Key Teaching Points.
-
•
Massive air embolism, especially when multifocal, should raise suspicion for atrioesophageal fistula (AEF) at the first medical contact. History of recent ablation for atrial fibrillation should be actively sought.
-
•
AEF might occur even after procedures in which power applied to the posterior left atrial wall is limited, in accordance with current recommendations.
-
•
Left atrial thrombosis at or close to the presumed location of the AEF could also occur.
Introduction
Catheter ablation with radiofrequency (RF) energy or cryoenergy is a well-established therapeutic method for symptomatic atrial fibrillation (AF), especially in its paroxysmal form.1, 2 The number of ablations of AF worldwide is growing rapidly, with more than 50,000 ablations performed in the United States on a yearly basis.3 The procedure is associated with a number of potential complications, occurring in 4.5% of cases.4 Atrioesophageal fistula (AEF) is a very rare but devastating complication of catheter ablation of AF. The reported incidence of this complication reaches up to 0.11% among patients ablated for AF.5 Owing to the very low total number of cases, exact preventive measures have not been systematically studied. Among the most important preventive measures applied in routine practice are esophageal temperature monitoring, limiting the power of delivered energy at the posterior left atrium (LA), and treatment with proton-pump inhibitors following catheter ablation.2
We describe a case of a patient developing an AEF resulting in massive multifocal air embolism following a routine pulmonary vein isolation (PVI) for paroxysmal AF.
Case report
A 62-year-old man was referred to our center for catheter ablation because of very frequent, symptomatic episodes of AF. He had previously undergone cavotricuspid isthmus ablation for typical atrial flutter. Following chest computed tomography (CT) for evaluation of LA anatomy, the patient underwent RF PVI. The procedure was carried out under general anesthesia. Following double transseptal puncture, a circular mapping catheter (Reflexion Spiral, Abbott, Minneapolis, MN) and a 4 mm irrigated-tip catheter (Flexability, Abbott, Minneapolis, MN) were introduced into the LA. A 3-dimensional anatomical map of the LA was created with the EnSite Velocity system (Abbott, Minneapolis, MN). Power delivered to the LA was 20 watts on the posterior wall and 30 watts at all other locations. Flow rate was set to 17 mL/min. Lesion duration was not limited and catheter dragging was applied constantly during RF power delivery, not allowing it to remain at one spot for more than 20 seconds. Esophageal temperature monitoring was not used. Wide-area circumferential ablation of left and right pulmonary venous antra was carried out, resulting in entrance and exit block to all 4 pulmonary veins. The procedure was carried out uneventfully. Total RF ablation time was 1962 seconds. Procedure time was 162 minutes. The patient was discharged on the following day and was prescribed propafenone, proton-pump inhibitor, and non-vitamin K antagonist oral anticoagulant as per protocol at our center.
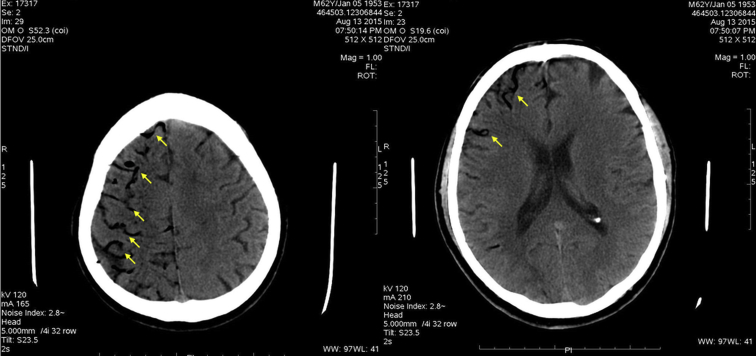
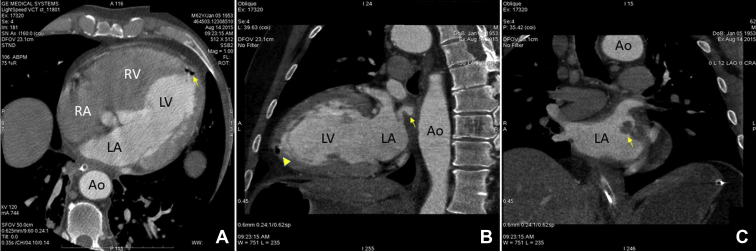
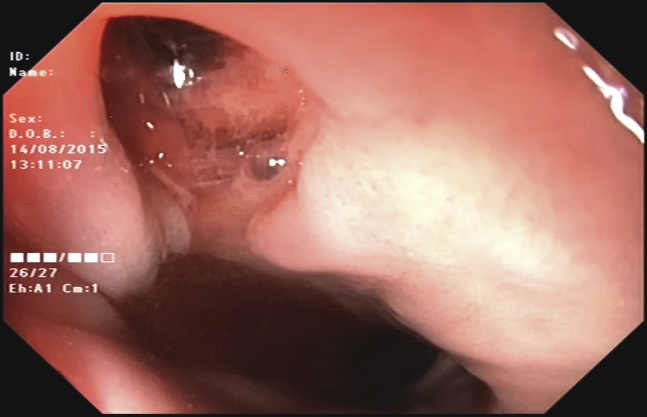
In the evening hours of day 21 following the procedure, the patient complained of sudden onset of malaise, dyspnea, and fever up to 38.9°C (102.02°F). He also reported vomiting, severe chest pain of sudden onset, and left arm numbness. Thirty minutes following initial symptom onset, the patient lost consciousness. An emergency unit was called in and the patient was taken to the hospital. At the emergency room he presented in deep coma (Glasgow coma scale, 3 points). The patient was intubated and laryngoscopy during intubation revealed severe edema of the oropharynx. Electrocardiogram at presentation demonstrated ST-segment elevation in leads I and aVL. Troponin was found to be elevated and ST-elevation myocardial infarction (STEMI) was diagnosed. No primary percutaneous coronary intervention strategy was undertaken because of the very poor condition of the patient. Owing to fever and neurological signs, a concomitant neuroinfection was suspected but was excluded following the results of lumbar puncture. As a part of the regular work-up of comatose conditions, a noncontrast head CT scan was performed shortly after presentation to the emergency room. It showed massive air embolism to the brain with clearly visible gas collections in the cerebral arteries (Figure 1). The patient was later admitted to the intensive care unit with an unclear diagnosis because the team on call was not aware of the potential complications of AF ablation. The following morning the patient had complete resolution of the electrocardiogram findings and deep coma still persisted. Because of cerebral air embolism and STEMI most likely due to coronary air embolism, AEF following catheter ablation of AF was strongly suspected and a cardiac CT scan was performed. It revealed an air collection in the left ventricular apex (Figure 2A). A leak of contrast to the periesophageal tissues was also clearly visible (Figure 2B). There was also a massive thrombosis of the LA attached to the posterior wall, close to the antrum of the left inferior pulmonary vein (Figure 2C). The patient was referred to a gastroenterologist, who performed esophagoscopy using carbon dioxide for insufflation. It revealed a deep lesion at the anterior wall of the esophagus forming a fistula to the adjacent LA, with active bleeding into the esophagus (Figure 3, Supplemental Video 1). Following all these findings, AEF resulting from previous catheter ablation of AF was diagnosed. The patient was also referred to a thoracic surgeon for further management, but an operation was deemed high risk because of the critical condition of the patient. Following diagnosis, the patient was treated conservatively. During the following 24 hours deep coma persisted, and the patient developed hemodynamic instability and succumbed to severe mediastinitis.
Figure 1.
Native head computed tomography scan demonstrating massive air collections in the cerebral arteries (arrows).
Figure 2.
Cardiac computed tomography scan. A: Horizontal plane section demonstrating air collection at the left ventricular apex (arrow). B: Sagittal plane section demonstrating contrast leakage from the left atrium to the periesophageal tissues (arrow). The air collection at the left ventricular apex is visible in this section as well (arrowhead). C: Frontal plane section at the level of left atrium demonstrating intracavitary thrombosis attached to the posterior wall close to the antrum of the left inferior pulmonary vein. Ao = aorta; LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
Figure 3.
Upper endoscopy demonstrating a deep ulcerative lesion at the anterior esophageal wall consistent with atrioesophageal fistula.
Discussion
Currently there are no data to support the benefit of catheter ablation of AF in terms of hard cardiovascular endpoints. Therefore, the procedure is directed mainly towards improvement of quality of life. Any complication in this setting has a major negative impact. AEF is associated with a high mortality, even after early surgical treatment, making it the most feared complication of catheter ablation of AF.6
Clinical manifestation of AEF includes a variety of symptoms, the most common of which are fever and neurological deficit.7 All symptoms develop some time after the procedure, in the range of 0–60 days, according to the results of 1 published series of patients with AEF.7 Late occurrence of symptoms might lead to late diagnosis and delayed treatment. In our case, symptoms suggestive of cerebral and possible coronary air embolism developed on day 21 following the ablation, which is in the typical time frame for AEF occurrence following catheter ablation of AF. The patient was diagnosed 10–12 hours after initial presentation, which probably reflects low awareness of this condition by the emergency and intensive care unit teams. Neurological impairment upon presentation is a very strong predictor of mortality, according to a recently published series.7 In our case the patient had severe neurological symptoms upon presentation, which determined poor outcome from the very beginning, and the 10–12 hours’ delay in the diagnosis would have been unlikely to change the therapeutic strategy. The treatment associated with the highest (although moderate in absolute numbers) survival rate in these patients is in the group of surgically treated patients.7, 8 In our case surgery was deemed very high risk and was not carried out because of the very poor condition of the patient.
Owing to its low incidence, management strategies and preventive measures to avoid AEF occurrence have not been extensively studied. Prevention of AEF during the procedure includes mainly limiting power to the posterior wall of the LA and implementing esophageal temperature monitoring, esophageal cooling, or mechanical displacement of the esophagus.9, 10, 11 The value of all these preventive measures has not been studied in large trials. Current practice guidelines recommend limiting the power on the posterior wall of the LA while applying RF energy. This was also done in our case and power was limited to 20 W on the LA posterior wall. Duration of RF energy delivery was not limited but was left to operator discretion. However, with constant catheter dragging during RF delivery the catheter was not allowed to remain at one spot for more than 20 seconds. It is logical to assume that shorter lesions, allowing more time between them for tissue cooling in the LA region overlying the esophagus, would potentially diminish the risk for esophageal injury. However, this would be accompanied by decreased lesion efficacy and durability. Given the lack of firm evidence, the benefits of such an approach in terms of safety remain unproven. Several studies have shown that esophageal temperature monitoring might potentially reduce the incidence of esophageal injury following ablation,9 and it has therefore been implemented as a routine practice in many centers worldwide.2 However, implementing all these measures is not able to completely eliminate the risk occurrence of AEF, and there are reports of high incidence of esophageal injury despite the use of esophageal temperature monitoring.5
Another widely used preventive measure to reduce the incidence of esophageal injury following catheter ablation is postprocedural administration of proton-pump inhibitors. Despite its wide adoption, its value has never been shown in trials, but it is currently recommended and used by many.2 Our postablation treatment protocol also included routine use of proton-pump inhibitors.
Performing the ablation under general anesthesia is reported to be among the factors associated with esophageal injury. A small randomized study demonstrated a higher rate of esophageal tissue damage, assessed by capsule endoscopy following the ablation.12 Whether this is translated to an increased rate of AEF formation in the patients ablated under general anesthesia remains unclear because no cases of AEF were reported in the studied population. Possible mechanisms associated with increased incidence of esophageal injury in anesthetized patients are absence of pain perception as a potential sign to limit or stop energy delivery, reduced esophageal motility, and the lack of swallowing. While there are no firm data showing higher incidence of AEF in these conditions, general anesthesia continues to be widely used in routine practice.2
In our case we observed AEF formation resulting in multifocal air embolism (cerebral and coronary) despite the preventive measures undertaken in accordance with the recommendations. The procedure was short lasting and RF time was also relatively short, although sufficient to achieve PVI. General anesthesia is a potential, although unproven, risk factor that could have aided the process. Diagnosis was made late after initial presentation, and surgery was not undertaken because of the poor condition of the patient. However, earlier recognition would have been unlikely to result in a surgical intervention because the patient was already in deep coma upon presentation.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2018.11.003.
Appendix. Supplementary data
Upper endoscopy demonstrating a deep lesion at the anterior esophageal wall consistent with fistula formation to the adjacent left atrium with signs of active bleeding.
References
- 1.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H., Hindricks G., Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e257–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman J.V., Wang Y., Akar J., Desai N., Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for medicare beneficiaries, 1999-2013. Circulation. 2017;135:1227–1239. doi: 10.1161/CIRCULATIONAHA.116.022388. [DOI] [PubMed] [Google Scholar]
- 4.Cappato R., Calkins H., Chen S.A. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros De Vasconcelos J.T., Filho S., Atie J., Maciel W., De Souza O.F., Saad E.B., Kalil C.A., De Castro Mendonca R., Araujo N., Pisani C.F., Scanavacca M.I. Atrial-oesophageal fistula following percutaneous radiofrequency catheter ablation of atrial fibrillation: the risk still persists. Europace. 2017;19:250–258. doi: 10.1093/europace/euw284. [DOI] [PubMed] [Google Scholar]
- 6.Singh S.M., d’Avila A., Singh S.K., Stelzer P., Saad E.B., Skanes A., Aryana A., Chinitz J.S., Kulina R., Miller M.A., Reddy V.Y. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm. 2013;10:1591–1597. doi: 10.1016/j.hrthm.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Han H.C., Ha F.J., Sanders P., Spencer R., Teh A.W., O’Donnell D., Farouque O., Lim H.S. Atrioesophageal fistula: clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol. 2017;10:e005579. doi: 10.1161/CIRCEP.117.005579. [DOI] [PubMed] [Google Scholar]
- 8.Chavez P., Messerli F.H., Casso Dominguez A., Aziz E.F., Sichrovsky T., Garcia D., Barrett C.D., Danik S. Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Heart. 2015;2:e000257. doi: 10.1136/openhrt-2015-000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leite L.R., Santos S.N., Maia H. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol. 2011;4:149–156. doi: 10.1161/CIRCEP.110.960328. [DOI] [PubMed] [Google Scholar]
- 10.Chugh A., Rubenstein J., Good E., Ebinger M., Jongnarangsin K., Fortino J., Bogun F., Pelosi F., Oral H., Nostrant T., Morady F. Mechanical displacement of the esophagus in patients undergoing left atrial ablation of atrial fibrillation. Heart Rhythm. 2009;6:319–322. doi: 10.1016/j.hrthm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Mateos J.C., Mateos E.I., Pena T.G., Lobo T.J., Vargas R.N., Pachon C.T., Acosta J.C. Simplified method for esophagus protection during radiofrequency catheter ablation of atrial fibrillation--prospective study of 704 cases. Rev Bras Cir Cardiovasc. 2015;30:139–147. doi: 10.5935/1678-9741.20150009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Biase L., Saenz L.C., Burkhardt D.J. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol. 2009;2:108–112. doi: 10.1161/CIRCEP.108.815266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Upper endoscopy demonstrating a deep lesion at the anterior esophageal wall consistent with fistula formation to the adjacent left atrium with signs of active bleeding.