Introduction
Key Teaching Points.
-
•
Some arrhythmogenic right ventricular dysplasia/cardiomyopathy might present as sustained ventricular tachycardia (VT) without overt structural heart disease.
-
•
Recurrent VTs suggest an epicardial disease that can be further suspected by biopsies.
-
•
Normal plakoglobin but reduced desmoplakin immunofluorescence staining signals on cardiac biopsies may be indicative of arrhythmogenic cardiomyopathy.
Arrhythmogenic cardiomyopathy (ACM), formerly known as arrhythmogenic right ventricular dysplasia, is a primary myocardial disorder characterized by ventricular arrhythmias and sudden cardiac death (SCD).1 Myocardial desmosomes are cell–cell junctions that reside within the intercalated disc. They consist of members of the cadherin family (desmocollin-2, desmoglein-2), which span the membrane mechanically coupling adjacent cells, and members of the plakin and armadillo families: plakoglobin (PKG), plakophilin-2 (PKP2), and desmoplakin (DSP1), which connect the cadherin complexes to the intermediate cytoskeleton filaments. A reduction in the PKG immunoreactive signal has been shown at intercalated discs of cardiomyocytes in ACM regardless of the underlying pathogenic mutation.1, 2 Herein we report 2 unusual cases of ACM with digenic heterozygosity showing normal immunoreactive PKG distribution but reduced DSP1 signal at myocardial cell–cell junctions without known pathogenicity in the DSP gene.
Case report
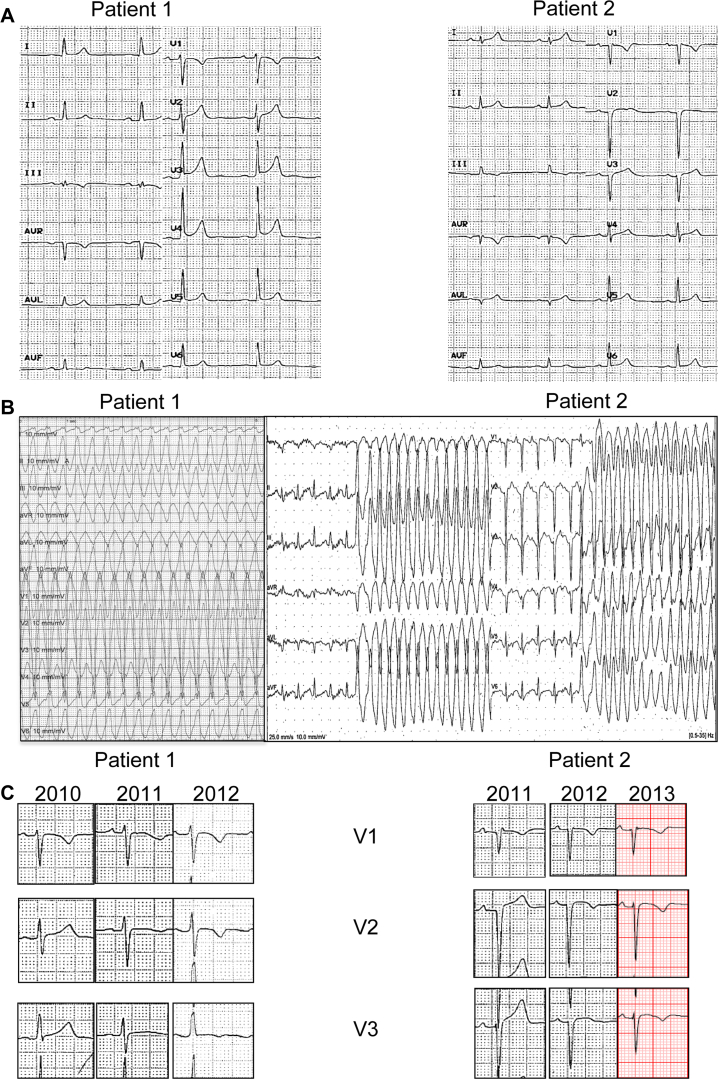
Two unrelated male patients, aged 28 and 31 years old at time of first symptoms, complained of syncope during strenuous exercise. They had no known comorbidities and no family history of SCD. Both practiced high-intensity sports. Figure 1A shows the resting electrocardiograms of both cases. Despite some minor anomalies, there was no sign suggestive of ACM. The echocardiogram, coronary angiogram, and laboratory work-up were normal. Cardiac magnetic resonance imaging (CMR) in patient 1 showed a limited (1-segment) nontransmural area of late gadolinium enhancement (LGE) in the inferolateral left ventricle (LV) wall and a local hypokinesia of the right ventricular outflow tract (RVOT) on the cine short axis as well as a discrete right ventricle (RV) dilation considered borderline in a sportive patient (Supplemental Figure 1A–C). The CMR in patient 2 was normal (Supplemental Figure 1E–F). A metabolic FDG18 PET scan showed no focal activity. Signal-averaged electrocardiogram as well as higher precordial or specific Fontaine leads were not obtained at time of referral, which might have revealed epsilon waves suggestive of a subclinical ACM.
Figure 1.
A: Baseline 12-lead electrocardiogram showing normal sinus rhythm with 1.5 mm J-point elevation in V3–V5 in patient 1 and normal sinus rhythm with late R-wave progression in precordial leads and incomplete right bundle branch block in V1 not fulfilling the criterion for an epsilon wave in patient 2. B: Left: Sustained monomorphic ventricular tachycardia (heart rate 214 beats/min) induced during an electrophysiological study in patient 1. Right: Nonsustained ventricular tachycardia (heart rate 230 beats/min) occurring during an exercise stress test in patient 2. C: Progressive occurrence of negative T waves up to V3 over a 3-year follow-up.
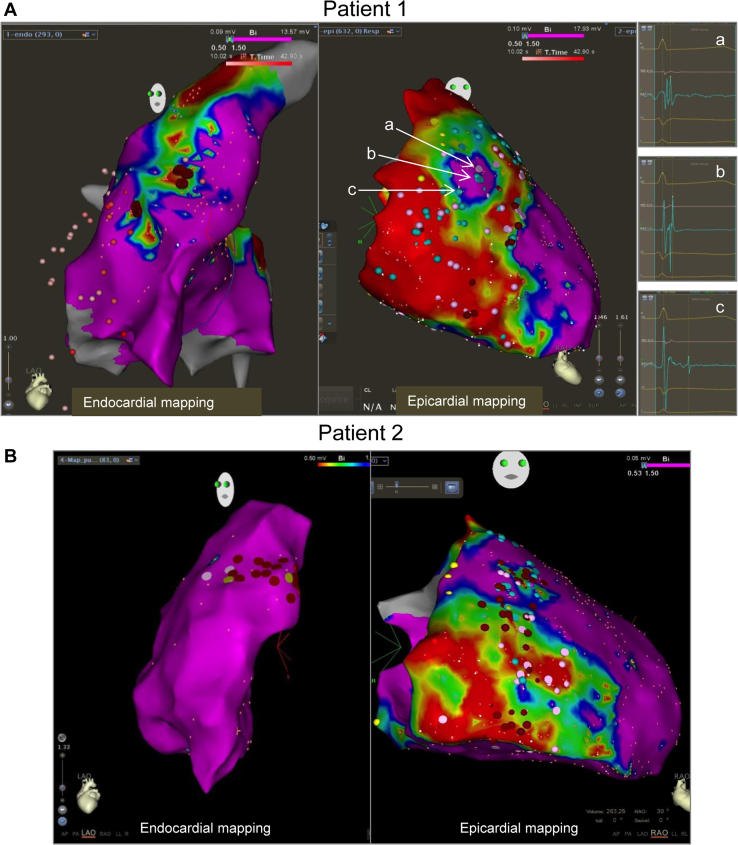
An electrophysiological study in patient 1 and a treadmill test in patient 2 revealed a monomorphic ventricular tachycardia (mVT) originating from the RVOT, as suggested by the inferior axis and the late transition occurring in lead V4 (Figure 1B). An ablation was attempted in both cases using a 3-dimensional electroanatomic system (Carto 3, Biosense Webster, Inc, Diamond Bar, CA). A limited area of reduced bipolar voltage suggestive of a scar was observed on the anterolateral RVOT (Figure 2, endocardial mapping sections). Importantly, these reduced potentials preceded the ventricular tachycardia (VT) by 30 ms and were successfully ablated with noninducibility at the end of the procedures.
Figure 2.
A: Left: Endocardial bipolar map based on a 3-dimensional electroanatomic system in patient 1. Note the presence of a limited area of low-voltage potentials in the anterolateral right ventricular outflow tract (RVOT). Right: Epicardial bipolar map showing an extended area of low-voltage potentials overlying most of the right ventricular (RV) epicardium. Examples of early (a), mid (b), and late (c) late abnormal ventricular activations are shown on the right-hand side. B: Left: Endocardial bipolar map in patient 2. A limited number of fragmented potentials in the anterolateral RVOT are shown as pink dots. Right: Epicardial bipolar map showing an extended area of low-voltage potentials overlying most of the RV epicardium.
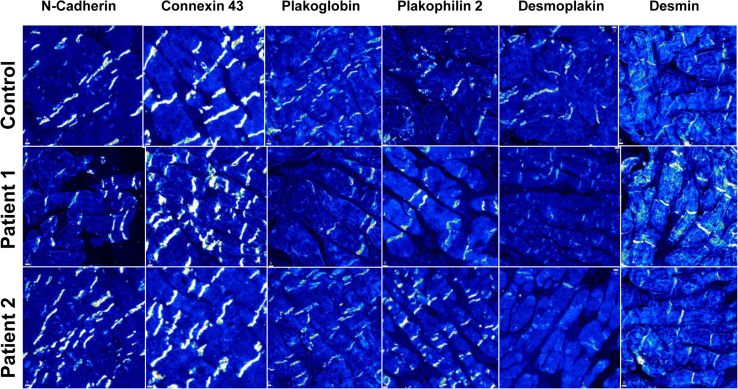
The initial diagnosis was idiopathic RVOT VT, and both patients were initiated on beta-blockers. After 2 and 9 months, respectively, both patients were referred again to our center for syncope during exercise. Symptomatic VT with a different morphology than the index arrhythmia in case 1, and VT of different cycle lengths in case 2 (Supplemental Figure 2), prompted a second endocardial ablation, deemed successful in case 1 and unsuccessful in case 2 because of inducibility at the end of the procedure. Amiodarone was then introduced. In view of early recurrence with different VT morphologies or cycle lengths than the index arrhythmia, there was increasing concern of an incipient ACM.3 An implantable cardioverter-defibrillator (ICD) was implanted and an epicardial ablation was programmed. Figure 2A and B shows an extended area overlying most of the RV epicardium (900 points) made of low-voltage electrograms depicted as a shade of colors from red (dense scar, <0.5 mV) to blue (<1.5 mV), and late abnormal ventricular activations (LAVA) and fragmented potentials (blue and pink dots, respectively). Ablation primarily targeted the “early” LAVA (Figure 2A), then whether mid and late LAVAs were still present was checked immediately, with noninducibility at the end of the procedure. Amiodarone was discontinued immediately after the epicardial VT ablation and beta-blocker treatment resumed. Septal RV biopsies, taken at the time of the second endocardial ablation, revealed no morphologic abnormality. Immunofluorescence staining (IF) of desmosomal proteins was performed on the biopsies and compared to a control sample. The following proteins were tested: PKG, PKP2, DSP1, desmin (DES), connexin 43, and N-cadherin. IF revealed in both cases a severely reduced signal for DSP1 at cell–cell junctions but normal distribution of other proteins (Figure 3). Interestingly, negative T waves occurred in leads V2 and V3 over a 3-year follow-up period before any epicardial procedure (Figure 1C).
Figure 3.
Immunofluorescence staining of intercalated disc proteins in a control subject and in patients 1 and 2.
Repeat imaging studies, performed at 10 and 12 months after symptom onset, respectively (before ICD implant in patient 1 and after ICD implant in patient 2), provided a major ACM criterion for patient 1 (segmental akinesia to dyskinesia with RV dilation, no change in the LV at CMR) and a minor criterion in patient 2 (RV free wall akinesia with normal function at echocardiogram). In the presence of 3 major criteria in patient 1 (imaging, sustained mVT, and repolarization abnormalities) and 1 major (repolarization abnormalities) + 2 minor criteria in patient 2 (sustained mVT and imaging), the diagnosis of ACM was established.4 Patients were advised to refrain from intense physical activity according to current guidelines.5 Over a 3- and 5-year follow-up no VT recurrence occurred after epicardial ablation.
Genetic analysis was performed in both cases (details in Supplemental Data). In patient 1, mutational analysis revealed 3 heterozygous variants: 1 likely pathogenic alteration in the DES gene: NM_001927.3:c.638C>T (p.Ala213Val) and 1 likely pathogenic variant in the CTNNA3 gene: NM_001127384.1:c.1603C>T (p.Arg535Cys); and an additional variant of unknown significance identified in the DSG2 gene: NM_001943.3:c.877A>G (p.Ile293Val).
In patient 2, mutational analysis also revealed 3 heterozygous variants: a pathogenic mutation in the PKP2 gene: NM_004572.3:c.2197_2202delCACACCinsG (p.His733AlafsTer8); and 2 variants of unknown significance in the DSG2 gene: NM_001943.3:c.2759T>G (p.Val920Gly) and DSP gene: NM_004415.2:c.6208G>A (p.Asp2070Asn).
Discussion
The diagnosis of ACM remains challenging owing to the age-related progression, vast phenotypic variation, and reduced genetic penetrance.1, 6 We report here 2 young patients presenting with arrhythmias displaying the typical morphology of RVOT VT. VT occurred years before any structural disease was declared. Importantly, none of the initial VTs displayed the typical features of a right epicardial origin, such as Q waves in II, III, aVF or Q waves in I.7 Cardiac sarcoidosis was reasonably excluded by multimodality imaging.1, 8
Importantly, IF of myocardial biopsies showed reduced DSP1 signal at junctional sites. Reduced DSP1 signal at intercalated discs has been recently reported in the majority of cases of dilated cardiomyopathy (DCM).9 In our cases, however, the normal RV and LV ejection fraction at the time of the biopsies and the lack of a clear DCM phenotype after several years of follow-up reasonably exclude DCM as the underlying causative disease. Interestingly, RV imaging abnormalities and T-wave inversion occurred more than 2 years after VT onset, confirming that some ACM might present with arrhythmic events in the absence of any structural heart disease.2, 6
Although not yet part of the current guidelines for the diagnosis of ACM,4 IF staining appears to be a promising tool giving insights into tissue characterization up to the desmosomal subunits.9 Intercalated disc anomalies occur in many diseases, including severe heart failure, but they are always accompanied by histologic abnormalities.6 The occurrence of an abnormal intercalated disc IF signal in histologically unaffected regions of the myocardium appears typical of ACM.1, 2, 6 IF of endomyocardial biopsies can reliably identify ACM even in the absence of genetic mutations.9, 10 The main intercalated disc culprits included PKG9 and connexin 43.10 While both were highly sensitive in the setting of ACM,9, 10 only reduced PKG signal was highly specific for the diagnosis of ACM.9 Our patients showed a selective reduction of desmosomal DSP1 expression, while the distribution of PKG appeared normal. IF bears some limitations that were addressed here to limit the risk of false-negatives. All biopsies were fixed within minutes after harvesting and compared to a control to ensure that the reduced DSP IF signal stemmed from the disease and not from the technique. DSP1 is the dominant isoform in cardiac tissue.6 Traditionally, loss of DSP1 was described by Alcalai and colleagues11 and Uzumcu and colleagues12 as part of a cardiocutaneus syndrome. In 2005, Norman and colleagues13 identified an ACM mutation in DSP1 with subsequent protein truncation,2, 6 which mainly involved the LV. To our knowledge, we report here the first cases of isolated reduced DSP1 signal that mainly affects the RV without associated skin and hair disease.
The discovery of causative mutations in desmosomal genes has shed light into ACM pathogenesis.2 Nevertheless, much less is known about how the mutant proteins actually cause the disease. A mutation in a single desmosomal protein can affect the localization of other proteins not genetically mutated.2 They appear to play a role in cell death through abnormal cell–cell adhesion and as signaling proteins. Interestingly, redistribution of PKG and DSP1 are involved in abnormal transcriptional activity and protein trafficking and in increased expression of fibrogenic and adipogenic genes.2 Our first case exhibited a mutation in the DES and catenin genes and the second case a mutation in the PKP2 gene, all encoding for proteins tightly connected to DSP1. Hence, it is possible that these abnormal proteins, although correctly expressed at the intercalated disc, might have limited the trafficking of DSP1. Another finding is the presence of multiple heterozygous gene variants, which carry a worse prognosis in terms of survival and disease progression.1, 14
Antiarrhythmic drugs are prescribed in ACM to reduce the arrhythmic burden, but they have no impact on preventing SCD.5 Catheter ablation has become an option for refractory VT.15 Substrate-based ablation has improved the procedural outcome by targeting multiple epicardial circuits typically seen in ACM.5, 15 Further studies are warranted to determine whether desmosome imaging techniques could predict the presence of an epicardial substrate suitable for ablation in suspected cases of ACM.
Conclusion
We report 2 ACM cases with RVOT VTs displaying an isolated reduced DSP1 signal at intercalated discs from histologically normal myocardial tissue. Although the RV might look unremarkable at histologic examination, the ability to sustain recurrent VT suggests a complex epicardial substrate that can be further suspected by IF studies of endomyocardial biopsies.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2018.06.013.
Appendix. Supplementary data
References
- 1.Asimaki A., Kleber A.G., Saffitz J.E. Pathogenesis of arrhythmogenic cardiomyopathy. Can J Cardiol. 2015;31:1313–1324. doi: 10.1016/j.cjca.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asimaki A., Saffitz J.E. Remodeling of cell-cell junctions in arrhythmogenic cardiomyopathy. Cell Commun Adhes. 2014;21:13–23. doi: 10.3109/15419061.2013.876016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niroomand F., Carbucicchio C., Tondo C., Riva S., Fassini G., Apostolo A., Trevisi N., Della Bella P. Electrophysiological characteristics and outcome in patients with idiopathic right ventricular arrhythmia compared with arrhythmogenic right ventricular dysplasia. Heart. 2002;87:41–47. doi: 10.1136/heart.87.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P., Fontaine G. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur Heart J. 2010;19:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrado D., Wichter T., Link M.S., Hauer R., Marchlinski F., Anastasakis A., Bauce B., Basso C., Brunckhorst C., Tsatsopoulou A., Tandri H. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. doi: 10.1093/eurheartj/ehv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmar M., McKenna W.J. The cardiac desmosome and arrhythmogenic cardiomyopathies from gene to disease. Circ Res. 2010;107:700–714. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- 7.Bazan V., Bala R., Garcia F.C., Sussman J.S., Gerstenfeld E.P., Dixit S., Callans D.J., Zado E., Marchlinski F.E. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3:1132–1139. doi: 10.1016/j.hrthm.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Ott P., Marcus F.I., Sobonya R.E., Morady F., Knight B.P., Fuenzalida C.E. Cardiac sarcoidosis masquerading as right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26:1498–1503. doi: 10.1046/j.1460-9592.2003.t01-1-00217.x. [DOI] [PubMed] [Google Scholar]
- 9.Asimaki A., Tandri H., Huang H., Halushka M.K., Gautam S., Basso C., Thiene G., Tsatsopoulou A., Protonotarios N., McKenna W.J., Calkins H. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 10.Noorman M., Hakim S., Cox M.G., van Rijen H.V., van der Heyden M.A., de Jonge N., van der Smagt J.J., Vink A., de Weger R.A., Varro A., de Bakker J.M. Connexin43 remodeling in septal biopsies as a sensitive marker for diagnosing arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2009;120:S905. [Google Scholar]
- 11.Alcalai R., Metzger S., Rosenheck S., Meiner V., Chajek-Shaul T. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol. 2003;42:319–327. doi: 10.1016/s0735-1097(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 12.Uzumcu A., Norgett E.E., Dindar A., Uyguner O., Nisli K., Kayserili H., Sahin S.E., Dupont E., Severs N.J., Leigh I.M., Yuksel-Apak M. Loss of desmoplakin isoform I causes early onset cardiomyopathy and heart failure in a Naxos-like syndrome. J Med Genet. 2006;43:e5. doi: 10.1136/jmg.2005.032904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norman M., Simpson M., Mogensen J., Shaw A., Hughes S., Syrris P., Sen-Chowdhry S., Rowland E., Crosby A., McKenna W.J. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation. 2005;112:636–642. doi: 10.1161/CIRCULATIONAHA.104.532234. [DOI] [PubMed] [Google Scholar]
- 14.Fressart V., Duthoit G., Donal E., Probst V., Deharo J.C., Chevalier P., Klug D., Dubourg O., Delacretaz E., Cosnay P., Scanu P. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. Europace. 2010;12:861–868. doi: 10.1093/europace/euq104. [DOI] [PubMed] [Google Scholar]
- 15.Garcia F.C., Bazan V., Zado E.S., Ren J.F., Marchlinski F.E. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–375. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.