Key Teaching Points.
-
•
TBX3 has dichotomous role in the establishment of atrioventricular conduction pathways in the human heart. TBX3 both specifies the development of the normal atrioventricular conduction system and suppresses abnormal, accessory atrioventricular pathways elsewhere. Patients with TBX3 mutations should be evaluated for conduction block and ventricular preexcitation.
-
•
TBX3 may maintain the postnatal health of the human conduction system. Patients with TBX3 mutations should be followed for the possibility of developing adult-onset conduction disease (eg, sinus node dysfunction and atrioventricular block).
-
•
The electrophysiological properties of an accessory pathway could depend upon its molecular genetic basis. Genetic studies in animal models and humans could lead to noninvasive methods of risk stratification in patients who have preexcitation.
Introduction
Mutations of the transcription factor gene TBX3 cause ulnar-mammary syndrome, a rare, autosomal dominant condition characterized by ulnar defects, apocrine and mammary gland hypoplasia, genital and dental abnormalities, and pituitary dysfunction.1, 2 We present a patient who has ulnar-mammary syndrome caused by a de novo mutation of TBX3. In addition to the congenital anomalies, she has ventricular preexcitation due to a left-sided accessory pathway and right bundle branch block. This unusual combination of conduction abnormalities highlights the dichotomous functions of TBX3 in specifying the development of the conduction system and suppressing accessory atrioventricular (AV) pathways elsewhere.
Case report
A 14-year-old female subject, who has ulnar-mammary syndrome, was referred to a pediatric cardiologist after she was discovered to have a de novo mutation of TBX3. Whole-exome sequencing of her and her parents revealed that she has a premature stop codon in the conserved TBX domain (E364X, c.1090 G>T) of TBX3. The mutation is predicted to cause loss of function. Her parents do not carry the mutation.
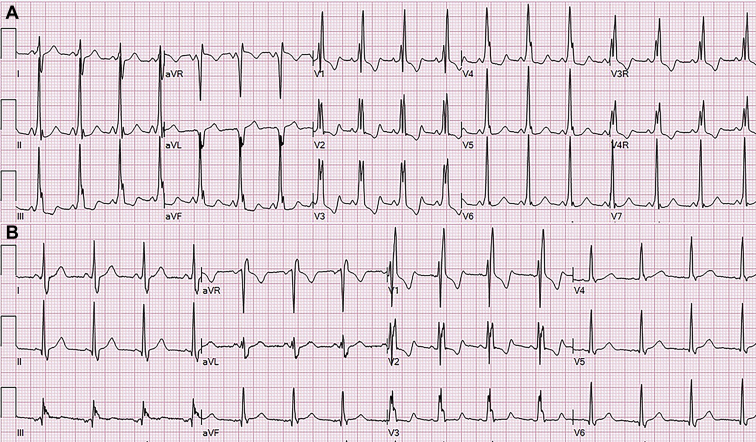
She had no physical findings or symptoms referable to the heart. An echocardiogram confirmed normal cardiac anatomy. The electrocardiogram, however, showed ventricular preexcitation consistent with a left-sided accessory pathway, as well as a right bundle branch block. The latter was apparent despite the ventricular preexcitation (Figure 1A). She underwent invasive electrophysiological study to stratify the risk of sudden death and transcatheter ablation of the accessory pathway. At baseline, there was a short HV interval (13 ms), and atrial pacing confirmed the presence of ventricular preexcitation.
Figure 1.
A: Ventricular preexcitation and a right bundle branch block were found in a 14-year-old girl who carries a de novo loss-of-function mutation of TBX3. The electrocardiogram (ECG) shown was her first. B: Preexcitation is no longer present after transcatheter ablation of a left-sided accessory pathway. The right bundle branch block remains. This ECG pattern has remained the same after 3 years of follow-up.
The accessory pathway appeared to be low risk.3 The accessory pathway block cycle length was 340 ms, and the accessory pathway effective refractory period was 340 ms at a paced drive cycle length of 600 ms. It exhibited nondecremental conduction. The retrograde pathway conduction time was somewhat long for a left-sided pathway, with a ventriculoatrial time of 170 ms. The upper limit of normal is 145 ms for a left lateral pathway.4 The retrograde accessory pathway effective refractory period was greater than 400 ms.
AV reciprocating tachycardia was not inducible in the baseline state, and only 1 beat of orthodromic tachycardia was inducible in the presence of isoproterenol.
Intracardiac mapping confirmed the presence of a single left anterior accessory pathway. Using a retrograde approach, radiofrequency energy applied under the anterior mitral valve leaflet at the site of the pathway was completely successful. There has been no recurrence of preexcitation in electrocardiograms obtained up to 3 years later.
Following the accessory pathway ablation, the AV node Wenckebach cycle length was 300 ms, and the AV node effective refractory period was 340 ms at a drive cycle length of 600 ms, indicating normal AV node function.
The right bundle branch block pattern persisted immediately after the ablation and 2 years later (Figure 1B). The time measured from the onset of the surface QRS to the local signal from the right ventricular apex catheter was 45 ms (normal ≤30 ms), consistent with proximal right bundle branch block. Although transient injury to the right bundle is common in electrophysiology studies owing to catheter contact, this is unlikely the cause of the right bundle branch block in this patient, given that it was present in the preprocedure electrocardiogram and has persisted up to 3 years after the ablation.
Ambulatory, 24-hour Holter monitoring, obtained 2 months after the ablation, documented normal sinus node function. There was no arrhythmia or new conduction defect. The sinus rate varied from 54 to 140 beats per minute (bpm). The mean rate was 90 bpm. The heart rate variability and diurnal variation were normal. The average heart rate was 79 bpm at night and 96 bpm during the day. A second Holter study, obtained 2 years after the ablation, showed similar results.
Discussion
TBX3 is a member of the T-box family of transcription factors, which regulate the embryonic development and postnatal function of diverse tissues. In the present case, a de novo loss-of-function mutation of TBX3 is associated with ventricular preexcitation and proximal right bundle branch block. This unusual pair of abnormalities illustrates the dichotomous functions of TBX3 in specifying the development of the conduction system and suppressing accessory AV pathways.
In a series of genetically engineered mice that have quantitative reductions in Tbx3 activity, mutants exhibit sinus node dysfunction, high-grade AV block, widening of the QRS interval, and ventricular preexcitation.5 The conduction defects correlate with the embryonic expression of Tbx3 in the developing sinus and AV nodes, His bundle, and proximal bundle branches.6 Tbx3-deficient mutants show cellular hypoplasia of the sinus node and the abnormal expression of ventricular genes in the AV bundle and bundle branches.5, 7, 8 The expression pattern and mutant phenotypes indicate the role of Tbx3 in the specification and development of the sinus node and central conduction system.
The developmental basis of ventricular preexcitation is attributed to the expression of Tbx3 in the AV canal myocardium, where it suppresses the persistence of accessory conduction pathways. In the early embryo, the atria are in electrical continuity with the ventricles around the circumference of the AV canal. Subsequently, the myocytes in the AV canal dissociate from each other, and the insulating fibrous annulus forms, leaving the His bundle as the sole AV connection.9 Tbx3-deficient mice have histologically confirmed AV myofibers.5
The patient in this case has some, but not all, of the conduction phenotypes described in Tbx3-deficient mice. For example, conditional deletion of the Tbx3 gene in adult mice causes sinus node dysfunction and AV block.5 TBX3 may maintain the postnatal health of the human conduction system as well. The possibility of adult-onset conduction disease thus remains a concern. The patient has normal sinus and AV node function, but she is still an adolescent.
Ulnar-mammary syndrome is rare. The published reports, consisting of a few dozen cases, have generally not described any evaluation of conduction or arrhythmia phenotypes. This suggests that most patients are asymptomatic, although 1 boy had Wolff-Parkinson-White syndrome and reentrant supraventricular tachycardia as an infant.1, 2 Thus, the present case may be the first, detailed electrophysiologic characterization of a person who has a TBX3 mutation. In particular, our patient was deemed to be at low risk for ventricular fibrillation and malignant arrhythmia because of the long accessory pathway effective refractory period and noninducible AV reentrant tachycardia.3 These signs are properties of the accessory pathway, which we speculate are the consequence of the abnormal regulation of genes that TBX3 positively or negatively regulates. In numerous different cell types, including stem and cancer cells, TBX3 integrates and responds to multiple cellular signaling pathways. TBX3 regulates gene expression and activity through direct effects on transcription, RNA splicing, and epigenetic modifications of chromatin state.10 Ectopic expression of Tbx3 in the myocardium of transgenic mice induces the expression of genes that confer a pacemaker-like phenotype and represses ventricular genes.11, 12 Similarly, Tbx3 knockout or deficiency causes loss of expression of genes specific to the sinus node and central conduction system and de-repression of atrial or ventricular genes.5, 7 Taken together, the observations suggest that the electrophysiological properties of an accessory pathway could depend upon its molecular genetic basis. Genetic studies in animal models and humans could lead to noninvasive methods of risk stratification in patients who have preexcitation.
References
- 1.Bamshad M., Lin R.C., Law D.J. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 2.Linden H., Williams R., King J., Blair E., Kini U. Ulnar Mammary syndrome and TBX3: expanding the phenotype. Am J Med Genet A. 2009;149A:2809–2812. doi: 10.1002/ajmg.a.33096. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M.I., Triedman J.K., Cannon B.C. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS) Heart Rhythm. 2012;9:1006–1024. doi: 10.1016/j.hrthm.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Ceresnak S.R., Doan L.N., Motonaga K.S., Avasarala K., Trela A.V., Reddy C.D., Dubin A.M. 50 is the new 70: Short ventriculoatrial times are common in children with atrioventricular reciprocating tachycardia. Heart Rhythm. 2015;12:1541–1547. doi: 10.1016/j.hrthm.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Frank D.U., Carter K.L., Thomas K.R., Burr R.M., Bakker M.L., Coetzee W.A., Tristani-Firouzi M., Bamshad M.J., Christoffels V.M., Moon A.M. Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis. Proc Natl Acad Sci U S A. 2012;109:E154–E163. doi: 10.1073/pnas.1115165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogaars W.M., Tessari A., Moorman A.F., de Boer P.A., Hagoort J., Soufan A.T., Campione M., Christoffels V.M. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc Res. 2004;62:489–499. doi: 10.1016/j.cardiores.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Hoogaars W.M., Engel A., Brons J.F. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakker M.L., Boukens B.J., Mommersteeg M.T., Brons J.F., Wakker V., Moorman A.F., Christoffels V.M. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ Res. 2008;102:1340–1349. doi: 10.1161/CIRCRESAHA.107.169565. [DOI] [PubMed] [Google Scholar]
- 9.Arguello C., Alanis J., Pantoja O., Valenzuela B. Electrophysiological and ultrastructural study of the atrioventricular canal during the development of the chick embryo. J Mol Cell Cardiol. 1986;18:499–510. doi: 10.1016/s0022-2828(86)80915-4. [DOI] [PubMed] [Google Scholar]
- 10.Papaioannou V.E. The T-Box gene family: emerging roles in development, stem cells and cancer. Development. 2014;141:3819–3833. doi: 10.1242/dev.104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakker M.L., Boink G.J., Boukens B.J. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc Res. 2012;94:439–449. doi: 10.1093/cvr/cvs120. [DOI] [PubMed] [Google Scholar]
- 12.Singh R., Hoogaars W.M., Barnett P. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cell Mol Life Sci. 2012;69:1377–1389. doi: 10.1007/s00018-011-0884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]