more than three times as long as that of P. o. wallikeri (31 days), which is similar to the work of Nolder et al.38. The longest latency period in this study was 1265 days, which happened in a case of P. o. curtisi; similarly, the longest was also a case of P. o. curtisi in the study of Nolder et al. (1083 days)38. The liver stage of malaria commonly exists for parasites of P. ovale and P. vivax; the hypnozoites in the liver last months or years after infection, which results in a long latency period. This makes it quite difficult to diagnose imported malaria cases in nonendemic countries because the presenting illness may be unrelated to recent travel abroad39–41. More studies about the latency period of P. ovale and P. vivax parasites will improve the diagnosis of imported malaria.
Henan Province, located in the Middle East of China, has a warm temperate zone, subtropical, humid-semi-humid monsoon climate. The climate and environment are suitable for the breeding of Anopheles, including Anopheles sinensis and Anopheles anthropophagus, so the threat of malaria reintroduction remains. Currently, for imported malaria, timely diagnosis, standardized treatment, and preventing further transmission are the key strategies to eliminate sources of infection in China.
Conclusion
This study illustrates that imported P. ovale cases account for a large proportion of the total cases in Henan Province, even larger than P. vivax cases. The proportion of P. ovale cases is underestimated in the origin countries of Africa, which is large and exceeded only by P. falciparum. We confirmed that the latency period of P. o. curtisi is much longer than that of P. o. wallikeri. It is recommended that more attention be paid to imported P. ovale cases to avoid reintroduction in China.
Methods
Data collection
Information on imported malaria in Henan Province was collected from case investigation reports and the Disease Surveillance Information Report Management system of the China Centers for Disease Control and Prevention (CCDC), including gender, age, occupation, travel history, date of arrival in China, date of symptom onset, date of diagnosis, and reported institution.
Species and subspecies confirmation
All of the cases were initially diagnosed by blood smear microscopy and/or rapid diagnostic tests (RDT) at the basic level of the hospital or CDC, then all of them were confirmed by the Henan Provincial Reference Laboratory for Malaria Diagnosis using nested polymerase chain reaction (PCR) and/or blood smear microscopy. The microscopy was performed by microscopists through the examination of thick and thin blood film at 1000×magnification. Rapid diagnostic tests were used to detect malaria antigens following the manufacturer’s instructions by laboratory staff. Nested PCR was performed using the methods published42,43. Subspecies of P. ovale were confirmed by nested PCR following sequencing according to previously published methods23,30.
Data analysis
All of the case information was collected and input into SSPS v.21.0 (Statistical Product and Service Solutions), in which all of the calculations and analyses were performed. The differences were compared by Wilcoxon rank-sum test, Chi-square, or Fisher’s exact tests, and a two-sided p value of <0.05 was considered statistically significant. Geographic distribution was conducted by geographic information system (GIS)-based spatial analysis, which was fulfilled by QGIS (Quantum GIS) v2.18.19.
Ethics approval and consent to participate
All of the identifiers of patients were removed from the data. Informed consent was obtained from all of the patients. This study was approved by the Ethical Committee of the Henan Province Center for Disease Control and Prevention. All of the methods were performed in accordance with the relevant guidelines and regulations of the Henan Province Center for Disease Control and Prevention.
Supplementary information
Characterization of Plasmodium ovale spp. imported from Africa to Henan Province, China
Acknowledgements
We would like to thank all the patients who participated in these surveys and the staff of the hospitals and centers for disease control and prevention in Henan Province. The study was supported by Science and Technology Project of Henan province (No. 182102310631, No. 162102310035) and Henan Provincial Medical Science and Technology Project (No. 201702274). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
R.M.Z. and H.W.Z. conceived and designed the study; R.M.Z. and S.U.L. performed the experiments; R.M.Z. was responsible for the data analysis and drafted the manuscript; Y.L.Z. and Y.L. participated in the sample collection; C.Y.Y. and D.Q contributed to the data collection; H.W.Z. revised the manuscript; H.W. and D.L.L. provided the administrative coordination. All authors reviewed and approved the final version of the manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the first/corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38629-0.
References
- 1.World Health Organization. World Malaria Report 2016. Geneva; World Health Organization (2017).
- 2.World Health Organization. World Malaria Report 2017. Geneva; World Health Organization (2018).
- 3.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.White NJ. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis. 2008;46:172–173. doi: 10.1086/524889. [DOI] [PubMed] [Google Scholar]
- 5.Jongwutiwes S, et al. Plasmodium knowlesi Malaria in humans and macaques, Thailand. Emerg Infect Dis. 2011;17:1799–1806. doi: 10.3201/eid1710.110349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–184. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Health of the People’s Republic of China. Action plan of China malaria elimination (2010–2020). http://www.malariaeradication.org/elimination-programmes/peoples-republic-china.
- 8.Liu Y, et al. Analysis of malaria epidemiological characteristics in Henan Province from 2005 to 2013. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2014;32:419–422. [PubMed] [Google Scholar]
- 9.Zhang L, et al. The progress of national malaria elimination and epidemiological characteristics of malaria in China in 2017. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2018;36:201–209. [Google Scholar]
- 10.Li Z, et al. Epidemiologic features of overseas imported malaria in the People’s Republic of China. Malar J. 2016;15:141. doi: 10.1186/s12936-016-1188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J, et al. Malaria epidemiological characteristics in China, 2005–2015. Zhongguo Re Dai Yi Xue. 2017;17:325–335. [Google Scholar]
- 12.Pan B, et al. Diagnosis and treatment of the first imported case of Plasmodium knowlesi infection in China. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2016;34:513–516. [PubMed] [Google Scholar]
- 13.Stephens JWW. A new malaria parasite of man. Ann Trop Med Parasitol. 1922;16:383–388. doi: 10.1080/00034983.1922.11684331. [DOI] [Google Scholar]
- 14.Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull World Health Organ. 1969;40:383–394. [PMC free article] [PubMed] [Google Scholar]
- 15.Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microb Rev. 2005;18:570–581. doi: 10.1128/CMR.18.3.570-581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AD, et al. Imported malaria and high risk groups: observational study using UK surveillance data 1987–2006. BMJ. 2008;337:a120. doi: 10.1136/bmj.a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roucher C, et al. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. Plos One. 2014;9:e87169. doi: 10.1371/journal.pone.0087169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trape JF, et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 years longitudinal study. Lancet Infect Dis. 2014;14:476–488. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 19.Doderer-Lang C, et al. The ears of the African elephant: unexpected high seroprevalence of Plasmodium ovale and Plasmodium malariae in healthy populations in Western Africa. Malar J. 2014;13:240. doi: 10.1186/1475-2875-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faye FB, et al. Diagnostic criteria and risk factors for Plasmodium ovale malaria. J Infect Dis. 2002;186:690–695. doi: 10.1086/342395. [DOI] [PubMed] [Google Scholar]
- 21.Tachibana M, et al. Two types of Plasmodium ovale defined by SSU rRNA have distinct sequences for ookinete surface proteins. Mol Biochem Parasitol. 2002;122:223–226. doi: 10.1016/S0166-6851(02)00101-9. [DOI] [PubMed] [Google Scholar]
- 22.Win TT, et al. Molecular analysis of Plasmodium ovale variants. Emerg Infect Dis. 2004;10:1235–1240. doi: 10.3201/eid1007.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland CJ, et al. Two non-recombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010;201:1544–1550. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- 24.Fuehrer HP, et al. Plasmodium ovale in Bangladesh: genetic diversity and the first known evidence of the sympatric distribution of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in southern Asia. Int J Parasitol. 2012;42:693–699. doi: 10.1016/j.ijpara.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Mehlotra RK, et al. Random distribution of mixed species malaria in fections in Papua New Guinea. Am J Trop Med Hyg. 2000;62:225–231. doi: 10.4269/ajtmh.2000.62.225. [DOI] [PubMed] [Google Scholar]
- 26.May J, et al. High rate of mixed and subpatient malarial infections in southwest Nigeria. Am J Trop Med Hyg. 1999;61:339–343. doi: 10.4269/ajtmh.1999.61.339. [DOI] [PubMed] [Google Scholar]
- 27.Calderaro A, et al. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J Clin Microbiol. 2007;45:1624–1627. doi: 10.1128/JCM.02316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y, et al. The increasing importance of Plasmodium ovale and Plasmodium malariae in a malaria elimination setting: an observational study of imported cases in Jiangsu Province, China, 2011–2014. Malar J. 2016;15:459. doi: 10.1186/s12936-016-1504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou S, et al. Trends of imported malaria in China 2010–2014: analysis of surveillance data. Malar J. 2016;15:39. doi: 10.1186/s12936-016-1093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou RM, et al. Polymorphisms analysis of the Plasmodium ovale tryptophan-rich antigen gene (potra) from imported malaria cases in Henan Province. Malar J. 2018;17:127. doi: 10.1186/s12936-018-2261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Xia ZG, Yan H. New type of SSUrDNA sequence was detected from both Plasmodium ovale curtisi and Plasmodium ovale wallikeri samples. Malar J. 2014;13:216. doi: 10.1186/1475-2875-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akerele D, et al. Molecular diagnosis of Plasmodium ovale by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One. 2017;12:e0179178. doi: 10.1371/journal.pone.0179178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joste V, et al. Distinction of Plasmodium ovale wallikeri and Plasmodium ovale curtisi using quantitative Polymerase Chain Reaction with High Resolution Melting revelation. Sci Rep. 2018;8:300. doi: 10.1038/s41598-017-18026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderaro A, et al. A new real-time PCR for the detection of Plasmodium ovale wallikeri. PLoS One. 2012;7:e48033. doi: 10.1371/journal.pone.0048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuehrer HP, Noedl H. Recent advances in detection of Plasmodium ovale: implications of separation into the two species Plasmodium ovale wallikeri and Plasmodium ovale curtisi. J Clin Microbiol. 2014;52:387–391. doi: 10.1128/JCM.02760-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alemu A, et al. Plasmodium ovale wallikeri and Plasmodium ovale curtisi in North-West Ethiopia. Malar J. 2013;12:346. doi: 10.1186/1475-2875-12-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diallo MA, et al. Plasmodium ovale wallikeri and Plasmodium ovale curtisi Malaria in Senegal in 2016. Bull Soc Pathol Exot. 2017;110:286–290. doi: 10.1007/s13149-017-0578-6. [DOI] [PubMed] [Google Scholar]
- 38.Nolder D, et al. An observational study of malaria in British travellers: Plasmodium ovale wallikeri and Plasmodium ovale curtisi differ significantly in the duration of latency. BMJ Open. 2013;3pii:e002711. doi: 10.1136/bmjopen-2013-002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis TM, Singh B, Sheridan G. Parasitic procrastination: late-presenting ovale malaria and schistosomiasis. Med J Aust. 2001;175:146–148. doi: 10.5694/j.1326-5377.2001.tb143063.x. [DOI] [PubMed] [Google Scholar]
- 40.Bottieau E, et al. Imported non-Plasmodium falciparum malaria: a five-year prospective study in a European referral center. Am J Trop Med Hyg. 2006;75:133–138. doi: 10.4269/ajtmh.2006.75.133. [DOI] [PubMed] [Google Scholar]
- 41.Coldren RL, et al. Apparent relapse of imported Plasmodium ovale malaria in a pregnant woman. Am J Trop Med Hyg. 2007;77:992–994. doi: 10.4269/ajtmh.2007.77.992. [DOI] [PubMed] [Google Scholar]
- 42.Snounou G, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Xia ZG, Tang LH. Establishment and application of multiplex PCR system for detecting four human Plasmodium species. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2015;33:91–95. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of Plasmodium ovale spp. imported from Africa to Henan Province, China
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the first/corresponding author on reasonable request.