Abstract
Recent data suggest that reduced sunlight exposure is associated with increased mortality in the general population. To date, the association between sunlight exposure and mortality in dialysis patients has not been examined. Among 134,478 dialysis patients in the Korean end-stage renal disease (ESRD) cohort from 2001 to 2014, 31,291 patients were enrolled from seven metropolitan cities, and data were analyzed using bi-directional case-crossover design. We examined the association between short-term sunlight exposure and mortality in ESRD patients. We adjusted for temperature, humidity, and daily concentrations of nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), carbon monoxide (CO), and particle matter (PM10) as confounders. The characteristics of the study population included age (65.6 ± 12.26 (mean ± standard deviation [SD]) years), sex (male, 59.96%; female, 41.04%), comorbidity (diabetes, 53.58%; hypertension, 40.5%), and kidney dialysis type (hemodialysis, 73.02%; peritoneal dialysis, 26.98%). The mean ± SD follow-up time was 4.68 ± 4.37 years. The daily sunlight exposure was significantly decreased in the case group compared with the control group (P = 0.004). Sunlight exposure was associated with all-cause death overall (ORs [95% CI]: 0.99 [0.98–0.99], P = 0.042) in a fully adjusted model. Patients with diabetes (ORs [95% CI]: 0.98 [0.97–0.99], P = 0.016) or aged higher than 75 years (ORs [95% CI]; 0.97 [0.96–0.99], P = 0.020) had higher risks of mortality than patients without diabetes or aged below 75 years, respectively. These findings suggest that sunlight exposure is inversely correlated with all-cause mortality in dialysis patients.
Introduction
There is a lively debate regarding the avoidance of sunlight and how this is a major risk factor for public health. Although high-intensity ultraviolet radiation can be a carcinogen to the skin1, there is growing scientific evidence that avoiding sunlight exposure is a major risk factor for various diseases and ultimately death2–4. Vitamin D is the “sunshine vitamin”, synthesized primarily in the skin during sunlight exposure, especially by ultraviolet B (UVB) radiation. In addition to observational studies, many randomized trials have demonstrated inverse associations of circulating vitamin D with the risks of cancer5, cardiovascular diseases6–8, infectious diseases9, metabolic disorders10, as well as mortality11,12. Prevalence of vitamin D deficiency is high in patients with chronic kidney disease (CKD) including end-stage renal disease (ESRD) patients undergoing dialysis13,14. In patients with CKD, conversion of serum 25-hydroxyvitamin D [25(OH)D] to 1,25(OH)2D, the active form of vitamin D, is insufficient owing to the loss of 1alpha-hydroxylase activity15.
Low circulating 25(OH)D concentration leads to mineral bone disease (MBD) such as secondary hyperparathyroidism, which is critically correlated with increased risks of coronary arterial calcification16, atherosclerosis, and endothelial cell dysfunction17, which result in cardiovascular events and mortality18,19. Although it is known that vitamin D supplementation improves serum 25(OH)D levels, there is still debate on whether this reduces mortality20,21.
Until recently, there have been few studies on the relationship between sunlight exposure and clinical prognosis in patients with ESRD who undergo renal replacement therapy, such as hemodialysis or peritoneal dialysis. Given this concern, this study was conducted to investigate the relationship of sunlight exposure and death in dialysis patients.
Results
Descriptive results
We identified 134,472 patients registered in the Korean Society of Nephrology for kidney dialysis registry. Among those, 31,291 (23.30%) patients died between 2001 and 2014 in the seven Korean metropolitan cities. The following characteristics of patients with all-cause deaths are shown in Table 1: sex (male, 59.96% and female, 41.04%), age at death (<75 years, 51.38%, and >75 years, 48.6%), comorbidity (diabetes, 53.58% and hypertension, 40.50%), kidney dialysis types (peritoneal dialysis, 26.98% and hemodialysis, 73.02%), primary disease (diabetes, 53.6%; hypertension, 15.2%; glomerulonephritis (GN), 8.16%; others, 7.31%; and unknown, 14.1%), and cause of death (cardiovascular disease, 28.31%; peripheral vessel disease, 12.7%; infection, 19.33%; cancer, 4.7%; and others, 34.9%). The average BMI in our study population was 21.21 for males and 21.20 for females. ESRD deaths occurred more frequently in males and those having diabetes as a primary disease. The number of ESRD deaths and the distributions by sunlight hours, temperature, humidity, and air pollutants (the daily concentrations of PM10, CO, NO2, SO2, and O3) concentrations by city in our study period are shown in Table 2.
Table 1.
Baseline characteristics of the study population (2001–2014).
| Variables | All-cause death |
|---|---|
| Overall | 31,291 |
| Sex | |
| Male (%) | 18,448 (58.96) |
| Female (%) | 12,843 (41.04) |
| Age (Death) (mean ± SD) | 65.6 ± 12.26 |
| <75 (%) | 16,076 (51.38) |
| ≥76 (%) | 15,208 (48.6) |
| Body mass index (BMI) | |
| Male (mean ± SD) | 21.21 ± 2.87 |
| Female (mean ± SD) | 21.20 ± 3.48 |
| Comorbidity | |
| Diabetes (%) | 53.58% |
| Hypertension (%) | 40.5% |
| Dialysis | |
| Peritoneal dialysis (%) | 8,442 (26.98) |
| Hemodialysis (%) | 22,849 (73.02) |
| Primary Disease | |
| Diabetes | 16,766 (53.6) |
| Hypertension | 4,753 (15.2) |
| Glomerulonephritis | 2,553 (8.16) |
| Others | 2,287 (7.31) |
| Unknown | 4,406 (14.1) |
| Cause of death | |
| CAD | 8,859 (28.31) |
| PVD | 3,976 (12.7) |
| Infection | 6,049 (19.33) |
| Cancer | 1,469 (4.7) |
| Others | 10,928 (34.9) |
Abbreviations: ESRD, end-stage renal disease; SD, standard deviation; BMI, body mass index; CAD, cardiovascular disease; PVD, peripheral vessel disease.
The mortality rate of this cohort was 23.3% among 134,472 ESRD patients.
Table 2.
City-specific descriptive information on the study period, all-cause death, and the levels of environmental variables in ESRD patients (2001–2014).
| City | Number of all-cause deaths | Number of monitoring sites | Sunlight (hrs.) | Temperature (°C) | Humidity (%) | PM10 (μg/m3) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percentiles | Percentiles | Percentiles | Percentiles | |||||||
| 50 | 90 | 50 | 90 | 50 | 90 | 50 | 90 | |||
| Seoul | 5,021 | 27 | 6.00 | 10.20 | 14.40 | 25.40 | 61.00 | 81.30 | 51.26 | 96.37 |
| Busan | 1,197 | 16 | 7.30 | 10.80 | 15.90 | 25.20 | 63.90 | 85.58 | 50.37 | 88.49 |
| Daegu | 856 | 11 | 7.20 | 10.80 | 15.65 | 26.80 | 57.90 | 79.28 | 50.18 | 88.49 |
| Incheon | 1,035 | 11 | 7.10 | 10.90 | 13.90 | 24.60 | 69.10 | 89.00 | 52.53 | 93.26 |
| Gwangju | 719 | 5 | 6.20 | 10.40 | 15.30 | 26.00 | 67.30 | 84.10 | 45.20 | 86.95 |
| Daejeon | 524 | 6 | 6.40 | 10.60 | 14.30 | 25.40 | 67.50 | 85.10 | 43.95 | 81.10 |
| Ulsan | 343 | 13 | 7.20 | 10.70 | 15.40 | 25.70 | 64.80 | 83.80 | 45.32 | 80.43 |
| City | Number of all-cause deaths | Number of monitoring sites | CO (ppm) | NO 2 (ppm) | SO 2 (ppm) | O 3 (ppm) | ||||
| Percentiles | Percentiles | Percentiles | Percentiles | |||||||
| 50 | 90 | 50 | 90 | 50 | 90 | 50 | 90 | |||
| Seoul | 5,021 | 27 | 0.607 | 1.002 | 0.037 | 0.056 | 0.005 | 0.008 | 0.053 | 0.087 |
| Busan | 1,197 | 16 | 0.473 | 0.786 | 0.024 | 0.037 | 0.006 | 0.009 | 0.056 | 0.087 |
| Daegu | 856 | 11 | 0.604 | 1.025 | 0.026 | 0.041 | 0.005 | 0.010 | 0.051 | 0.086 |
| Incheon | 1,035 | 11 | 0.596 | 0.958 | 0.028 | 0.046 | 0.007 | 0.011 | 0.055 | 0.089 |
| Gwangju | 719 | 5 | 0.597 | 0.976 | 0.023 | 0.035 | 0.004 | 0.006 | 0.048 | 0.075 |
| Daejeon | 524 | 6 | 0.588 | 1.096 | 0.021 | 0.035 | 0.004 | 0.008 | 0.018 | 0.035 |
| Ulsan | 343 | 13 | 0.479 | 0.772 | 0.020 | 0.031 | 0.006 | 0.010 | 0.024 | 0.037 |
Abbreviations: ESRD, end-stage renal disease; PM10, particulate matter less than 10 μm in diameter; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone.
Effect modification by meteorological and air pollution factors
The distributions of the meteorological variables (daily sunlight hour, ambient temperature and humidity) and five major pollutants (the daily concentrations of PM10, CO, NO2, SO2, and O3) differed by city and are shown in Table 2. As shown in Supplementary Fig. S1, Temperature, humidity and O3 showed strong seasonal patterns. The time-series plots of both the number of deaths and exposure (sunlight hours) over time for the entire study period are shown in Fig. 1.
Figure 1.
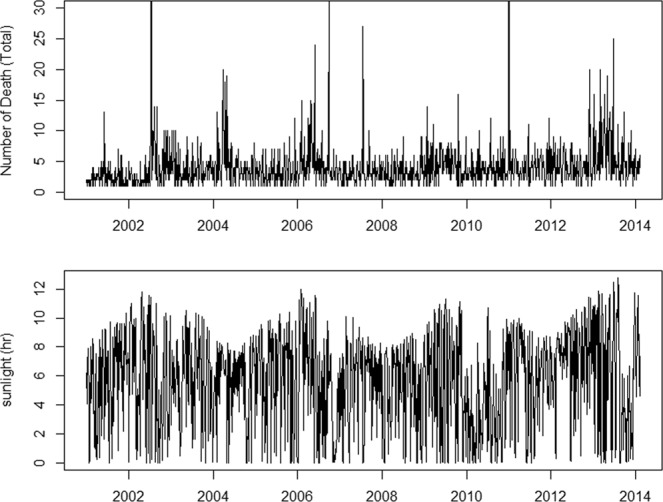
The trend of all-cause mortality and sunlight. The time-series plot indicates the daily number of mortality cases of ESRD patients from 2001 to 2014, (top panel) and daily hours of sunlight during 2001–2014 (bottom panel) which was collected from the Korea Meteorological Administration.
Difference in daily levels of environmental variables between case and control periods (2001–2014)
Table 3 shows the daily levels of weather variables and the major five pollutants in the case and control periods. The difference in the daily level and our main exposure variable, sunlight hours per day, was significantly less in the case period compared to the control period, as shown by a t-test (p = 0.004).
Table 3.
Difference in daily levels of environmental variables between case and control periods (2001–2014).
| Case Period | Control Period | Mean difference | 95% CI | P for t-test | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Sun light (hrs.) | 5.638 | 3.906 | 5.777 | 3.935 | −0.139 | (−0.233, −0.046) | 0.004 |
| Temperature (°C) | 13.813 | 10.148 | 13.824 | 10.191 | −0.010 | (−0.253, 0.233) | 0.934 |
| Humidity (%) | 62.849 | 15.988 | 62.533 | 15.992 | 0.315 | (−0.067, 0.698) | 0.106 |
| PM10 (μg/m3) | 53.711 | 30.357 | 53.695 | 35.809 | 0.016 | (−0.742, 0.774) | 0.967 |
| CO (ppm) | 0.623 | 0.255 | 0.618 | 0.242 | 0.005 | (−0.001, 0.011) | 0.128 |
| NO2 (ppm) | 0.032 | 0.017 | 0.032 | 0.014 | 0.000 | (0.032, 0.032) | 0.600 |
| SO2 (ppm) | 0.006 | 0.002 | 0.006 | 0.002 | 0.000 | (−0.00007, 0.00004) | 0.652 |
| O3 (ppm) | 0.991 | 0.093 | 0.991 | 0.095 | 0.000 | (−0.002, 0.003) | 0.674 |
Abbreviations: SD, standard deviation; CI, confidence interval; PM10, particulate matter less than 10 μm in diameter; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone.
Effect of sunlight exposure on ESRD death
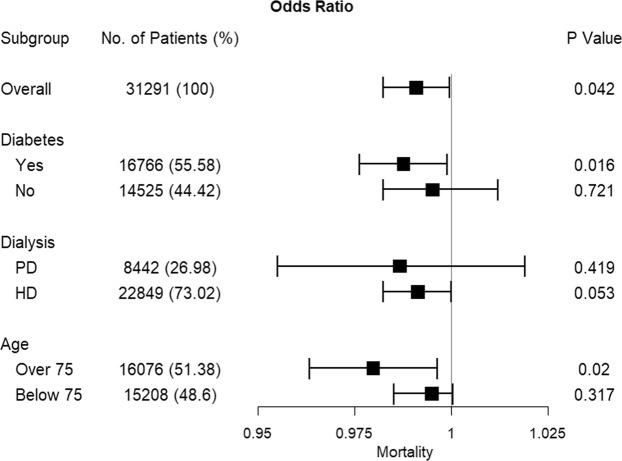
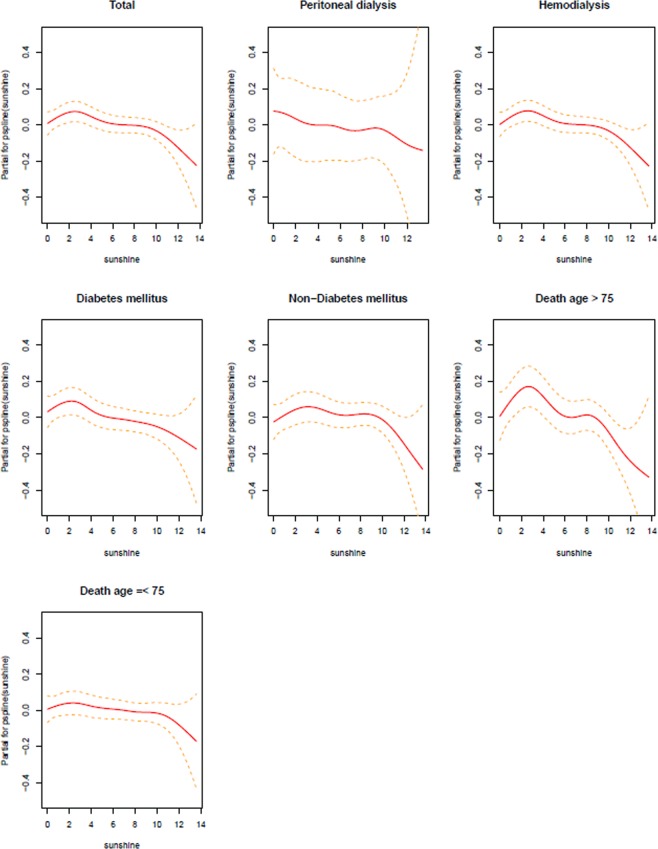
There was a statistically significant negative association between daily sunlight hours and all-cause mortality in ESRD patients (Table 4 and Fig. 2). An increase in daily sunlight hours was associated with a decrease in the ESRD-related deaths (overall OR [95% CI]: 0.99 [0.98–0.99]). Moreover, in sub-group analysis we identified that patients with diabetes or being older than 75 years have higher mortality with lower sunlight exposure which means the risk of mortality was different across these groups (OR [95% CI] for diabetes: 0.98 [0.97–0.99], OR [95% CI] for being older than 75 years: 0.97 [0.96–0.99]). However, there was no difference of risk of all-cause mortality according to the dialysis types (OR [95% CI] for peritoneal dialysis: 0.98 [0.95–1.01], OR [95% CI] for hemodialysis: 0.99 [0.98–1.00]). Although patients receiving hemodialysis had a higher relative risk, as indicated in Table 4, it was not statistically significant (p = 0.054). These analyses were performed with PM10 as an air-pollutant potential confounder in Models 1 to 5, with a similar pattern in all models (Table 4 and Fig. 2). The penalized smoothing spline between sunlight exposure and ESRD death in Fig. 3 also illustrates that lower sunlight exposure was associated with increased risk of mortality.
Table 4.
Effect modification of association between all-cause death and sunlight exposure with environmental confounders in conditional logistic regression models.
| Total | DM | Non-DM | PD | HD | Over 75 | Below 75 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | ||
| Model 1 | Sunlight (hrs.) | −0.009 | 0.042 | −0.015 | 0.016 | −0.002 | 0.721 | −0.013 | 0.419 | −0.009 | 0.053 | −0.021 | 0.02 | −0.005 | 0.317 |
| Temperature (°C) | 0.001 | 0.781 | 0.001 | 0.888 | 0.002 | 0.788 | 0.013 | 0.351 | −0.001 | 0.765 | 0.012 | 0.117 | −0.004 | 0.411 | |
| Humidity (%) | −0.001 | 0.667 | −0.002 | 0.251 | 0.001 | 0.666 | −0.003 | 0.561 | 0.000 | 0.796 | −0.003 | 0.244 | 0.001 | 0.541 | |
| PM10 (μg/m3) | 0.000 | 0.838 | 0.0004 | 0.459 | 0.000 | 0.624 | −0.001 | 0.675 | 0.000 | 0.889 | 0.0004 | 0.653 | 0.000 | 0.791 | |
| Model 2 | Sunlight (hrs.) | −0.009 | 0.044 | −0.015 | 0.018 | −0.003 | 0.672 | −0.015 | 0.359 | −0.009 | 0.060 | −0.021 | 0.017 | −0.005 | 0.341 |
| Temperature (°C) | 0.002 | 0.647 | 0.000 | 0.999 | 0.004 | 0.560 | 0.022 | 0.152 | −0.002 | 0.617 | 0.014 | 0.077 | −0.004 | 0.321 | |
| Humidity (%) | −0.001 | 0.654 | −0.002 | 0.309 | 0.001 | 0.609 | −0.002 | 0.619 | 0.000 | 0.818 | −0.003 | 0.277 | 0.001 | 0.560 | |
| NO2 (ppm) | 0.427 | 0.707 | 1.383 | 0.266 | −2.463 | 0.263 | −8.970 | 0.099 | 0.958 | 0.388 | −2.848 | 0.311 | 1.059 | 0.369 | |
| Model 3 | Sunlight (hrs.) | −0.009 | 0.039 | −0.015 | 0.014 | −0.002 | 0.717 | −0.013 | 0.439 | −0.009 | 0.048 | −0.021 | 0.020 | −0.005 | 0.303 |
| Temperature (°C) | 0.002 | 0.647 | 0.002 | 0.755 | 0.002 | 0.733 | 0.010 | 0.478 | 0.000 | 0.945 | 0.012 | 0.104 | −0.003 | 0.488 | |
| Humidity (%) | −0.001 | 0.661 | −0.002 | 0.314 | 0.001 | 0.646 | −0.003 | 0.561 | 0.000 | 0.805 | −0.003 | 0.003 | 0.001 | 0.553 | |
| SO2 (ppm) | 5.125 | 0.482 | −2.914 | 0.769 | −7.830 | 0.467 | 16.750 | 0.519 | −7.269 | 0.326 | −11.51 | 0.454 | −2.924 | 0.717 | |
| Model 4 | Sunlight (hrs.) | −0.009 | 0.041 | −0.015 | 0.014 | −0.002 | 0.748 | −0.012 | 0.458 | −0.009 | 1.504 | −0.021 | 0.019 | −0.006 | 0.237 |
| Temperature (°C) | 0.001 | 0.719 | 0.001 | 0.875 | 0.002 | 0.726 | 0.017 | 0.243 | −0.001 | 0.762 | 0.010 | 0.192 | −0.003 | 0.434 | |
| Humidity (%) | −0.001 | 0.641 | −0.002 | 0.345 | 0.001 | 0.721 | −0.004 | 0.459 | 0.001 | 0.707 | −0.003 | 0.296 | 0.001 | 0.442 | |
| O3 (ppm) | −0.178 | 0.791 | 0.434 | 0.633 | −0.888 | 0.380 | −3.987 | 0.136 | 1.504 | 0.428 | 1.086 | 0.457 | 3.110 | 0.146 | |
| Model 5 | Sunlight (hrs.) | −0.009 | 0.046 | −0.015 | 0.017 | −0.002 | 0.739 | −0.014 | 0.402 | −0.009 | 0.060 | −0.021 | 0.020 | −0.005 | 0.352 |
| Temperature (°C) | 0.000 | 0.966 | −0.001 | 0.884 | 0.001 | 0.912 | 0.016 | 0.266 | −0.003 | 0.388 | 0.013 | 0.097 | −0.006 | 0.156 | |
| Humidity (%) | −0.001 | 0.569 | −0.002 | 0.251 | 0.001 | 0.671 | −0.002 | 0.637 | 0.000 | 0.987 | −0.003 | 0.296 | 0.001 | 0.721 | |
| CO (ppm) | 0.093 | 0.221 | 0.146 | 0.154 | 0.027 | 0.812 | −0.268 | 0.361 | 0.158 | 0.033 | −0.117 | 0.445 | 0.192 | 0.020 | |
Abbreviations: DM, diabetes mellitus; PD, peritoneal dialysis; HD, hemodialysis; PM10, particulate matter less than 10 μm in diameter.
Figure 2.
Percent decrease in odds ratios of sunlight exposures for all-cause death. Forest plot showing the associations of sunlight exposures and mortality of ESRD patients (adjusted for temperature, humidity and PM10), according to diabetes, dialysis modality, and old age (over 75 years). Abbreviations: ESRD, end-stage renal disease; PD, peritoneal dialysis; HD, hemodialysis.
Figure 3.
Penalized spline terms on exposure. An estimated exposure-response curve for short-term exposures to sunlight was created to assess the percentage increase in daily mortality at various daily sunlight exposure hours. The solid line represents the predicted log relative risk, and dashed lines indicate 95% confidence intervals.
Discussion
This is the first study, to our knowledge, to examine the association between short-term sunlight exposure and mortality, in a nationwide ESRD cohort in Korea using a bi-directional case-crossover method. In the present study, the daily sunlight exposure hours were measured in the seven metropolitan cities along with ambient temperature and relative humidity from 2001 to 2014 (14 years). The daily concentrations of PM10, NO2, SO2, O3 and CO were adjusted for as confounders. We have demonstrated that low-level daily sunlight exposures significantly increased the risk of all-cause mortality in dialysis patients, especially in high-risk patients with diabetes and old age >75 years.
To date, there has been limited research on the relationship between sunlight exposure and mortality in ESRD patients. In 2014, Shapiro et al. reported that among 47,286 US dialysis patients, those residing in higher ultraviolet (UV) index regions had lower all-cause mortality compared to those living in moderate-high UV regions22. The average of the annual noon-time UV index value was calculated in each patient during the follow-up periods. In our study, we applied a case crossover method and compared the daily sunlight exposure on the day of the mortality event with the daily sunlight exposure for 1 or 2 weeks on the same day of the week. Findings from our study are similar to Shapiro’s report that sunlight or UV exposure has a negative correlation with mortality in dialysis patients, but differed in that we also observed the effect of short-term sunlight exposure on mortality.
The principal response to sunlight exposure is elevation of vitamin D status. It is reported that the mean serum concentration of 25(OH)D was about 115 mmol/L in two traditionally living populations in East Africa with lifelong exposure to tropical sunlight23. In a cross-sectional study of patients with CKD inhabiting a subtropical region of Brazil where sunlight exposure is elevated throughout the year, the serum 25(OH)D levels were higher than those found in patients residing in higher-latitude regions24. Moreover, narrow-band UVB exposure is known to increase serum 25(OH)D and 1,25(OH)2D in dialysis patients25.
It is well known that vitamin D deficiency, which is caused by limited sunlight exposure, is highly prevalent in patients with ESRD and is associated with various adverse outcomes including death14,26,27. Cardiovascular disease is one of the most common cause of death in ESRD, which is in concordance with our data, while moderate to severe vitamin D deficiency is a risk factor for developing cardiovascular disease. Previous studies showed that a lower serum 25(OH)D concentration is associated with an increased risk of cardiovascular events, in not only peritoneal dialysis28, but also hemodialysis patients26.
Infectious disease remains the leading cause of death in ESRD patients. Increasing evidence demonstrates that vitamin D has immune-modulatory effects on both the innate and adaptive immune systems29. 1,25(OH)2D is known to suppress adaptive immunity by inhibiting the proliferation and differentiation of CD4 cells into T helper cell type 1 (Th1) and Th17 cells and by promoting the production of Th2 and regulatory T cells30. Macrophages activated by toll-like receptors (TLRs) promote the production of 1,25(OH)2D, which then induces the expression of the antimicrobial peptides, cathelicidin31. The association of vitamin D deficiency and infectious events, such as septic shock32, respiratory infection33, and influenza34, is supported by a large number of epidemiologic studies.
As reported previously, several observational studies have shown that vitamin D supplementation may be associated with a reduced risk for cardiovascular and all-cause death among ESRD patients35–38. Contrary to this, a recent review article with 17 randomized controlled trials (RCTs) involving 1,819 patients demonstrated that vitamin D treatment did not affect the risk of cardiovascular or all-cause death20. These differences between observational studies and RCTs might be explained by selection bias, because patients taking vitamin D supplements are generally healthier than untreated patients. Therefore, large-scale RCTs are needed to assess the efficacy of vitamin D treatment for ESRD patients.
Although there has been substantial body of evidence that sunlight exposure, through synthesis of vitamin D, might have a beneficial effects including survival gain in many kinds of diseases, controversies still exists with “vitamin D hypothesis”. In a large prospective study of Swedish women, natural sunlight exposure was inversely associated with all-cause mortality and cardiovascular mortality, whereas artificial UV exposure using indoor tanning devices4. Furthermore, in a recent study, overall cancer mortality was not associated with baseline 25(OH)D status in the general population of NHANES III cohort39. It is important to note that vitamin D might not be the only pathway whereby sunlight exposure, especially natural source of sunlight might have beneficial effects on human health. It has been reported that sunlight induces Nitric Oxide (NO) synthesis in the skin and it is released the systemic vasculature which acts as a vasodilator and lowers blood pressure40. Furthermore, UV radiation-induced NO showed suppressive effect in developing obesity and metabolic syndrome in a mouse model which of the two mechanism were independent of vitamin D supplementation41.
The strengths of our study include the examination of a large, contemporary Korean dialysis population with long-term follow-up of 14 years, and the comprehensive availability of clinical data allowing for adjustment of multiple weather and air-pollution confounders. Moreover, this study employed a case-crossover design, which is particularly powerful for matching potential confounding factors individually and avoiding the selection bias, healthy-day bias, and healthy-volunteer bias that are limitations of ecological epidemiologic studies42. Although sunlight exposure has seasonality, such as shorter sunlight hours during the winter period (December, January, and February) compared to other seasons, we did not need to adjust for the season or holiday as cofounders because we made the controls in the same day of the week within two weeks in the case-crossover design. However, several limitations of our study bear mention. First, we were unable to measure the serum vitamin D concentrations in our study population. Second, given that the Korean National Institute of Environmental Research measures the sunlight exposure time only in major cities, our study cohort may not be representative of dialysis patients living in outlying or rural regions.
For the selection of control days in the case-crossover design, several selection schemes have been used and compared. A recent study showed that the bi-directional and three different time-stratified (day of the week) methods yielded no difference in results in the assessment of the association between air pollution exposure and acute myocardial infarction43. This experimental comparison of the control selection schemes in the case-crossover study design could support the fact that our use of the bi-directional method in selecting control days would likely produce unbiased estimates.
In conclusion, in the Korean ESRD population from 2001 to 2014, sunlight exposure was inversely correlated with increased risk of all-cause mortality. Further studies are needed to evaluate the effect of extended sunlight exposure and vitamin D supplement on survival.
Methods
Study population
The Korean Society of Nephrology (KSN) end-stage renal disease (ESRD) registry was established in 1985. All KSN members contribute voluntarily to the ‘Insan Prof. Byung-Suk Min Memorial ESRD Patient Registry44,45. The KSN ESRD Registry Committee has been collecting data on dialysis through an online internet program that was opened in 2001 and revised in 2013. Informed written consent was obtained from all the patients who were enrolled in the registry. The Korean ESRD registry covers about two-thirds of all dialysis patients in Korea because the enrollment is voluntarily. The present study enrolled patients who died from 2001 through 2014, according to KSN data. The study protocol complies with the Declaration of Helsinki and received full approval from the institutional review board at the Seoul National University Boramae Medical Center (20180410/10-2018-50/051). Clinical information, including the date of death, age, sex, body mass index (BMI), comorbidities (hypertension and diabetes mellitus) and type of kidney dialysis (peritoneal dialysis and hemodialysis), the causes of ESRD, and the cause of death were obtained from the registry.
Weather and air pollution data
The seven metropolitan cities in Korea were selected as our study area, and 2001 to 2014 was chosen as the study period. We obtained hourly data on ambient temperature, relative humidity, and hours of sunlight from the Korea Meteorological Administration. To adjust for potential confounding factors, we also obtained the daily (24-hour) concentrations of nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), carbon monoxide (CO), and particulate matter less than 10 μm in diameter (PM10) measured at 89 monitoring sites located within the seven cities. These were provided by the Korean National Institute of Environmental Research. As short-term confounder measures, we used the daily max concentration of ozone (O3) and the daily mean concentration for the other air pollutants (PM10, NO2, SO2, O3 and CO) within same city.
Study design
We applied a bi-directional case-crossover design (Fig. 4) to estimate the short-term association between sunlight and ESRD death. A case-crossover design, which was described by Maclure for evaluating a transient acute effect, is a variant of the case-control design and produces sufficient statistical power with small cases46. It has recently been used as an alternative to time series, and an extension of this approach has also been used for observational studies in areas such as clinico-epidemiology, impairment epidemiology, pharmaco-epidemiology and environmental epidemiology. The case period was defined as the day ESRD led to the death of the patient. We performed the bi-directional case-crossover study as a two-to-one matched case-control study that sampled control periods as the exposure, seven days before and seven days after the date of event (ESRD death); resulting in four control days per case47 (Fig. 1). For example, if ESRD death occurred on April 13, the four control days were selected as follows: March 30, April 6, April 20, and April 27. In the case-crossover design, the time-invariant individual characteristics, such as sex and genetic predisposition, and the slowly varying characteristics, such as age, marital status, employment status, and seasonality, can be controlled48. In our study, the daily sunlight exposure hours during the case and control periods were compared.
Figure 4.
Bi-directional sampling of control time in the case-crossover study. A bi-directional case-crossover study was conducted, which sampled control periods as the exposure seven days before and seven days after the event day (the day of mortality), producing four control days per case.
Statistical analysis
The conditional logistic regression analytic method is an extension of the logistic regression method, which allows one to take into account the stratification and matching that is usually utilized in observational studies. We investigated the association between sunlight and ESRD death risk by conditional logistic regression performed via the Cox proportional hazard function. The comparisons within subject were made between the case and control periods. The odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of ESRD death on sunlight exposure were calculated by conditional logistic regression analysis. We used ambient temperature and humidity as potential confounders, and five other air pollutants (PM10, NO2, SO2, O3 and CO) were also used to have five different models as a potential effect modification. The conditional logistic model can be simplified by the following formula after matching for the time-invariant individual risk factors;
where β1, β2, β3 and β4 represent the vectors whose components denote the log odds of mortality associated with sunlight, ambient temperature, humidity, and air pollutants separately as confounders. Using the formula above, represents the case status (case = 1, control = 0) of the jth observation of ith strata where αi is constant term of ith strata. The ambient temperature and humidity are stationary confounder factors and five air pollutants (PM10, NO2, SO2, O3 and CO) were applied in each model.
For subgroup analysis, we defined higher risk patients in the ESRD cohort as those with diabetes, older adults (over 75 years), or those undergoing peritoneal dialysis or hemodialysis, and we compared them to the lower-risk group. The statistical analysis was performed using the statistical programing language R, version 3.4.0.
Supplementary information
Acknowledgements
This research was supported by the Global Research Lab (#K210040000001-10A0500-00710; H Kim) through the National Research Foundation of Korea funded by the Ministry of Science and ICT (Information and Communication Technologies). We express our gratitude to the ESRD registry Committee of the Korean Society of Nephrology.
Author Contributions
Y.S.K., C.S.L., J.P.L. and H.K. designed the study; U.A.Y. and Y.C.K. carried out experiments; U.A.Y., Y.C.K. and H.L. analyzed the data; U.A.Y., S.K. and J.N.A. created the figures; U.A.Y., Y.C.K. and D.K.K. drafted and revised the paper; all authors approved the final version of the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Una Amelia Yoon and Yong Chul Kim contributed equally.
Contributor Information
Jung Pyo Lee, Email: nephrolee@gmail.com.
Ho Kim, Email: hokim@snu.ac.kr.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38522-w.
References
- 1.IARC monographs on the evaluation of carcinogenic risks to humans. Solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum55, 1–316 (1992). [PMC free article] [PubMed]
- 2.Lindqvist PG, et al. Avoidance of sun exposure as a risk factor for major causes of death: a competing risk analysis of the Melanoma in Southern Sweden cohort. J Intern Med. 2016;280:375–387. doi: 10.1111/joim.12496. [DOI] [PubMed] [Google Scholar]
- 3.Lindqvist PG, et al. Avoidance of sun exposure is a risk factor for all-cause mortality: results from the Melanoma in Southern Sweden cohort. J Intern Med. 2014;276:77–86. doi: 10.1111/joim.12251. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, et al. Ultraviolet exposure and mortality among women in Sweden. Cancer Epidemiol Biomarkers Prev. 2011;20:683–69. doi: 10.1158/1055-9965.EPI-10-0982. [DOI] [PubMed] [Google Scholar]
- 5.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siadat ZD, et al. Association of vitamin D deficiency and coronary artery disease with cardiovascular risk factors. J Res Med Sci. 2012;17:1052–1055. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromso study. Eur J Endocrinol. 2010;162:935–942. doi: 10.1530/EJE-09-1041. [DOI] [PubMed] [Google Scholar]
- 12.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayedi, A., Soltani, S. & Shab-Bidar, S. Vitamin D status and all-cause mortality in patients with chronic kidney disease: A systematic review and dose-response meta-analysis. J Clin Endocrinol Metab (2017). [DOI] [PubMed]
- 14.Pilz S, et al. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603–3609. doi: 10.1093/ndt/gfr076. [DOI] [PubMed] [Google Scholar]
- 15.Pitts TO, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67:876–881. doi: 10.1210/jcem-67-5-876. [DOI] [PubMed] [Google Scholar]
- 16.de Boer IH, et al. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.London GM, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 18.Ravani P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 19.Mailliez, S. et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int74, 389; author reply 389 (2008). [DOI] [PubMed]
- 20.Lu RJ, et al. Effects of vitamin D or its analogues on the mortality of patients with chronic kidney disease: an updated systematic review and meta-analysis. Eur J Clin Nutr. 2017;71:683–693. doi: 10.1038/ejcn.2017.59. [DOI] [PubMed] [Google Scholar]
- 21.Kandula P, et al. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro BB, et al. The relationship between ultraviolet light exposure and mortality in dialysis patients. Am J Nephrol. 2014;40:224–232. doi: 10.1159/000367903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012;108:1557–1561. doi: 10.1017/S0007114511007161. [DOI] [PubMed] [Google Scholar]
- 24.Cuppari L, Carvalho AB, Draibe SA. Vitamin D status of chronic kidney disease patients living in a sunny country. J Ren Nutr. 2008;18:408–414. doi: 10.1053/j.jrn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Ala-Houhala MJ, et al. Narrow-band ultraviolet B exposure increases serum vitamin D levels in haemodialysis patients. Nephrol Dial Transplant. 2012;27:2435–2440. doi: 10.1093/ndt/gfr700. [DOI] [PubMed] [Google Scholar]
- 26.Wolf M, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 27.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Wang AY, et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87:1631–1638. doi: 10.1093/ajcn/87.6.1631. [DOI] [PubMed] [Google Scholar]
- 29.Sterling KA, Eftekhari P, Girndt M, Kimmel PL, Raj DS. The immunoregulatory function of vitamin D: implications in chronic kidney disease. Nat Rev Nephrol. 2012;8:403–412. doi: 10.1038/nrneph.2012.93. [DOI] [PubMed] [Google Scholar]
- 30.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 32.Danai PA, Sinha S, Moss M, Haber MJ, Martin GS. Seasonal variation in the epidemiology of sepsis. Crit Care Med. 2007;35:410–415. doi: 10.1097/01.CCM.0000253405.17038.43. [DOI] [PubMed] [Google Scholar]
- 33.Grant WB. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27:853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 34.Cannell JJ, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoji T, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 36.Teng M, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 37.Naves-Diaz M, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–1078. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]
- 38.Cozzolino M, et al. VDRA therapy is associated with improved survival in dialysis patients with serum intact PTH</=150 pg/mL: results of the Italian FARO Survey. Nephrol Dial Transplant. 2012;27:3588–3594. doi: 10.1093/ndt/gfs108. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988–2006) Cancer Res. 2010;70:8587–8597. doi: 10.1158/0008-5472.CAN-10-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weller RB. Sunlight Has Cardiovascular Benefits Independently of Vitamin D. Blood Purif. 2016;41:130–134. doi: 10.1159/000441266. [DOI] [PubMed] [Google Scholar]
- 41.Geldenhuys S, et al. Ultraviolet radiation suppresses obesity and symptoms of metabolic syndrome independently of vitamin D in mice fed a high-fat diet. Diabetes. 2014;63:3759–3769. doi: 10.2337/db13-1675. [DOI] [PubMed] [Google Scholar]
- 42.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 43.Collart P, Coppieters Y, Mercier G, Massamba Kubuta V, Leveque A. Comparison of four case-crossover study designs to analyze the association between air pollution exposure and acute myocardial infarction. Int J Environ Health Res. 2015;25:601–613. doi: 10.1080/09603123.2014.1003037. [DOI] [PubMed] [Google Scholar]
- 44.Jin DC. Dialysis registries in the world: Korean Dialysis Registry. Kidney Int Suppl (2011) 2015;5:8–11. doi: 10.1038/kisup.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin DC. Current characteristics of dialysis therapy in Korea: 2016 registry data focusing on diabetic patients. Kidney Res Clin Pract. 2018;37:20–29. doi: 10.23876/j.krcp.2018.37.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 47.Fung KY, Krewski D, Chen Y, Burnett R, Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003;32:1064–1070. doi: 10.1093/ije/dyg246. [DOI] [PubMed] [Google Scholar]
- 48.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.