Abstract
Background
Diagnosis of tuberculosis (TB) in human immunodeficiency virus (HIV)-coinfected individuals is challenging. We hypothesized that combinations of inflammatory markers could facilitate identification of active TB in HIV-positive individuals.
Methods
Participants were HIV-positive, treatment-naive adults systematically investigated for TB at Ethiopian health centers. Plasma samples from 130 subjects with TB (HIV+/TB+) and 130 subjects without TB (HIV+/TB−) were tested for concentration of the following markers: CCL5, C-reactive protein (CRP), interleukin (IL)-6, IL12-p70, IL-18, IL-27, interferon-γ-induced protein-10 (IP-10), procalcitonin (PCT), and soluble urokinase-type plasminogen activator receptor (suPAR). Analyzed markers were then assessed, either individually or in combination, with regard to infection status, CD4 cell count, and HIV ribonucleic acid (RNA) levels.
Results
The HIV+/TB+ subjects had higher levels of all markers, except IL12p70, compared with HIV+/TB− subjects. The CRP showed the best performance for TB identification (median 27.9 vs 1.8 mg/L for HIV+/TB+ and HIV+/TB−, respectively; area under the curve [AUC]: 0.80). Performance was increased when CRP was combined with suPAR analysis (AUC, 0.83 [0.93 for subjects with CD4 cell count <200 cells/mm3]). Irrespective of TB status, IP-10 concentrations correlated with HIV RNA levels, and both IP-10 and IL-18 were inversely correlated to CD4 cell counts.
Conclusions
Although CRP showed the best single marker discriminatory potential, combining CRP and suPAR analyses increased performance for TB identification.
Keywords: biomarker, CRP, HIV, Mycobacterium tuberculosis, sub-Saharan Africa
Human immunodeficiency virus (HIV)-related cellular immunodeficiency increases the risk of both primary and reactivated tuberculosis (TB) and is associated with greater likelihood of extrapulmonary and disseminated disease [1]. Rates of TB coinfection among people living with HIV (PLHIV) are highest in sub-Saharan Africa [2], and TB is the leading cause of death among PLHIV globally [3]. Autopsy studies suggest that considerable proportions of HIV-related TB may be missed in TB-endemic settings [4]. Contributing factors for this are atypical disease presentation [5] and suboptimal performance of commonly available diagnostic methods for active TB, such as smear microscopy [6] and chest radiography [7]. A clinical symptom algorithm is recommended by the World Health Organization (WHO) for excluding TB among PLHIV [8]. Although this algorithm is sensitive, it has low specificity, limiting its usefulness as a screening tool [9]. Alternative strategies for screening in populations at high risk of TB could improve case finding.
These circumstances have prompted investigations of plasma biomarkers for identification of active TB. C-reactive protein (CRP) has been shown to have promising capacity as a point-of-care biomarker for TB screening among PLHIV in different settings [10–15]. Although the capacity of panels of inflammatory markers for TB identification has been investigated [16–18], this concept has not been assessed in a large PLHIV cohort systematically categorized for active TB. Because HIV leads to dysregulation of the TB immune response [19, 20], we hypothesized that HIV-positive individuals with active TB have inflammatory reactions that could be used to distinguish such cases from HIV-positive persons without TB, and that combinations of inflammatory markers would provide stronger discriminatory capacity than single markers.
To explore this hypothesis, we designed a nested case-control study based on participants from a cohort of antiretroviral therapy (ART)-naive patients systematically investigated for active TB. We characterized plasma profiles of 9 markers of inflammation, selected on the basis of their previously reported associations with TB. We analyzed the performance for TB identification of these markers separately as well as in different combinations. Furthermore, we evaluated whether plasma levels of these markers were related to CD4 cell count and HIV-ribonucleic acid (RNA) levels, respectively.
METHODS
Study Participants
Participants for this study were identified from a prospective cohort of 812 HIV-positive adults subjected to intensified TB case finding before ART initiation at 5 public health centers in the Oromia region, Ethiopia (included between 2011 and 2013). Details of this cohort have been presented previously [9, 21]. In brief, for inclusion in the cohort, subjects were required to be ≥18 years old and meet Ethiopian criteria for ART initiation at the time of the study (CD4 cell count ≤350 cells/mm3 and/or WHO stage 4). Individuals with previous ART experience and/or ongoing treatment for TB for ≥2 weeks, as well as those who did not provide sputum samples for TB analyses, were excluded.
At inclusion, cohort participants underwent physical examination and interview based on a structured questionnaire covering clinical and sociodemographic data. All subjects were instructed to submit 2 spontaneously expectorated morning sputum samples (irrespective of symptoms or physical findings), which were examined with Ziehl-Neelsen smear microscopy, liquid TB culture, and Xpert MTB/RIF (Cepheid, Sunnyvale, CA). Fine-needle aspirations were obtained from persons with lymphadenopathy. Venous blood was collected for analysis of CD4 cell count, complete blood count, and HIV RNA quantification. Aliquots of plasma were stored at −80°C and transported with intact cold chain to the Biomedical Center, Lund, Sweden, where laboratory analyses were undertaken after data collection and subject selection. A nested case-control design was chosen for the current study. All participants with bacteriologically confirmed TB (HIV+/TB+) and available plasma samples were included as cases, and, for each of these, 1 control without active TB was selected, matched for age and gender (HIV+/TB−). Plasma samples were available from 130 of 137 HIV+/TB+ subjects. Control subjects (HIV+/TB−) were required to have negative bacteriological results for TB and not to be diagnosed with bacteriologically confirmed nor clinically suspected TB during the first 6 months of follow-up. Participants without active TB at inclusion who were lost to follow-up or died within 6 months of follow-up were not eligible as controls, because unrecognized active TB could not be reliably excluded.
Quantification of Inflammatory Markers
Concentrations of inflammatory markers were determined using the Magnetic Luminex Assay (R&D Systems Inc., Minneapolis, MN) and Bio-Plex 200 reader (Bio-Rad Laboratories, Hercules, CA). In an initial exploratory experiment, the following 14 markers were analyzed in 20 HIV+/TB+ and 20 HIV+/TB− subjects: CC-chemokine ligand 5 (CCL5, a.k.a. RANTES), CRP, granzyme B, interferon (IFN)-β, IFN-γ, IFN-γ-induced protein 10 (IP-10), interleukin (IL)-10, IL-12p70, IL-18, IL-27, IL-6, procalcitonin (PCT), soluble urokinase-type plasminogen activator receptor (suPAR), and tumor necrosis factor (TNF)-α. For Granzyme B, IFN-β, IFN-γ, IL-10, and TNF- α plasma levels were uniformly low or undetectable; therefore, we excluded these markers from further experiments.
C-reactive protein and CCL5 were analyzed in separate plates, diluted 1:50 and 1:200, respectively, and CRP was later diluted 1:2000 for values beyond the standard curve. The remaining markers were analyzed in 1:2 dilution in a 7-plex plate. All plates contained equal proportions of HIV+/TB+ and HIV+/TB− samples, randomly placed on the plate. Laboratory procedures were performed according to the manufacturer’s instructions.
Each plate included a standard curve of 6 standard points and 2 internal controls (1 TB+, 1 TB−). All samples were analyzed in duplicates. Mean intra-assay variation for concentrations was 7% coefficient of variation (%CV). Mean interassay variation for median fluorescence intensity (MFI) values for all markers except suPAR was 11.3%CV (range, 6–17) and increased to 19.6%CV (range, 11–27) after translation to concentrations. For suPAR, both MFI and concentration values showed greater variability between runs (%CV 29 and 30.5, respectively).
Statistical Analysis
Comparison of marker levels between HIV+/TB+ and HIV+/TB− subjects was evaluated using the Mann-Whitney U test. P < .05 (adjusted for multiple testing using the Holm-Bonferroni method) were considered significant. Receiver operating characteristics (ROC) curves were constructed for each marker. In addition, marker levels were correlated to CD4 cell count and HIV RNA levels using Spearman’s rank-order correlation. To adjust for lower CD4 cell counts among HIV+/TB+ subjects, markers were dichotomized in 2 groups, with regard to median levels for the respective marker, and logistic regression was performed for each marker in respect to TB, with and without adjustment for CD4 cell count.
To assess the best combination of inflammatory markers to distinguish between HIV+/TB+ and HIV+/TB−, backward stepwise logistic multivariate analysis was performed using continuous variables. From this multivariate analysis, predicted probabilities were calculated. A ROC curve describing sensitivity and specificity of the combination of markers that constituted the best model was then fitted. This model was subsequently assessed in an analysis of subjects with CD4 cell count <200/mm3. All statistical analyses were conducted using SPSS version 24.0 (IBM, Armonk, NY).
Ethics Statement
Ethical approval was obtained from both the National Research Ethics Review Committee at the Ministry of Science and Technology of Ethiopia (3.10/825/05) and the Regional Ethical Review Board at Lund University, Sweden (2010/672). Written informed consent was obtained from all participants at inclusion in the cohort study.
RESULTS
Participant Characteristics
The characteristics of study participants at the time of sampling (130 HIV+/TB+ and 130 HIV+/TB−) are presented in Table 1. All patients had pulmonary TB, in addition 12 subjects had bacteriologically confirmed concomitant TB lymphadenopathy. The HIV+/TB+ subjects were more likely to be malnourished (lower body mass index and mid-upper arm circumference). In addition, they had lower median CD4 cell count (173 vs 224 cells/mm3), lower hemoglobin levels (10.4 vs 12 g/dL), and higher viral load (5.3 vs 5.0 log10 RNA copies/mL). In both groups, a majority reported positive WHO-TB symptom score results (presence of ≥1 symptom; cough, weight loss, night sweating, and/or fever [93% of HIV+/TB+, 79% of HIV+/TB−]).
Table 1.
Characteristics of 130 HIV-Positive Participants With TB Coinfection (HIV+/TB+) and 130 HIV-Positive Matched Controls Without TB Coinfection (HIV+/TB−) Selected From a Cohort of 812 ART-Naive HIV-Positive Patients Investigated for Active TBa
| TB+ | TB− | ||||
|---|---|---|---|---|---|
| Characteristics | Included | Excluded | Included | Excluded | Total |
| Female | 63/130 (49) | 3/7 (43) | 60/130 (46) | 341/524 (65) | 467/791 (59) |
| Age, years | 35 (28–42) | 30 (29–37) | 35 (28–41) | 31.5 (28–38) | 32 (28–40) |
| CD4 count, cells/mm3 | 173 (94.5–271) | 91 (88–197) | 224 (146–328) | 220 (121–327) | 211 (119–320) |
| Hemoglobin, g/dL | 10.4 (9.1–11.8) | 10.9 (8.5–14.2) | 12 (10.9–13.2) | 11.8 (10.3–12.7) | 11.6 (10.2–12.7) |
| HIV RNA, log10 copies/ mL | 5.3 (4.7–5.6) | 5.4 (5.2–5.8) | 5 (4.5–5.5) | 5 (5.5–5.5) | 5.1 (4.5–5.6) |
| On TB treatment at enrollment | 15/130 (12)b | 0/7 (0) | 0 (130) | 0/524 (0) | 15/791 (2) |
| Bacteriological results | |||||
| Sputum smear positive | 29/129 (23) | 2/7 (29) | 0/130 | 0/518 (0) | 31/784 (4) |
| Sputum Xpert MTB/RIF positive | 90/129 (70) | 6/7 (86) | 0/130 | 0/518 (0) | 96/783 (12) |
| Sputum culture positive | 118/129 (92) | 6/7 (86) | 0/130 | 0/523 (0) | 124/788 (17) |
| Lymph node culture positive | 4/5 (80) | 0/0 (0) | 0/3 (0) | 0/1 (0) | 4/9 (44) |
| Lymph node Xpert MTB/RIF positive | 11/12 (92) | 0 (0) | 0/1 (0) | 0/7 (0) | 13/20 (65) |
| Clinical findings | |||||
| WHO-TB symptom screening positivec | 120/129 (93) | 6/7 (86) | 101/128 (79) | 398/520 (77) | 625/784 (80) |
| MUAC cm | 21 (19.4–23) | 22 (18.8–22.5) | 23 (21–25) | 23 (21–24.5) | 22.5 (20.5–24) |
| BMI kg/m2 | 17.7 (16.1–19.7) | 17.6 (16.7–20.7) | 19.5 (17.8–22) | 19.1 (17.6–21.1) | 19 (17.4–21) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; MUAC, mid-upper arm circumference; RNA, ribonucleic acid; TB, tuberculosis; WHO, World Health Organization.
Twenty-one subjects with TB diagnosed on clinical criteria are not presented in this table. For continuous variables, median and interquartile range are presented; for categorical variables, absolute numbers and percentage of total are presented.
Median number of days on treatment before enrollment: 7.
Presence of either cough, weight loss, night sweating, or fever.
Individual Inflammation Markers in Respect to Active Tuberculosis
Plasma concentrations of investigated markers are shown in Table 2, and differences are graphically presented in Figure 1. With the exception of IL-12p70, HIV+/TB+ subjects had significantly higher levels of all markers compared with HIV+/TB− (all P < .01). The difference between HIV+/TB+ and HIV+/TB− were most pronounced for CRP (median concentration, 27.9 vs 1.8 mg/L; P < .001), suPAR (median concentration, 2.6 vs 1.1 ng/mL; P < .001), and IL-6 (median concentration, 9.7 vs 2.1 ng/L; P < .001). The IL-27 and IL-12-p70 concentrations could not be extrapolated due to large proportions of values below the standard curve; therefore, these markers were excluded from further analyses.
Table 2.
Levels of Investigated Inflammatory Markers (Median, IQR in Brackets) in HIV+ Subjects in Whom TB Was Excluded (HIV+/TB−) and HIV+ Subjects With Bacteriologically Confirmed TB (HIV+/TB+) With Regard to Distribution of Bacteriological Results and Presence of Clinical Symptoms
| HIV+/TB− (n = 130) | |||||||
|---|---|---|---|---|---|---|---|
| Inflammatory Marker | HIV+/TB+ (n = 130) | All | Smear+a (n = 29) | Gene Xpert+b (n = 90) | Culture+/ AFB−/Gene Xpert− (n = 38) | WHO Score+c (n = 120) | WHO Score− (n = 9) |
| CCL5, µg/mL | 40.8 (14.9–98.9) | 73.1(27.9–154.7) | 59.8 (12.2–12.7) | 73.1 (26.5–130) | 91.9 (34.9–17.4) | 70.3 (27.3–153.5) | 91.2 (40.6–141.4) |
| CRP, mg/L | 1.8 (0.6–6.9) | 27.9 (5.9–50) | 50 (31.7–50) | 49.5 (15.3–50) | 9.1 (1.4–49.7) | 38.9 (10–50) | 1 (0.6–3.8) |
| IL-6, pg/mL | 2.1 (0.5–4.5) | 9.7(3.2–18.4) | 14.1 (7.4–32.4) | 12.3 (4.7–22.6) | 3.6 (1–10.5) | 10.4 (3.7–18.7) | 1.1 (0.6–2.9) |
| IL12p70, MFId | 12.5 (11.5–13.5) | 13 (12–14) | 13 (11.5–15) | 13.3 (12–14.5) | 12.65 (11.5–13.5) | 13 (12–14) | 12.3 (11.1–13.4) |
| IL-18, pg/mL | 672 (469–1 023) | 1098 (665–1 956) | 1432 (830–2 982) | 1356 (832–2394) | 831 (521–1301) | 1107 (670–2 106) | 648 (436–1 216) |
| IL-27, MFId | 35.4 (27.0–43.4) | 44.0 (32.9–52.9) | 46.0 (35.0–59.3) | 46.3 (34.7–54.3) | 40.9 (29.1–49.3) | 45.7 (33.3–53.6) | 32.7 (25.4–46) |
| IP-10, pg/mL | 143 (83–251) | 241 (154–414) | 319 (185–509) | 295.3 (167–466) | 208 (101–254) | 253 (167– 418) | 103 (50.1– 197) |
| PCT, ng/mL | 0.04 (0.03–0.07) | 0.08 (0.04–0.14) | 0.11 (0.08–0.2) | 0.08 (0.05–0.19) | 0.05 (0.03–0.09) | 0.08 (0.05–0.15) | 0.04 (0.03–0.04) |
| suPAR, ng/mL | 1.1 (0.7–1.7) | 2.6 (1.3–5.5) | 3 (1.7–10.1) | 3.2 (1.5–6.7) | 1.8 (1–2.6) | 2.7 (1.3–5.8) | 1.3 (0.9–2) |
Abbreviations: CCL5, CC-chemokine ligand 5; CRP, C-reactive protein; HIV, human immunodeficiency virus; IL, interleukin; IP-10, interferon-γ-induced protein-10; IQR, interquartile range; MFI, median fluorescence intensity; PCT, procalcitonin; suPAR, soluble urokinase-type plasminogen activator receptor; TB, tuberculosis; WHO, World Health Organization.
Positive Zieh-Neelsen-stained direct microscopy.
Positive Gene Xpert MTB/RIF.
Presence of either cough, fever, weight loss, or night sweating.
For these markers, it was not possible to extrapolate reliable concentrations; hence, MFI values are presented instead.
Figure 1.
Levels of 9 markers of inflammation in plasma from 130 human immunodeficiency virus (HIV)+/tuberculosis (TB)+ and 130 HIV+TB− subjects. Boxes represent median and interquartile range. Whiskers have been graphically cut for soluble urokinase-type plasminogen activator receptor (suPAR), interleukin (IL)-6, and procalcitonin (PCT). Mann-Whitney U test P values are indicated in each graph. All markers remained significantly associated with TB after Holm-Bonferroni correction, except IL-12p70. For IL-12p70 and IL-27, the graphs represent mean fluorescence intensity (MFI) due to unreliable conversions to concentrations.
We compared levels of inflammatory markers with regard to bacteriological results (reflecting bacterial load in sputum) and presence of TB symptoms. Levels of CRP, IL-6, IL-18, IP-10, PCT, and suPAR were significantly higher in Xpert MTB/RIF-positive HIV+/TB+ subjects, compared with Xpert MTB/RIF-negative HIV+/TB+ subjects, and CRP and IL-6 were significantly higher in smear-positive HIV+/TB+ subjects, compared with smear-negative HIV+/TB+ subjects. The HIV+/TB+ subjects without TB symptoms had low levels of the markers investigated (Table 2). Adjustments for CD4 cell count in logistic regression did not significantly change the odds ratio for any marker (Table 3).
Table 3.
Odds Ratios for HIV+/TB+ Versus HIV+/TB− for Marker Levels Above Median Level of the Total Study Population, Compared to Levels Below Median, With and Without Adjustments for CD4 Cell Counta
| Inflammatory Marker | Crude OR | 95% CI | Adjusted OR | 95% CI | AUC | 95% CI | AUC in Subjects With CD4 <200 Cells/mm3 | 95% CI |
|---|---|---|---|---|---|---|---|---|
| CCL5 | 2.1 | 1.3–3.5 | 2.1 | 1.2–3.4 | 0.62 | 0.55–0.68 | 0.62 | 0.52–0.71 |
| CRP | 8.6 | 4.9–15.1 | 8.0 | 4.5–14.1 | 0.80 | 0.75–0.86 | 0.89 | 0.84–0.95 |
| IL-6 | 5.4 | 3.2–9.3 | 5.0 | 2.9–8.5 | 0.76 | 0.71–0.82 | 0.87 | 0.8–0.93 |
| IL-18 | 3.6 | 2.1–6.1 | 3.1 | 1.8–5.4 | 0.71 | 0.65–0.77 | 0.71 | 0.63–0.8 |
| IP-10 | 2.9 | 1.7–4.8 | 2.5 | 1.5–4.2 | 0.67 | 0.6–0.73 | 0.65 | 0.55–0.75 |
| PCT | 3.1 | 1.9–5.2 | 2.8 | 1.7–4.8 | 0.68 | 0.61–0.74 | 0.73 | 0.64–0.81 |
| suPAR | 4.4 | 2.6–7.4 | 3.9 | 2.3–6.7 | 0.77 | 0.71–0.83 | 0.87 | 0.81–0.93 |
Abbreviations: AUC, area under the curve; CI, confidence intertval; CRP, C-reactive protein; HIV, human immunodeficiency virus; IL, interleukin; IP-10, interferon-γ-induced protein-10; OR, odds ratio; PCT, procalcitonin; suPAR, soluble urokinase-type plasminogen activator receptor; TB, tuberculosis.
Area under the curve values are for all markers in whole material and in subjects with CD4 <200 cells/mm3.
Correlation of Inflammation Markers to Other Parameters
Because the incidence of active TB increases with HIV-related immunosuppression, we determined whether biomarker levels were independently associated with TB or with surrogate markers of HIV disease progression. For this purpose, we performed logistic regression with adjustments for CD4 cell count. This did not significantly change the odds ratio for any marker (Table 3). In addition, Spearman’s rank-order correlations were performed between plasma levels of each marker and CD4 cell count and HIV-RNA levels, respectively. When the results from all 260 study participants were analyzed, all markers, except CCL5, exhibited significant inverse Spearman rank correlations to CD4 cell count (ρ: −0.34 to −0.41, all P < .01). The same markers were significantly inversely correlated to CD4 cell count in separate analysis of HIV+/TB+ subjects (ρ: −0.22 to −0.52, all P < .05). Among HIV+/TB− subjects, IL-18 (ρ: −0.29, P < .01) and IP-10 (ρ: −0.35, P < .01) were significantly inversely correlated to CD4 cell count, whereas other markers did not show such correlation.
When assessed for correlation with HIV-RNA levels, IL-6 (ρ: 0.2, P < .01), IL-18 (ρ: 0.27, P < .01), IP-10 (ρ: 0.43, P < .01), and suPAR (ρ: 0.2, P < .01) exhibited significant correlation when all subjects were included. In HIV+/TB+ subjects, IL-18 (ρ: 0.34, P < .01), IP-10 (ρ: 0.42, P < .01), and suPAR (ρ: 0.27, P < .01) correlated to HIV RNA, whereas only IP-10 exhibited significant correlation among HIV+/TB− subjects (ρ: 0.4, P < .01) (Tables 1 and 12; Supplementary Material). C-reactive protein and PCT were higher in male subjects (P < .01; median 10 vs 4 mg/L and 74 vs 44 pg/mL, respectively). Marker levels were not significantly altered in the 15 subjects who had received TB treatment before collection of samples.
Discriminatory Potential of Inflammation Markers for Tuberculosis
To explore the discriminatory potential for TB identification, ROC curves were constructed for both individual markers and combinations of markers. Individual area under the curve (AUC) values were highest for the following markers: CRP (AUC = 0.80; 95% confidence interval [CI], 0.75–0.86), suPAR (AUC = 0.77; 95% CI, 0.71–0.83), IL-6 (AUC = 0.76; 95% CI, 0.71–0.82), and IL-18 (AUC = 0.71; 95% CI, 0.65–0.77). For all AUC of all markers, see Table 3. In subjects with CD4 cell count <200 cells/mm3, the corresponding AUC values were as follows: CRP 0.89 (95% CI, 0.84–0.95), suPAR 0.87 (95% CI, 0.81–0.93), IL-6 0.87 (95% CI, 0.8–0.91), and IL-18 0.71 (95% CI, 0.63–0.80). For the remaining markers, the AUC values were <0.7. The ROC curves of MFI are shown in Supplementary Figure 1.
Tuberculosis Discriminatory Potential of C-Reactive Protein and Soluble Urokinase-Type Plasminogen Activator Receptor, Individually and in Combination
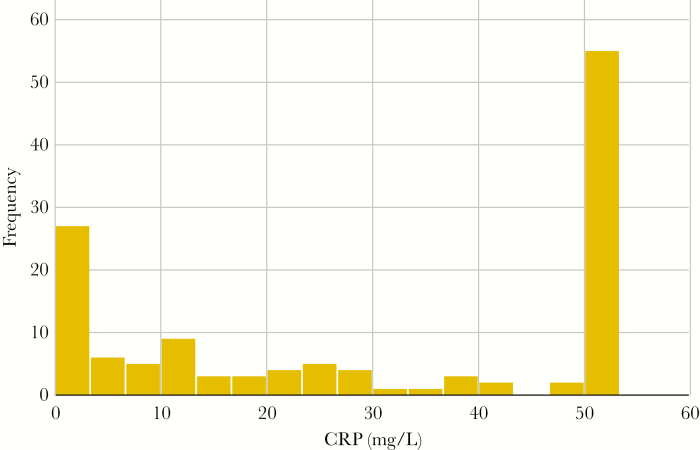
Although CRP had the best discriminatory potential among the markers investigated, 24% of HIV+/TB+ subjects had CRP levels <5 mg/L, and 29% had levels <10 mg/L. Hence, using previously suggested cutoffs of 10 or 5 mg/L in this population would result in a sensitivity of 71% or 76%, respectively. Because a high proportion of HIV+/TB+ subjects in this population had CRP levels <5 mg/L, a cutoff with the requested 90% sensitivity would be as low as 1.2 mg/L. Figure 2 shows the distribution of CRP among HIV+/TB+ subjects. Median CRP levels were low in asymptomatic subjects (1.0 mg/L) and in subjects in whom TB was only detected by liquid culture (smear and Xpert negative; 9.1 mg/L). In logistic stepwise regression analysis, 2 markers, CRP and suPAR, remained independently associated with TB (both P < .001). The AUC of this combination was 0.83 (CI, 0.78–0.88). In subjects with CD4 cell count <200 cells/mm3, the AUC for this combination was 0.93 (CI, 0.87–0.97). For ROC curves of CRP and suPAR alone and in combination, see Figure 3.
Figure 2.
C-reactive protein levels (mg/L) subjects with human immunodeficiency virus and tuberculosis coinfection, note that the highest detectable level in our assay was 50 mg/L.
Figure 3.
(A) Receiver operating characteristics (ROC) curves for C-reactive protein (CRP) (area under the curve [AUC], 0.80; 95% confidence interval [CI], 0.75–0.86), soluble urokinase-type plasminogen activator receptor (suPAR) (AUC, 0.77; 95% CI, 0.71–0.83), interleukin (IL)-6 (AUC, 0.76; 95% CI, 0.71–0.82), and IL-18 (AUC, 0.71; 95% CI, 0.65–0.78). (B) The ROC curve for the combination of CRP and suPAR in all subjects (n = 260; AUC, 0.83; 95% CI, 0.78–0.88). (C) The ROC curve for the combination of CRP and suPAR in subjects with CD4 cell count <200 cells/mm3 (n = 132; AUC, 0.93; 95% CI, 0.89–0.97).
DISCUSSION
In this nested case-control study, based on a large cohort of ART-naive PLHIV subjected to intensified TB case finding, we hypothesized that profiles of inflammatory markers could distinguish individuals with active TB. Indeed, plasma levels of most inflammatory markers investigated were significantly elevated in participants with active TB compared with those without TB, with the most prominent differences found for CRP, suPAR, and IL-6.
A potential confounder for associations with TB in HIV-positive individuals is the degree of immunosuppression, which is linked to the risk of active TB. For this reason, we correlated levels of inflammatory markers to CD4 cell counts. Although most investigated markers showed inverse correlation to CD4 cell count, the relationships to TB coinfection remained significant after adjustment for this factor. Most notably, we observed that the associations with CD4 cell count differed with regard to TB coinfection. Whereas inverse correlation with CD4 cell count was observed for several markers among HIV+/TB+ subjects, only IP-10 and IL-18 showed such an association in HIV+/TB- patients. Both of these markers have previously been associated with HIV disease progression [22, 23]. The finding of lower CD4 cell counts in HIV+/TB+ subjects may be explained by the increasing risk of TB in relation to decreasing CD4 count [24], but this could also be due to mechanisms related to TB disease per se, such as redistribution of CD4 cells [25, 26].
In agreement with previous reports, we found that CRP, suPAR, and IL-6 had the strongest associations with active TB [10–13, 16, 27–29]. Interleukin-6 has been suggested as a potential biomarker for TB [27, 28, 30]. However, in our study, IL-6 did not add discriminative capacity in multivariate analysis, which might be due to covariance with CRP (Spearman’s ρ: 0.7).
Currently, CRP appears to be the most promising biomarker for TB screening [29]. In addition, point-of-care assays for CRP testing are available. Its performance for this purpose has been validated among both TB suspects and PLHIV in cohorts from South Africa and Uganda [10–12, 15, 29]. Our results, showing an overall AUC of 0.80, support the discriminative capacity of CRP for TB identification. In a recent prospective cohort study, Yoon et al [13] found an AUC of 0.81 for a cutoff of 10 mg/L, with 90.2% sensitivity and 69.6% specificity. Although we did not aim to define a cutoff level for TB identification, a threshold value of 10 mg/L had considerably reduced sensitivity in our population. Median CRP levels in HIV+/TB+ subjects in our cohort were lower than those reported by Yoon et al [13] (median CRP for HIV+/TB+ 28 mg/L compared with 52 mg/L, respectively); with 29% having CRP levels <10 mg/L.
In agreement with Lawn et al [10], we observed that CRP levels varied with regard to bacteriological TB results, reflecting bacterial load in sputum. Persons with paucibacillary TB (Xpert negative, culture positive) had lower median CRP levels (9.1 mg/L compared with 50 mg/L in Xpert-positive patients). We have previously reported that HIV+/TB+ individuals with negative Xpert and smear microscopy results had less advanced disease characteristics, and they were often asymptomatic [31]. However, despite ART initiation before establishment of TB diagnosis and delayed initiation of TB therapy, these patients did not have inferior treatment outcomes. Similar observations have been made by other researchers among HIV-positive persons living in TB-endemic settings [32, 33]. It is plausible that these cases represent early active TB, and that the inflammatory response characteristic of HIV/TB coinfection is not fully evolved. Accurate identification of early asymptomatic active TB is challenging by clinical examination as well as radiology and bacteriological methods. Our findings also show low levels of inflammatory markers (including CRP) in the small number of patients with asymptomatic TB (9 of 130), implying that CRP screening may be inadequate in this situation.
Similar to our study, Shapiro et al [15] recently reported inadequate performance for CRP screening using a cutoff of 10 mg/L. However, these investigators found a sensitivity of 90.5% using a cutoff level of 5 mg/L. The corresponding sensitivity for this cutoff in our population was <80%. These results suggest that CRP, although strongly associated with active TB, might not optimally identify all TB+/HIV+ cases as a single biomarker.
An alternative option for TB identification among PLHIV might be the use of combinations of biomarkers. Apart from CRP, suPAR showed strong independent discriminative capacity. Besides associations with increased mortality in several conditions, including both HIV [34, 35] and TB [36], elevated suPAR levels have been associated with TB coinfection in PLHIV [36, 37]. Although both CRP and suPAR individually had discriminative performance in our population, a combination of both these markers showed higher discriminatory capacity than when either marker was used separately. The best performance was found in subjects with severe immunosuppression (CD4 <200 cells/mm3; AUC = 0.93). These results imply a potential for improved identification of TB using a combination of biomarkers, especially in this subset of patients who represent those at highest risk of TB. The combination might be constructed as a composite score; alternatively, the combination could be used as a 2-step process, in which subjects with low CRP levels are further investigated with suPAR. Further studies are required (1) to define optimal cutoff levels and (2) to validate the proposed combination of CRP and suPAR prospectively in different populations before it is implemented in clinical care. Our results support the concept of combining biomarkers for TB identification, and further studies, based on relevant clinical populations, are indicated.
Although other markers investigated did not show adequate discriminatory capacity as biomarkers for TB screening, we did observe significant differences related to TB coinfection. Two of those markers, IP-10, a chemokine secreted after IFN-γ stimulation [38], and IL-18, which promotes IFN-γ secretion by T cells [39], were both associated with active TB and with CD4 cell count. Previous studies have linked levels of IP-10 and IL-18 to accelerated HIV disease progression [22, 23]. Whereas IP-10 has been evaluated as a TB biomarker in both HIV-negative and HIV-positive subjects [30, 40, 41], the association between IL-18 and active TB in PLHIV has not previously been investigated to our knowledge.
Three additional markers (CCL5, PCT, and IL-27) were significantly elevated in HIV+/TB+ subjects, but their performance for TB identification was relatively low. Whereas most studies indicate that TB only leads to slightly elevated PCT [42, 43], raised levels of PCT have been associated with poor prognosis in TB patients [43]. Thus, PCT may serve as a prognostic marker in cases with established TB diagnosis. Although IL-27 may promote differentiation of IFN-γ-producing Th1 cells, it appears to favor bacterial survival in the setting of mycobacterial infection [44], possibly by suppressing T-cell migration into infected tissue [45]. It has been suggested as a biomarker for TB in pleural effusions [46], and it has previously been shown to be elevated in plasma from TB subjects [45, 47].
We observed differences in the distribution of CRP and PCT with regard to gender, with significantly higher median levels in men than women. Similar findings with regard to CRP in TB have been reported in a study by Lawn et al [10], where male gender was associated with CRP >50 mg/L. This phenomenon might reflect later presentation and more advanced TB and HIV disease at inclusion among men. Indeed, the male subjects had higher viral load, lower CD4 cell count, and higher proportion sputum smear and Xpert MTB/RIF-positive results, albeit none of these findings were significant. Chavez et al [48] described different cytokine profiles between males and females with TB. It is possible that different biomarkers are optimal for TB screening in men and women.
A major strength of this study was access to samples from a large prospective cohort of ART-naive PLHIV, in which all subjects had been systematically investigated for active TB. In particular, this allowed for inclusion of HIV-positive controls in whom active TB was excluded at inclusion and who were followed for 6 months without displaying clinical manifestations suggestive of active TB. Hence, we consider the risk of misclassification to be minimal. Furthermore, we explored a range of potential biomarkers for TB, including several that have not previously been investigated for the purpose of TB identification among PLHIV. We also correlated the levels of these biomarkers to CD4 cell counts and HIV-RNA levels, to determine whether the associations with TB were explained by more advanced HIV disease and not directly related to TB coinfection.
Although we assessed a panel of TB-associated immune markers and explored the possibility of combing these, other markers involved in the host response to TB infection might increase the discriminatory capacity. With regard to the laboratory analyses, high interassay variability occurred for some markers, especially suPAR. However, MFI values were consistent between runs, and the results in concentrations correspond well to the MFI results in terms of discriminatory capacity (Supplementary Figure 1). In addition, samples were handled randomly in plates containing both HIV+/TB+ and HIV+/TB− samples, making false-positive findings less likely. We did not aim to define cutoff levels for clinical use in this exploratory study of potential biomarkers. This will be necessary to translate these results into clinical practice.
CONCLUSIONS
We found significant differences in the inflammatory profiles in HIV-positive ART-naive individuals with regard to concomitant active TB. Whereas CRP had the strongest discriminatory potential for TB identification as a single marker, 29% of subjects with bacteriologically confirmed active TB had CRP below the previously proposed cutoff level of 10 mg/L The best discriminative performance was observed for a combination of CRP and suPAR, suggesting a potential for combinations of biomarkers for TB screening among PLHIV.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the patients participating in this study. We also extend our gratitude to the staff at the Adama Regional Laboratory and the investigators at the health centers involved in the study (Adama, Geda, Mojo, Wolenchiti, and Dhera). We acknowledge the Oromia Health Bureau for valuable support and the Adama Lund University research site, led by Mr. Gadisa Merga, for help with this study. In addition, we thank the National Bioinformatics Infrastructure Sweden for support with the statistical analysis and the Department of Clinical Microbiology, Region Skåne, for help with laboratory analyses.
Financial support. Funding for this project was provided by the Swedish Civil Contingency Agency (Grant no. 2010-7551; to P. B.), Young researcher ALF regional grant of the Skåne Region (to S. S.), Doctors against AIDS foundation (to S. S.), Physiographical Society in Lund (to S. S.), the Swedish Research Council (Grant no. 2014-3239; to F. C.), the Foundation of Alfred Österlund (to F. C.), the Foundation of Emil and Wera Cornell (to F. C.), and a private donation to the Faculty of Medicine, Lund University.
Potential conflicts of interest. M. J. received grants from the Swedish Research Council, and P. B. reports grants from the Swedish Civil Contingency Agency and grants from a private donation to Lund University, during the conduct of the study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med 2013; 2013:828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Heath Organization. Global tuberculosis report 2018. Available at: https://www.who.int/tb/publications/global_report/en/ Accessed 31 January 2019. [Google Scholar]
- 3. UNAIDS. Global HIV & AIDS statistics — 2018 fact sheet. Available at: http://www.unaids.org/en/resources/fact-sheet Accessed 31 January 2019. [Google Scholar]
- 4. Cox JA, Lukande RL, Lucas S, et al. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev 2010; 12:183–94. [PubMed] [Google Scholar]
- 5. Raviglione MC, Narain JP, Kochi A. HIV-associated tuberculosis in developing countries: clinical features, diagnosis, and treatment. Bull World Health Organ 1992; 70:515–26. [PMC free article] [PubMed] [Google Scholar]
- 6. Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006; 6:570–81. [DOI] [PubMed] [Google Scholar]
- 7. Piccazzo R, Paparo F, Garlaschi G. Diagnostic accuracy of chest radiography for the diagnosis of tuberculosis (TB) and its role in the detection of latent TB infection: a systematic review. J Rheumatol Suppl 2014; 91:32–40. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. Latent TB Infection: Updated and consolidated guidelines for programmatic management. Available at: http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ Accessed 6 October 2018. [PubMed] [Google Scholar]
- 9. Balcha TT, Skogmar S, Sturegård E, et al. A clinical scoring algorithm for determination of the risk of tuberculosis in HIV-infected adults: a cohort study performed at Ethiopian health centers. Open Forum Infect Dis 2014; 1:ofu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis 2013; 17:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drain PK, Mayeza L, Bartman P, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis 2014; 18:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One 2011; 6:e15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoon C, Semitala FC, Atuhumuza E, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis 2017; 17:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bedell RA, van Lettow M, Meaney C, et al. Predictive value of C-reactive protein for tuberculosis, bloodstream infection or death among HIV-infected individuals with chronic, non-specific symptoms and negative sputum smear microscopy. Trop Med Int Health 2018; 23:254–62. [DOI] [PubMed] [Google Scholar]
- 15. Shapiro AE, Hong T, Govere S, et al. C-reactive protein as a screening test for HIV-associated pulmonary tuberculosis prior to antiretroviral therapy in South Africa. AIDS 2018; 32:1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu J, Wang S, Lu C, et al. Multiple cytokine responses in discriminating between active tuberculosis and latent tuberculosis infection. Tuberculosis (Edinb) 2017; 102:68–75. [DOI] [PubMed] [Google Scholar]
- 17. Wergeland I, Assmus J, Dyrhol-Riise AM. Cytokine patterns in tuberculosis infection; IL-1ra, IL-2 and IP-10 differentiate borderline QuantiFERON-TB samples from uninfected controls. PLoS One 2016; 11:e0163848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamakia R, Kiazyk S, Waruk J, et al. Potential biomarkers associated with discrimination between latent and active pulmonary tuberculosis. Int J Tuberc Lung Dis 2017; 21:278–85. [DOI] [PubMed] [Google Scholar]
- 19. Skogmar S, Schön T, Balcha TT, et al. Plasma levels of neopterin and C-reactive protein (CRP) in tuberculosis (TB) with and without HIV coinfection in relation to CD4 cell count. PLoS One 2015; 10:e0144292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wyndham-Thomas C, Corbière V, Selis E, et al. Immune activation by Mycobacterium tuberculosis in HIV-infected and -uninfected subjects. J Acquir Immune Defic Syndr 2017; 74:103–11. [DOI] [PubMed] [Google Scholar]
- 21. Reepalu A, Balcha TT, Sturegard E, et al. Long-term outcome of antiretroviral treatment in patients with andc without concomitant tuberculosis receiving health center-based care-results from a prospective cohort study. Open Forum Infect Dis 2017; 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liovat AS, Rey-Cuillé MA, Lécuroux C, et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One 2012; 7:e46143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiercinska-Drapalo A, Jaroszewicz J, Flisiak R, Prokopowicz D. Plasma interleukin-18 is associated with viral load and disease progression in HIV-1-infected patients. Microbes Infect 2004; 6:1273–7. [DOI] [PubMed] [Google Scholar]
- 24. Lawn SD, Myer L, Edwards D, et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; 23:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skogmar S, Schön T, Balcha TT, et al. CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS One 2013; 8:e83270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kony SJ, Hane AA, Larouzé B, et al. Tuberculosis-associated severe CD4+ T-lymphocytopenia in HIV-seronegative patients from Dakar. SIDAK Research Group. J Infect 2000; 41:167–71. [DOI] [PubMed] [Google Scholar]
- 27. Anbarasu D, Raja CP, Raja A. Multiplex analysis of cytokines/chemokines as biomarkers that differentiate healthy contacts from tuberculosis patients in high endemic settings. Cytokine 2013; 61:747–54. [DOI] [PubMed] [Google Scholar]
- 28. Phalane KG, Kriel M, Loxton AG, et al. Differential expression of host biomarkers in saliva and serum samples from individuals with suspected pulmonary tuberculosis. Mediators Inflamm 2013; 2013:981984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoon C, Chaisson LH, Patel SM, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2017; 21:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zambuzi FA, Cardoso-Silva PM, Espindola MS, et al. Identification of promising plasma immune biomarkers to differentiate active pulmonary tuberculosis. Cytokine 2016; 88:99–107. [DOI] [PubMed] [Google Scholar]
- 31. Balcha TT, Sturegård E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLoS One 2014; 9:e85478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lawn SD, Kerkhoff AD, Vogt M, et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis 2012; 54:1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oni T, Burke R, Tsekela R, et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax 2011; 66:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirkegaard-Klitbo DM, Langkilde A, Mejer N, et al. Soluble urokinase plasminogen activator receptor is a predictor of incident non-AIDS comorbidity and all-cause mortality in human immunodeficiency virus type 1 infection. J Infect Dis 2017; 216:819–23. [DOI] [PubMed] [Google Scholar]
- 35. Ostrowski SR, Piironen T, Høyer-Hansen G, et al. High plasma levels of intact and cleaved soluble urokinase receptor reflect immune activation and are independent predictors of mortality in HIV-1-infected patients. J Acquir Immune Defic Syndr 2005; 39:23–31. [DOI] [PubMed] [Google Scholar]
- 36. Eugen-Olsen J, Gustafson P, Sidenius N, et al. The serum level of soluble urokinase receptor is elevated in tuberculosis patients and predicts mortality during treatment: a community study from Guinea-Bissau. Int J Tuberc Lung Dis 2002; 6:686–92. [PubMed] [Google Scholar]
- 37. Rudolf F, Wagner AJ, Back FM, et al. Tuberculosis case finding and mortality prediction: added value of the clinical TBscore and biomarker suPAR. Int J Tuberc Lung Dis 2017; 21:67–72. [DOI] [PubMed] [Google Scholar]
- 38. Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 1985; 315:672–6. [DOI] [PubMed] [Google Scholar]
- 39. Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995; 378:88–91. [DOI] [PubMed] [Google Scholar]
- 40. Guo SJ, Jia LQ, Hu QJ, et al. Diagnostic accuracy of interferon gamma-induced protein 10 for tuberculosis: a meta-analysis. Int J Clin Exp Med 2014; 7:93–100. [PMC free article] [PubMed] [Google Scholar]
- 41. Wergeland I, Pullar N, Assmus J, et al. IP-10 differentiates between active and latent tuberculosis irrespective of HIV status and declines during therapy. J Infect 2015; 70:381–91. [DOI] [PubMed] [Google Scholar]
- 42. Ugajin M, Miwa S, Shirai M, et al. Usefulness of serum procalcitonin levels in pulmonary tuberculosis. Eur Respir J 2011; 37:371–5. [DOI] [PubMed] [Google Scholar]
- 43. Rasmussen TA, Søgaard OS, Camara C, et al. Serum procalcitonin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2011; 15:251–6, i. [PubMed] [Google Scholar]
- 44. Abdalla AE, Li Q, Xie L, Xie J. Biology of IL-27 and its role in the host immunity against Mycobacterium tuberculosis. Int J Biol Sci 2015; 11:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torrado E, Fountain JJ, Liao M, et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J Exp Med 2015; 212:1449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Porcel JM. Advances in the diagnosis of tuberculous pleuritis. Ann Transl Med 2016; 4:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao J, Zhang L, Li D, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest 2012; 141:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chavez K, Ravindran R, Dehnad A, Khan IH. Gender biased immune-biomarkers in active tuberculosis and correlation of their profiles to efficacy of therapy. Tuberculosis (Edinb) 2016; 99:17–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.