Abstract
Objective
This study evaluated the diagnostic value of measuring the levels of procalcitonin (PCT) and C‐reactive protein (CRP) to differentiate children co‐infected with H1N1 influenza and bacteria from children infected with H1N1 influenza alone.
Methods
Consecutive patients (children aged < 5 years) with laboratory‐confirmed H1N1 influenza who were hospitalized or received outpatient care from a tertiary‐care hospital in Canton, China, between January 1, 2012, and September 1, 2017, were included in the present study. Laboratory results, including serum PCT and CRP levels, white blood cell (WBC) counts, and bacterial cultures, were analyzed. The predictive value of the combination of biomarkers versus any of the biomarkers alone for diagnosing bacterial co‐infections was evaluated using logistic regression analyses.
Results
Significantly higher PCT (1.46 vs 0.21 ng/mL, P < 0.001) and CRP (19.20 vs 5.10 mg/dL, P < 0.001) levels were detected in the bacterial co‐infection group than in the H1N1 infection‐alone group. Using PCT or CRP levels alone, the areas under the curves (AUCs) for predicting bacterial co‐infections were 0.801 (95% CI, 0.772‐0.855) and 0.762 (95% CI, 0.722‐0.803), respectively. Using a combination of PCT and CRP, the logistic regression‐based model, Logit(P) = −1.912 + 0.546 PCT + 0.087 CRP, showed significantly greater accuracy (AUC: 0.893, 95% CI: 0.842‐0.934) than did the other three biomarkers.
Conclusions
The combination of PCT and CRP levels could provide a useful method of distinguishing bacterial co‐infections from an H1N1 influenza infection alone in children during the early disease phase. After further validation, the flexible model derived here could assist clinicians in decision‐making processes.
Keywords: bacterial co‐infection, C‐reactive protein, H1N1 influenza, procalcitonin
1. INTRODUCTION
Co‐infections with bacterial pathogens are a major cause of morbidity and mortality in children with H1N1 influenza infections worldwide.1 Most deaths that occurred during several H1N1 influenza pandemics in 1918‐1919 were due to bacterial co‐infections rather than direct effects of the virus.2 A recent study estimated a bacterial co‐infection rate of approximately 33% in patients hospitalized with H1N1 infection,3 a second study shows that more than 74% of patients receive antibiotic therapy after admission for initial H1N1 influenza infection,4 despite the adverse effects, high costs, and contribution to antibiotic resistance. Therefore, early and rapid diagnosis has been recognized as a priority in managing bacterial co‐infections, which may assist clinicians in initiating appropriate antibiotic treatments to improve patient outcomes.5 An early diagnosis of bacterial co‐infections among patients with H1N1 influenza is challenging, because of the many overlapping symptoms and the lack of specific clinical manifestations of bacterial co‐infections compared with H1N1 infection alone.6 Furthermore, young children cannot accurately describe their own disease symptoms, making the diagnosis even more difficult. Microbiological culture is the gold standard for diagnosing bacterial co‐infections; however, current microbiological culture involves time‐consuming cultivation of bacteria before identification via colony and biochemical profiling, and routine testing procedures may take several days and can also result in false‐negative results.
Consequently, the availability of an efficient biomarker system would be crucial in helping to quickly differentiate bacterial co‐infections from H1N1 infections alone. Recently, several inflammatory biomarkers have been evaluated for their abilities to distinguish co‐infections with H1N1 and bacteria from H1N1 infections alone. Among these biomarkers, traditional biomarkers such as a white blood cell (WBC) count7 and C‐reactive protein (CRP) levels8 are commonly used to differentiate between bacterial and viral etiologies. Although previous studies have focused on using CRP levels to detect bacterial co‐infections in patients with H1N1 infections, the evidence from these studies is inconsistent. Studies suggested serum CRP as a potential diagnostic biomarker,9, 10, 11 whereas Piacentini et al12 found that CRP levels were unable to distinguish bacterial co‐infections from H1N1 infections. Another interesting biomarker is procalcitonin (PCT), the prohormone of calcitonin produced by C cells in the thyroid. Plasma PCT concentrations are low in healthy individuals and increase during bacterial, parasitic, or fungal infections, whereas they remain at normal levels during viral infections or non‐infectious inflammatory reactions.13 Studies attempting to assess PCT levels in patients with H1N1 infection have found that PCT helped to distinguish bacterial co‐infections from H1N1 infections alone.14, 15 Nevertheless, to the best of our knowledge, previous studies published to date have focused on adults14, 15 and patients with severe disease16 but have included few pediatric patients with H1N1 infections.
Thus, in the present study, we conducted a retrospective analysis of 3180 children with H1N1 infection to evaluate the diagnostic levels of serum PCT, CRP and WBC alone and in combination for differentiating bacterial co‐infections from H1N1 influenza infections alone in children. These results could be used to provide a reliable clinical diagnostic support system for improving diagnostic accuracy and enabling the early treatment of bacterial co‐infections accompanying H1N1 influenza infections.
2. METHODS
2.1. Settings and participants
We performed a retrospective cohort study of consecutive patients with laboratory‐confirmed H1N1 influenza infections, all of whom were children <5 years old who were hospitalized or received outpatient care from a tertiary‐care hospital in Canton, China, between January 1, 2012, and September 1, 2017. Demographic and clinical characteristics, including age, gender, weight, diagnoses, total length of hospital stay, intensive care unit (ICU) admission, total length of ICU stay, total cost, and in‐hospital mortality, were recorded. Data from initial laboratory examinations, including serum PCT and CRP levels, WBC counts, and bacterial cultures, were collected. The ethics committee of Guangzhou Woman and Children's Medical Center approved our study, and written informed consent was obtained from all the participants' parents or designated guardians.
2.2. Definitions
Patients diagnosed with H1N1 influenza infection confirmed by real‐time reverse transcriptase polymerase chain reaction (RT‐PCR)17 of nasopharyngeal secretions or bronchoalveolar lavage (BAL) fluid samples within the first 48 hours of hospitalization were included in the study. Bacterial co‐infection was defined as a positive H1N1 influenza viral PCR result with one or more bacterial pathogens detected. Bacterial cultures were obtained from blood, valid sputum, and BAL fluid within the first 48 hours of hospitalization. We excluded patients who received antibiotics prior to hospitalization or who lacked bacterial cultures.
2.3. Inflammatory biomarker (PCT, CRP, and WBC) measurements
Venous blood samples were collected from H1N1‐infected patients upon admission. Serum PCT levels were determined using an enzyme‐linked fluorescence analysis (ELFA, VIDAS BRAHMS PCT kit, bioMerieux SA, Marcy‐l'Etoile, France). Serum CRP levels were determined using a BNPProSpec automatic protein analyzer (Dade Behring BN Prospec, Deerfield, IL, USA),18 and WBC counts were analyzed by using a Sysmex XE‐2100 hematology analyzer (Sysmex, Kobe, Japan).
2.4. Statistical analysis
Categorical variables are summarized using absolute values and percentages, and continuous variables are presented as medians and interquartile ranges (IQR). The chi‐square test (for nominal variables) or the Wilcoxon rank‐sum test (for continuous variables) was employed for between‐group comparisons. Univariate logistic regression analysis was used to assess the ability of each biomarker (PCT, CRP, and WBC) to diagnose bacterial co‐infections. Furthermore, iterative biomarker(s) were selected (including biomarkers with P < 0.10) using automatic forward stepwise regression, and the multivariate logistic regression model was built. The performance of the models was then assessed by calculating the area under the receiver operating characteristic (ROC) curve (AUC). The AUC values were compared for each biomarker individually and in conjunction with biomarker models using the Hanley and McNeil method.19 The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also reported. The Youden index (sensitivity + specificity − 1) was used to determine the optimal ROC cutoff value. Moreover, 10‐fold cross‐validation to evaluate the robustness of the estimates obtained from the constructed model was performed as described previously.20 Then, we averaged the AUC, sensitivity, and specificity values obtained from the 10‐fold cross‐validations to generate summary performance estimates.
All statistical analyses were performed using R Software, version 3.4.2 (www.r-project.org). A two‐tailed P value < 0.05 was considered significant.
3. RESULTS
3.1. Study population and bacterial pathogen characteristics
Figure S1 shows the flowchart for the patients included in the study. During the study period, 3180 children with laboratory‐confirmed H1N1 influenza infection were included, with a median age of 3.6 years (IQR, 1.8‐4.7 years); 1784 (52.3%) were males. Among these patients, 226 (7.1%) had a proven bacterial co‐infection. Streptococcus pneumoniae was the most frequent pathogen causing the bacterial co‐infection in 82 (36.2%) cases, followed by Staphylococcus aureus in 55 (24.3%) cases and Pseudomonas aeruginosa in 34 (15.0%) cases (Table S1). Eight children (3.5%) displayed two positive respiratory tract bacterial cultures.
When the baseline characteristics and clinical outcomes of the H1N1 plus bacterial co‐infection group were compared, children in the H1N1‐alone group were older, but this result was not significant (median age, 2.5 vs 2.4 years, P = 0.197). Differences in gender or weight were not observed between the two groups; however, the bacterial co‐infection group showed significantly higher inpatient admission (14.3% vs 50.4%, P < 0.001) and ICU admission rates (2.6% vs 36.3%, P < 0.001) than patients in the H1N1‐alone group. The bacterial co‐infection group also required longer hospital stays (5 vs 10 days, P = 0.003) than H1N1‐alone group and thus had much higher hospital costs (median hospital cost, 1213.2 vs 3467.3 RMB, P < 0.001). Moreover, a higher in‐hospital mortality rate was noted for the bacterial co‐infection group than the H1N1‐alone group (0.1% vs 4.8%, P < 0.001; Table 1).
Table 1.
Demographic and clinical characteristics of patients with H1N1 influenza who presented with and without bacterial co‐infections
| Characteristic | H1N1 alone (n = 2954) | Bacterial co‐infection (n = 226) | P a |
|---|---|---|---|
| Age (y), median (IQR) | 2.5 (1.2, 4.0) | 2.4 (1.0, 4.1) | 0.197 |
| Male, n (%) | 1794 (49.5) | 105 (47.6) | 0.240 |
| Weight (kg), median (IQR) | 9.4 (5.2, 20.8) | 9.6 (4.8, 21.5) | 0.368 |
| Patients, n (%) | |||
| Inpatient | 432 (14.3) | 114 (50.4) | <0.001 |
| Outpatient | 2522 (85.4) | 112 (49.6) | |
| Total length of hospital stays (d), median (IQR) | 5.0 (2.0, 9.0) | 10.00 (6.0, 18.3) | <0.001 |
| ICU admission, n (%) | 77 (2.6) | 82 (36.3) | <0.001 |
| Total length of ICU stays (d), median (IQR) | 6.0 (3.8, 10.0) | 11.0 (6.3, 19.8) | <0.001 |
| Total cost (RMB), median (IQR) | 1213.2 (205.5, 3041.7) | 3467.3 (1302.3, 41321.6) | <0.001 |
| In‐hospital mortality, n (%) | 3 (0.1) | 11 (4.8) | <0.001 |
IQR, interquartile range; ICU, intensive care unit.
The differences between the H1N1‐alone group and bacterial co‐infection group were examined using the Wilcoxon rank‐sum test or chi‐square tests.
3.2. Comparison of serum PCT, CRP, and WBC levels between H1N1 alone and H1N1 with bacterial co‐infection groups
Serum PCT, CRP, and WBC levels were analyzed to identify potential biomarkers that distinguished between H1N1 infections and H1N1 and bacterial co‐infections. The median serum PCT, CRP, and WBC levels were all significantly higher in the H1N1 with bacterial co‐infection group than in the H1N1‐alone group (median PCT level, 1.46 vs 0.21 ng/mL, P < 0.001; median CRP level, 19.20 vs 5.10 mg/dL, P < 0.001, median WBC count, 8.50 vs 6.90 × 109 cells/L, P = 0.019; Figure 1).
Figure 1.
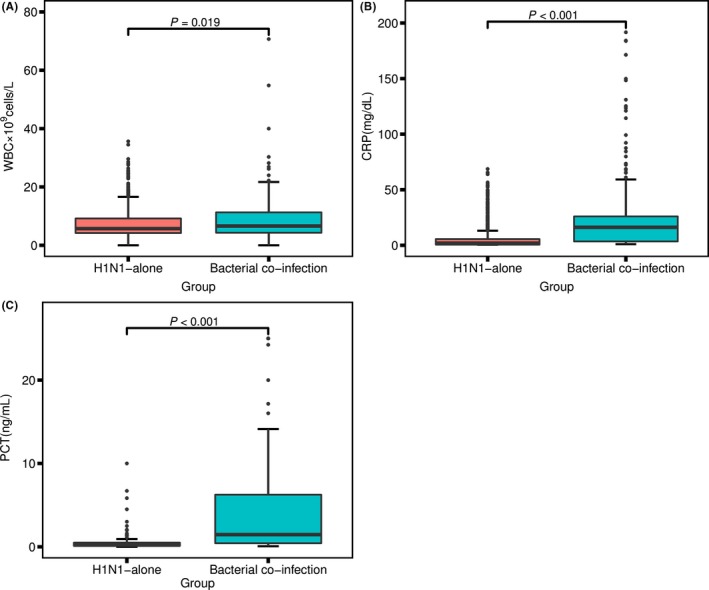
Serum PCT (A), CRP (B), and WBC (C) levels in patients with H1N1 influenza who presented with and without bacterial co‐infections. The differences between the H1N1‐alone group and bacterial co‐infection group were examined using the Wilcoxon rank‐sum test
3.3. Univariate and multivariate logistic regression analyses
Univariate analysis revealed significant associations of serum PCT, CRP, and WBC levels with co‐infections with H1N1 and bacteria (odds ratio [OR]: 1.65, 95% confidence interval [CI], 1.34‐2.06, P < 0.001; OR: 1.08, 95% CI, 1.06‐1.09, P < 0.001; OR:1.06, 95% CI, 1.04‐1.09, P = 0.02, respectively). The associations with PCT and CRP levels remained statistically significant (P < 0.05) after the application of the forward regression model, whereas WBC counts were excluded from the model (P < 0.05). Then, multivariate logistic regression analysis showed that CRP (OR:1.09, 95% CI, 1.06‐1.13, P < 0.001) and PCT levels (OR:1.73, 95% CI, 1.34‐2.42, P < 0.001) were significant independent diagnostic biomarkers. (Table 2).
Table 2.
Univariate and multivariate logistic regression analyses of biomarkers for of bacterial co‐infection in H1N1 patients infected with H1N1
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| β | OR | 95% CI | P | β | OR | 95% CI | P | |
| WBC | 0.060 | 1.06 | 1.04‐1.09 | <0.001 | ‐a | ‐ | ‐ | ‐ |
| PCT | 0.498 | 1.65 | 1.34‐2.06 | <0.001 | 0.546 | 1.73 | 1.34‐2.42 | <0.001 |
| CRP | 0.073 | 1.08 | 1.06‐1.09 | <0.001 | 0.087 | 1.09 | 1.06‐1.13 | <0.001 |
CI, confidence interval; CRP, C‐reactive protein; OR, odds ratio; PCT, procalcitonin; WBC, white blood cell; β, regression coefficient.
In the multivariate logistic regression analysis, WBC counts (P > 0.05) were excluded from the final model based on the results of the forward stepwise analysis.
3.4. Comparison and validation of the model's diagnostic ability
Because serum PCT and CRP levels were independent predictors that differentiated patients with bacterial co‐infections from patients infected with H1N1 alone, we constructed a new model, PCT&CRP [Logit(P) = −1.912 + 0.546 PCT + 0.087 CRP], that combined the PCT and CRP levels. The performance of the ROC curves of the constructed model, PCT, CRP, and WBC levels for differentiating children with H1N1 and bacterial co‐infections from children infected with H1N1 alone was compared. The AUC, sensitivity, specificity, PPV, and NPV are shown in Table 3. The constructed model exhibited the largest AUC (0.893, 95% CI, 0.852‐0.934). The P values of the ROC curve comparison between the constructed model and CRP and PCT levels were all less than 0.01. The AUCs for PCT, CRP, and WBC levels were 0.801 (95% CI, 0.772‐0.855), 0.762 (95% CI, 0.722‐0.803), and 0.551 (95% CI, 0.502‐0.592), respectively. The optimum cutoff values for PCT, CRP, and WBC were 0.52 ng/mL, 13.55 mg/L, and 11.56 × 109 cells/L, respectively. Significant differences were observed among the ROC curves of PCT, CRP, and WBC (P < 0.05). The diagnostic ability of each model followed the order of PCT&CRP > PCT > CRP > WBC (Figure 2). The PCT&CRP was superior to use of the PCT, CRP and WBC alone in differentiating patients with bacterial co‐infections from those infected with H1N1 alone. The robustness of PCT&CRP was internally evaluated through 10‐fold cross‐validation. On average, the constructed model presented an AUC of 0.872, a sensitivity of 0.754, and a specificity of 0.896.
Table 3.
Discriminatory performance of WBC, CRP, PCT and the constructed model for detecting patients with H1N1 influenza and a bacterial co‐infection
| Variables | AUC (95% CI) | Cutoff level | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| WBC | 0.551 (0.502‐0.592) | 11.56 | 0.267 | 0.887 | 0.144 | 0.910 |
| CRP | 0.762 (0.722‐0.803) | 13.55 | 0.633 | 0.856 | 0.330 | 0.971 |
| PCT | 0.801 (0.772‐0.855) | 0.52 | 0.643 | 0.886 | 0.773 | 0.852 |
| PCT&CRP* | 0.893 (0.852‐0.934) | ‐ | 0.830 | 0.868 | 0.854 | 0.846 |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; CRP, C‐reactive protein; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value; WBC, white blood cell. *PCT&CRP: Logit (P) = −1.912 + 0.546 PCT + 0.087 CRP.
Figure 2.
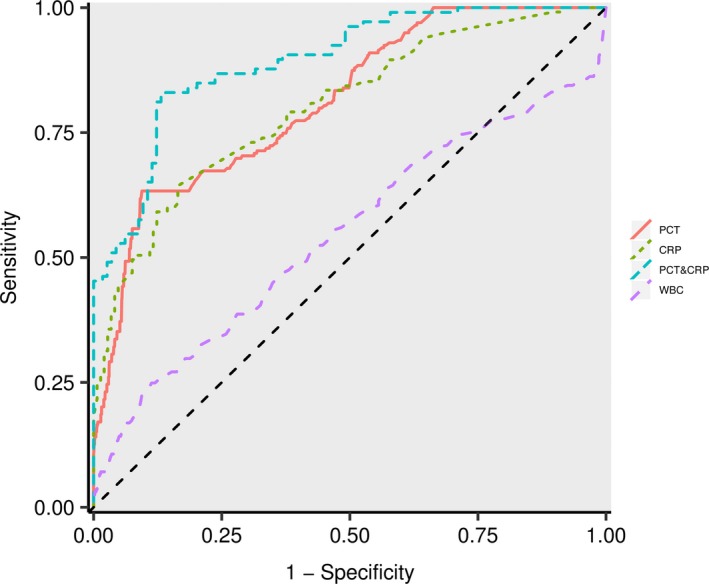
ROC curves of PCT, CRP, WBC, and PCT&CRP (Logit(P) = −1.912 + 0.546 PCT + 0.087 CRP) for differentiating patients with bacterial co‐infections from those infected with H1N1 alone
4. DISCUSSION
Bacterial co‐infection is especially known to increase the mortality and morbidity of H1N1 influenza. Unfortunately, it is difficult to correctly diagnose bacterial co‐infection based on only clinical criteria, while bacterial culturing is time‐consuming. There is a crucial need to differentiate H1N1 patients with bacterial co‐infection from those with H1N1 infection alone. The diagnostic and predictive value of serum PCT and CRP levels as biomarkers has been discussed in several studies.10, 14, 15, 21 Shin et al10 found that serum PCT was a good indicator in discriminating bacterial co‐infections from H1N1 infection alone in 60 adult patients in ICU. Guervilly et al21 reported that PCT values were significantly higher in patients with bacterial co‐infections. In addition, PCT has been suggested to exclude bacterial co‐infections in patients with H1N1 infection and to reliably and accurately reduce inappropriate antibiotic exposure.14 Our results showed that serum PCT levels were significantly higher in patients with bacterial co‐infection compared with those infected with H1N1 alone, confirming that PCT is associated with bacterial co‐infection. Furthermore, the results of ROC curve analyses indicated that an AUC value of 0.801 (95% CI, 0.772‐0.855), with a cutoff value of 0.52 ng/mL, supported the diagnostic value of PCT in children with or without bacterial co‐infection.
The diagnostic utility of CRP to differentiate bacterial co‐infection from H1N1 infection is disputed.9, 10, 11, 15 Haran et al11 found that CRP served as a predictor of bacterial infection among patients with H1N1 infection. Similarly, Shin et al10 reported that serum CRP levels were significantly higher in patients with bacterial co‐infection compared with those infected with H1N1 alone. However, another study suggested that CRP levels were not useful for distinguishing bacterial co‐infections from H1N1 infections.9 Our study showed that serum CRP levels were significantly higher in patients with bacterial co‐infection compared with those infected with H1N1 alone, indicating that these biomarkers may useful in discriminating between these conditions. Interestingly, for the optimal cutoff value, the NPV of CRP (0.971) alone is considerably higher, in line with one previous study.10 This suggests that clinicians might consider using CRP to rule out patients who are free of co‐infections. In the present study, the PPV of CRP&PCT (0.854) and the PPV of PCT (0.773) were much higher than the PPV of CRP (0.331), indicating that CRP&PCT or PCT alone might help clinicians to avoid unnecessary antibiotic therapy. Furthermore, our study showed that the diagnostic efficacy of PCT for bacterial co‐infection in H1N1 infection was better than that of CRP (AUC 0.801 and 0.783, respectively; P < 0.05), consistent with the results of a previous study.11 However, the AUC of WBC counts in diagnosing bacterial co‐infections was 0.551 (95% CI, 0.502‐0.592), indicating that WBC may not be a valuable biomarker for our cohort of children.
A previous study using a combination of CRP and PCT levels to evaluate bacterial co‐infections observed increased accuracy in differentiating children with bacterial co‐infections from those infected with H1N1 alone.10 Similar observations were reported in the present study, in which we used a multivariate logistic regression analysis to construct a new model using PCT and CRP levels: [Logit(P) = −1.912 + 0.546 PCT + 0.087 CRP]. The ROC curve analysis yielded an AUC value for the model of up to 0.893, which was clearly superior to PCT or CRP levels alone (P < 0.05). Furthermore, the constructed model [Logit(P) = −1.912 + 0.546 PCT + 0.087 CRP] was internally validated through 10‐fold cross‐validation, resulting in high diagnostic accuracy. Therefore, the joint detection of PCT and CRP levels clearly improves the diagnosis of children with H1N1 bacterial co‐infection. Based on the results from our study, the combination of serum PCT and CRP levels may help clinicians determine whether antibiotic therapy is appropriate,22 thus potentially improving patient outcomes and reducing antibiotic overuse.5
The present study involved 3180 children with H1N1, 7.11% of whom presented a confirmed bacterial co‐infection, after including both outpatients and inpatients. The proportion of bacterial co‐infection was similar to that previously reported for H1N1. Nevertheless, previous studies of children with H1N1 influenza infection reported a bacterial co‐infection rate ranging from 18% to 60%.23, 24 These rates may be overestimated because the previous studies were limited to pediatric patients in the ICU, which represent a population with moderate to severe H1N1 influenza infection. Moreover, children with bacterial co‐infections exhibited a higher percentage of ICU admission rates in the current study.
Our study showed that Streptococcus pneumoniae was the leading cause of bacterial co‐infection with H1N1, followed by Staphylococcus aureus and Streptococcus pyogenes, consistent with the results from previous studies.25, 26 Additionally, children with H1N1 infection and bacterial co‐infection have been reported to exhibit a higher risk of severe outcomes.26, 27, 28 In our study, patients co‐infected with bacteria and H1N1 exhibited increased percentages of inpatient and ICU admissions, higher costs, and longer hospital stays. Furthermore, a significantly higher hospital mortality rate was observed in children with H1N1 and bacterial co‐infections because bacterial co‐infections represent an important mortality risk factor, suggesting that early empiric antibiotic treatment in severe patients may improve outcomes.
The potential limitations of our study should be mentioned. First, the levels of selected biomarkers (PCT, CRP, and WBC) were evaluated only once. Second, our diagnostic model was derived and validated at a single hospital center and should be validated in a multicenter trial before its broad application. Finally, we also acknowledge that we may have created bias, as bacterial organisms cannot be confirmed solely through blood, sputum, and BAL culture.
5. CONCLUSION
In conclusion, we detected serum PCT and CRP levels and revealed that they represent promising biomarkers and useful clinical tools for differentiating pediatric patients with bacterial co‐infections from those infected with H1N1 alone. Furthermore, the combination of PCT and CRP levels could represent a useful method for screening bacterial co‐infections from H1N1 influenza infections alone in children during the early disease phase. After further validation, the flexible model reported here may assist clinicians with decision‐making processes.
Supporting information
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Li Z, He L, Li S, et al. Combination of procalcitonin and C‐reactive protein levels in the early diagnosis of bacterial co‐infections in children with H1N1 influenza. Influenza Other Respi Viruses. 2019;13:184–190. 10.1111/irv.12621
Zhihao Li, and Liya He are contributed equally to this work.
Contributor Information
Xin Sun, Email: doctorsunxin@hotmail.com.
Huiying Liang, Email: lianghuiying@hotmail.com.
REFERENCES
- 1. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta‐analysis. The Lancet. 2011;378:1917‐1930. [DOI] [PubMed] [Google Scholar]
- 2. McCullers JA. The co‐pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12:nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 3. Cillóniz C, Ewig S, Menéndez R, et al. Bacterial co‐infection with H1N1 infection in patients admitted with community acquired pneumonia. J Infect. 2012;65:223‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Eng J Med. 2009;361:1935‐1944. [DOI] [PubMed] [Google Scholar]
- 5. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275‐282. [DOI] [PubMed] [Google Scholar]
- 6. Libster R, Bugna J, Coviello S, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Eng J Med. 2010;362:45‐55. [DOI] [PubMed] [Google Scholar]
- 7. Lindbäck S, Hellgren U, Julander I, Hansson L. The value of C‐reactive protein as a marker of bacterial infection in patients with septicaemia/endocarditis and influenza. Scand J Infect Dis. 1989;21:543‐549. [DOI] [PubMed] [Google Scholar]
- 8. Sanders S, Barnett A, Correa‐Velez I, Coulthard M, Doust J. Systematic review of the diagnostic accuracy of C‐reactive protein to detect bacterial infection in nonhospitalized infants and children with fever. J Pediatr. 2008;153:570‐574. e573. [DOI] [PubMed] [Google Scholar]
- 9. Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C‐reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med. 2010;36:528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn S, Kim WY, Kim SH, et al. Role of procalcitonin and C‐reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respir Viruses. 2011;5:398‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haran JP, Beaudoin FL, Suner S, Lu S. C‐reactive protein as predictor of bacterial infection among patients with an influenza‐like illness. Am J Emerg Med. 2013;31:137‐144. [DOI] [PubMed] [Google Scholar]
- 12. Piacentini E, Sánchez B, Arauzo V, Calbo E, Cuchi E, Nava JM. Procalcitonin levels are lower in intensive care unit patients with H1N1 influenza A virus pneumonia than in those with community‐acquired bacterial pneumonia, A pilot study. J Crit Care. 2011;26:201‐205. [DOI] [PubMed] [Google Scholar]
- 13. Gendrel D, Raymond J, Assicot M, et al. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997;24:1240‐1242. [DOI] [PubMed] [Google Scholar]
- 14. Rodríguez AH, Avilés‐Jurado FX, Díaz E, et al. Procalcitonin (PCT) levels for ruling‐out bacterial coinfection in ICU patients with influenza: a CHAID decision‐tree analysis. J Infect. 2016;72:143‐151. [DOI] [PubMed] [Google Scholar]
- 15. Cuquemelle E, Soulis F, Villers D, et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicenter study Intensive Care Med. 2011;37:796‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfister R, Kochanek M, Leygeber T, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta‐analysis. Crit Care. 2014;18:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duncan C, Guthrie JL, Tijet N, et al. Analytical and clinical validation of novel real‐time reverse transcriptase–polymerase chain reaction assays for the clinical detection of swine‐origin H1N1 influenza viruses. Diagn Microbiol Infect Dis. 2011;69:167‐171. [DOI] [PubMed] [Google Scholar]
- 18. Esfahani A, Makhdami N, Faramarzi E, et al. Prealbumin/CRP based prognostic score, a new tool for predicting metastasis in patients with inoperable gastric cancer. Gastroenterol Res Prac. 2016;2016:4686189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNeil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making. 1984;4:137‐150. [DOI] [PubMed] [Google Scholar]
- 20. Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci. 2002;99:6567‐6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guervilly C, Coisel Y, Botelho‐Nevers E, et al. Significance of high levels of procalcitonin in patients with influenza A (H1N1) pneumonia. J Infect. 2010;61:355‐358. [DOI] [PubMed] [Google Scholar]
- 22. Albrich WC, Harbarth S. Pros and cons of using biomarkers versus clinical decisions in start and stop decisions for antibiotics in the critical care setting. Intensive Care Med. 2015;41:1739‐1751. [DOI] [PubMed] [Google Scholar]
- 23. Carr SB, Adderson EE, Hakim H, Xiong X, Yan X, Caniza M. Clinical and demographic characteristics of seasonal influenza in pediatric patients with cancer. Pediatr Infect Dis J. 2012;31:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cordero E, Pérez‐Romero P, Moreno A, et al. Pandemic influenza A (H1N1) virus infection in solid organ transplant recipients: impact of viral and non‐viral co‐infection. Clin Microbiol Infect. 2012;18:67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Q, Ji W, Guo Z, Bai Z, MacDonald NE. Risk factors and outcomes for pandemic H1N1 influenza compared with seasonal influenza in hospitalized children in China. Can J Infect Dis Med Microbiol. 2012;23:199‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reed C, Kallen AJ, Patton M, et al. Infection with community‐onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. 2009;28:572‐576. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen T, Kyle UG, Jaimon N, et al. Coinfection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med. 2012;40:3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhat N, Wright JG, Broder KR, et al. Influenza‐associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559‐2567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


