Abstract
Objectives and methods:
The Furosemide Stress Test (FST) is a novel dynamic assessment of tubular function that has been shown in preliminary studies to predict patients who will progress to advanced stage acute kidney injury, including those who receive renal replacement therapy (RRT). The aim of this study is to investigate if the urinary response to a single intraoperative dose of intravenous furosemide predicts delayed graft function (DGF) in patients undergoing deceased donor kidney transplant.
Results:
On an adjusted multiple logistic regression, a single 100 mg dose of intraoperative furosemide after the anastomosis of the renal vessels (FST) predicted the need for RRT at 2 and 6 h post kidney transplantation (KT). Recipient urinary output was measured at 2 and 6 h post furosemide administration. In receiver-operating characteristic (ROC) analysis, the FST predicted DGF with an area-under-the curve of 0.85 at an optimal urinary output cut-off of <600 mls at 6 h with a sensitivity of and a specificity of 83% and 74%, respectively.
Conclusions:
The FST is a predictor of DGF post kidney transplant and has the potential to identify patients requiring RRT early after KT.
Keywords: Biomarker, delayed graft function, dialysis, kidney transplant, furosemide, stress test, urinary output
Introduction
Delayed graft function (DGF), a clinical syndrome commonly defined as the requirement of dialysis within 7 days of kidney transplantation (KT), is associated with significant morbidity and mortality (Ojo et al. 1997, Perico et al. 2004, Tapiawala et al. 2010). Prompt diagnosis and management of DGF post KT is of the utmost importance as the diagnosis and duration of DGF is associated with; increased in hospital monitoring, increased length of hospital stay, worse long-term allograft function and increased risk of death (Dominguez et al. 2009, Jayaram et al. 2012, Marek et al. 2014) (Giral-Classe et al. 1998, Dominguez et al. 2009, Yarlagadda et al. 2009, Tapiawala et al. 2010). There is a paucity of evidence regarding the optimal timing for initiation of renal replacement therapy (RRT) in DGF and other causes of acute kidney injury (AKI) (Gibney et al. 2008, Karvellas et al. 2011, Vaara et al. 2014, Wald et al. 2015, Gaudry et al. 2016, Zarbock et al. 2016). Early diagnosis of DGF requiring RRT is vitally important as it will improve risk stratification and would help guide physicians on the timing of RRT in this cohort.
Via inhibition of the Na-K-Cl cotransporter (NKCC) in the thick ascending limb of the loop of Henle, loop diuretics inhibit sodium reabsorption, giving rise to natriuresis and an increase in urine flow (Dirks and Seely 1970, Burg et al. 1973, Brater et al. 1979). Based on these properties, furosemide-stimulated increases in urine output (UO) may represent a useful technique to assess the integrity of renal tubular function in the setting of AKI. In preliminary studies, Chawla et al. have standardized this methodology which has been coined the furosemide stress test (FST) (Chawla et al. 2013). Increased UO after the intravenous administration of furosemide (1.0 or 1.5 mg/kg) predicted the progression of early AKI in this study (Chawla et al. 2013). The area under the receiver-operating characteristic curve (AUC) for UO at 2 h post FST to predict progression from AKI network (AKIN) stage 1 to AKIN stage 3 AKI in 77 patients was 0.87 (p = 0.001). The ideal cut-off for predicting progression of AKI during the first 2 h was a urine volume of 200 mL (100 mL/hr.) with a sensitivity and specificity of 87.1% and 84.1%, respectively (Chawla et al. 2013).
The aim of this study was to investigate if the urinary response to a single intraoperative dose of intravenous furosemide predicts DGF in patients undergoing deceased donor kidney transplantation (DDKT).
Methods
This is a single centre retrospective cohort analysis of a random sample of 200 patients who underwent KT from January 2012 to October 2015 at Johns Hopkins Hospital, Baltimore, Maryland. Patients were selected at random from the entire cohort of patients who underwent KT during the time period using a random sequence generator. As standard of care, all patients undergoing KT received an intraoperative single 100 mg dose of intravenous furosemide after the anastomosis of the renal vessels. No other diuretics were administered during KT. In these patients, UO was measured hourly via an indwelling bladder catheter. Inclusion criteria for the study were (1) DDKT, (2) age 18 years, (3) patients who received 100 mg of furosemide intraoperatively. Living kidney recipients were excluded from the study. Donor and recipient clinical variables were obtained from prospectively maintained institutional transplant database and anaesthesia information management system (Metavision) (Motamed and Bourgain 2016). The Institutional Review Board (IRB) at the Johns Hopkins Hospital approved the study (IRB00068004). Preoperative recipient baseline urinary flow rate (UFR) was established from observed urinary output immediately prior to KT. Hourly recipient UO was measured at 2 and 6 h post furosemide administration. Information regarding donor’s history of furosemide use was not available in this study.
Outcomes
Consistent with prior publications, we defined DGF as the receipt of RRT within the first 7 days post transplantation (Yarlagadda et al. 2008, Yarlagadda et al. 2009, Decruyenaere et al. 2016). The primary outcome was the development of DGF within 1 week of DDKT after receiving intraoperative FST. Other secondary outcomes were hypokalaemia and hypotension (within 24 h of furosemide), length of hospital stay, graft loss, rejection, death with graft function and death. Patients were followed for a median follow-up of 1.76 years (interquartile range [IQR] 1.08–2.7 years). Although DGF traditionally is defined as dialysis requirement in the first-week post transplantation, this definition has been criticized for its subjectivity and whether dialysis requirement, especially in the first 24 h post transplantation, truly reflects graft dysfunction or dialysis inadequacy pre-transplantation (Jayaram et al. 2012). For this reason, another secondary outcome was the need for dialysis within 1 week of DDKT excluding those patients who were dialyzed within the first 24 h post transplantation after receiving intraoperative FST.
Statistics
Parametric data were compared using an unpaired t-test. Non-parametric data were compared using the Wilcoxon–Mann–Whitney test. Binomial data were compared using Pearson’s coefficient. Continuous variables are presented as mean (± standard deviation) or median (IQR). The Skewness-Kurtosis test was used to check for distribution of continuous variables. Using logistical regression analysis, we tested the ability of variable UO cut-offs to predict DGF following FST post DDKT: UO at 2 h (50 mL, 100 mL, 150 mL, 200 mL and 300 mL) and 6 h (300 mL, 400 mL, 500 mL, 600 mL, 700 mL and 800 mL). Odds ratios for developing DGF using different values of total UO at 2 h (listed above) and 6 h (listed above) were then adjusted for known predictors of DGF in a multiple logistical regression (MLR) analysis. Known predictors of DGF used in the MLR were donor age, donor race, donor terminal creatinine (Roodnat et al. 2003), cold ischemic time (CIT) (Ojo et al. 1997, Debout et al. 2015), recipient weight and baseline UFR (defined as non-oliguria UO > 400 mL/24 h versus oliguria UO < 400 mL/24 h). Unadjusted and adjusted AUC were calculated for the prediction of DGF at all UO values at 2 h and 6 h. Adjusted AUC that predicted DGF for different values of UO at 2 and 6 h were generated based on the result of the MLR analysis for each UO value. A base model for DGF was initially established. The known predictors of DGF were assessed in a MLR analysis (donor terminal creatinine, donor age, donor race, recipient baseline UFR (oliguria versus non-oliguria), CIT, recipient weight). Using a combination of forward and backward stepwise regression, eliminating predictor variable with p-value >0.2, the base model for DGF was generated. Subsequently, each value of urinary output was evaluated in a MLR model adjusted for the factors established in the base model. A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed using STATA version 13 (College Station, TX, USA).
Results
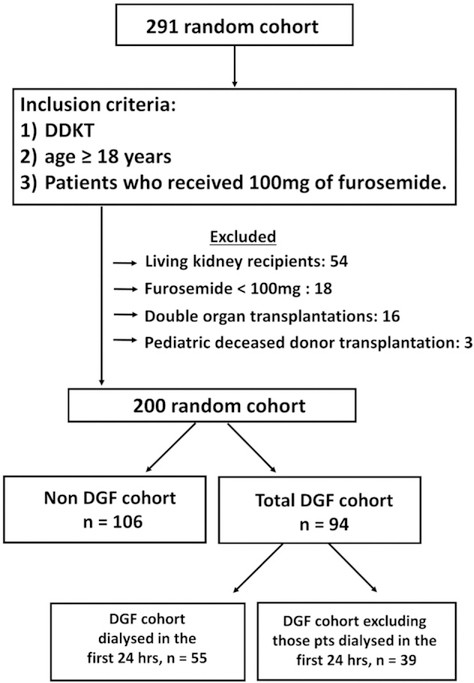
A random cohort of 291 patients who underwent DDKT from January 2012 to October 2015 at Johns Hopkins Hospital was assessed in order to identify 200 patients who fulfilled the inclusion and exclusion criteria. A total of 54 patients were excluded as they were living donor recipients, 18 were excluded due to receiving a dose of furosemide less than 100 mg, 16 were excluded due to dual organ transplantation including kidney and 3 were excluded due to paediatric age (Figure 1). Patient characteristics of DGF and non-DGF cohort are shown in Table 1. The mean age in the entire cohort was 55 years ± standard deviation (SD) 13 years and 71 (36%) were male. Among the entire cohort of patients, 128 (64%) were African-American, 72 (36%) were Caucasian (Table 1). 94 of 200 DDKT patients (48%) developed DGF. Among these patients who developed DGF, 55 (59%) developed DGF within the first 24-h post DDKT. The most common indication for dialysis was hyperkalaemia 68 (72%) followed by volume overload 14 (15%) (Table 1). Patients with or without DGF had similar clinical demographic with regards to recipient age, race, weight, and aetiology of end stage renal disease (ESRD) (Table 1). The mean dose of furosemide per preoperative weight was not significantly different between the DGF group (1.28 mg/kg, SD 0.33) and non-DGF group (1.31 mg/kg, SD 0.34). Similarly, there was no difference in the prevalence of donor age, donor sex and donor hypertension in those who developed DGF and those who did not (Table 1). Patients with DGF had a higher median CIT (30 h (IQR 23–39 h) vs. 25 h (IQR 20–31 h), p < 0.001) than in those patients who did not develop DGF.
Figure 1.
Enrolment of deceased kidney donors and recipients into the study cohort.
Table 1.
Patient characteristics.
| Combined n=200 | Non-DGF cohort n=106 | DGF-cohort n=94 | p-Value | |
|---|---|---|---|---|
| Recipient details | ||||
| Age, years | 55 (±13) | 56 (±13) | 54 (±14) | 0.32 |
| Gender, male (%) | 71 (36) | 46 (43) | 25 (27) | 0.01 |
| Race, n (%) | ||||
| Caucasian, (%) | 72 (36) | 40 (38) | 32 (34) | 0.65 |
| African American, (%) | 128 (64) | 62 (58) | 56 (60) | |
| Weight, kgs | 82 (±20) | 81 (±20) | 83 (±20) | 0.54 |
| Primary aetiology of ESRD | ||||
| Hypertension, n (%) | 59 (30) | 28 (26) | 31 (33) | 0.69 |
| DM, n (%) | 45 (23) | 23 (22) | 22 (23) | |
| Glomerular diseases | 45 (23) | 24 (23) | 21 (22) | |
| Polycystic kidneys | 11 (6) | 7 (7) | 4 (4) | |
| Miscellaneous renal disease | 36 (18) | 22 (21) | 14 (15) | |
| Donor details | ||||
| Age, years | 36 (±16) | 36 (±16) | 36 (±15) | 0.73 |
| Race, Caucasian (%) | 146 (73) | 74 (70) | 72 (77) | 0.55 |
| Gender, male (%) | 74 (37) | 39 (37) | 35 (37) | 0.95 |
| Hypertension (%) | 54 (27) | 30 (28) | 24 (26) | 0.66 |
| Terminal creatinine, mg/dL | 2.0 (0.9–2.0) | 2.0 (0.8–2.0) | 2.0 (1.2–2.1) | 0.08 |
| Clinical data | ||||
| Pre-emptive (%) | 21 (11) | 18 (17) | 3 (3) | 0.001 |
| CDC (%) | 57 (29) | 31 (29) | 26 (28) | 0.67 |
| DCD (%) | 41 (21) | 15 (14) | 26 (28) | 0.03 |
| UFR-Raw, mL/h | 4.5 (0–38) | 9 (0–50) | 0 (0–10) | <0.001 |
| UFR-ABW, mL/kg/h | 0.06 (0–0.4) | 0.11 (0.6 – 1.0) | 0 (0–0.1) | <0.001 |
| CIT, hours | 27 (21–35) | 25 (20–31) | 30 (23–39) | <0.001 |
| Furosemide dose, mg/kg | 1.29 (±0.33) | 1.31 (±0.34) | 1.28 (±0.33) | 0.55 |
| Dialysis first 24h, n (%) | – | – | 55 (59) | – |
| Indication for dialysis within 7 days | ||||
| 1. Hyperkalaemia, n (%) | – | – | 68 (72) | – |
| 2. Volume overload, n (%) | – | – | 14 (15) | – |
| 3. Uraemia, n (%) | – | – | 12 (13) | – |
Continuous variables are presented as mean (± standard deviation) or median (interquartile range) unless otherwise stated. Parametric data were compared with an unpaired t-test. Non-parametric data were compared using the Wilcoxon–Mann–Whitney test. Binomial data were compared using a Persons coefficient. Glomerular diseases include FSGS. membranous nephropathy, Henoch-Schonlein Purpura, Ig A nephropathy, Fibrillary glomerulonephritis, Alports Disease, Wegners granulomatosis. Miscellaneous Renal Disease includes renal cancer including renal cell cancer and myeloma, obstructive uropathy, graft failure and nephrotoxicity. Nephrotoxicity includes analgesic nephropathy, calcineurin toxicity and lithium toxicity. Pre-emptive is defined as, chronic kidney disease stage V who receive kidney transplantation and have not yet started dialysis. CDC: centre for disease control criteria; DCD: donation after cardiac death criteria; DM: Diabetes Mellitus (Type 1 or Type 2); FSGS: Focal Segmental Glomerular Sclerosis; UFR: urinary flow rate; UFR-Raw: baseline UFR prior to transplantation; UFR-ABW: UFR adjusted for body weight; CIT: cold ischemic time; MAP: mean arterial pressure.
Baseline urinary flow rates
Recipient baseline UFR was established from observed urinary output 4–24 h prior to transplantation. Patients who developed DGF had a lower median baseline UFR-raw (0 mL/h, (IQR 0–10 mL/h) compared to those patients who did not develop DGF (9 mL/h, (IQR 0–50 mL/h), p < 0.001) (Table 1). After correcting for actual body weight (UFR-ABW), the difference in UFR-raw between the DGF and non-DGF cohort persisted, 0.0 mL/kg/h (IQR 0–0.1 mL/kg/h) and 0.11 mL/kg/h (IQR 0.6–1.0 mL/kg/h), respectively (Table 1). As a result of this, we adjusted for baseline UFR in response to the FST in our multilogistical regression analysis.
Furosemide stress test characteristics for prediction of DGF
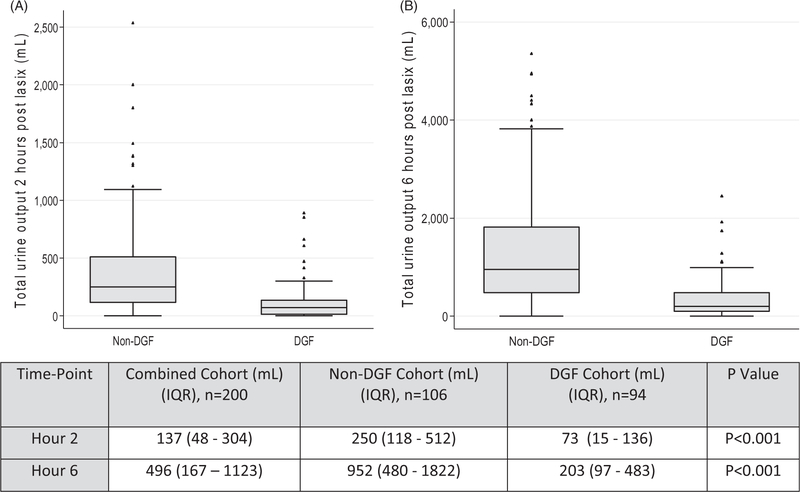
Patients who developed DGF had a median UO of 73 mL (IQR 15–136) at 2 h post FST compared to the non-DGF cohort who had a median UO of 250 mL (IQR 118–512) at 2 h post FST, p < 0.001 (Figure 2). At 6 h post FST, the median UO in the DGF cohort was 203 mL (IQR 97–483) and 952 mL (IQR 480–1822) in the non-DGF cohort, p < 0.001. We tested the sum of the recipient urinary output in the first 2 and 6 h in response to the FST to assess which had the best discriminative capacity for DGF (Table 2). The 2-h UO of <150 mL and 6-h UO of <600 mL after FST was associated with the development of DGF with an AUC of 0.79 and 0.85, respectively (p < 0.001) (Table 2). We assessed the sensitivity and specificity of various 2-h urine volumes and 6-h urine volumes to predict DGF (Table 2). We assessed the capacity of oliguria (defined as daily baseline UFR as less than 400 mL a day) to predict DGF. The ROC AUC for oliguria was 0.62 (p < 0.05). This indicates that the FST offers additional clinical information that cannot be explained by the recipient’s UFR.
Figure 2.
(A) 2 h (B) 6 h urinary output in response to intraoperative furosemide stress test. Box and whisker plots for 2 h and 6 h urinary output in response to the furosemide stress test (FST). Urine volume in mL is shown as median (IQR, interquartile range). p-Value calculated by Wilcoxon rank sign test. n: number of patients; DGF: delayed graft function. Hour 2 is defined as the first 2 h after the FST and is the sum of the urine from the first and second hour after the FST. Hour 6 is the first 6 h after the FST and is the sum of the urine from the first, second, third, fourth, fifth, and sixth hour after the FST.
Table 2.
Furosemide stress test (FST) receiver operation characteristics, sensitivity and specificity for development of DGF at 2 and 6 h. The primary outcome was delayed graft function (DGF) the requirement for dialysis (A) during the first 7 days of transplantation (B) during the first 7 days of transplantation excluding those dialyzed during the first 24 h
| ROC | Sensitivity | Specificity | p-Value | |
|---|---|---|---|---|
| (A) DGF during the first 7 days post transplantation | ||||
| (A) Total urine output over 2 h | ||||
| <50 | 0.73 | 67.0 | 76.4 | 0.06 |
| <100 | 0.78 | 73.4 | 77.4 | <0.001 |
| <150 | 0.79 | 82.0 | 69.0 | <0.001 |
| <200 | 0.76 | 72.3 | 69.8 | <0.001 |
| <300 | 0.79 | 76.0 | 66.0 | <0.001 |
| (B) Total urine output over 6 h | ||||
| <300 | 0.80 | 71.3 | 80.2 | <0.001 |
| <400 | 0.83 | 82.0 | 75.4 | <0.001 |
| <500 | 0.84 | 81.0 | 75.4 | <0.001 |
| <600 | 0.85 | 83.0 | 74.0 | <0.001 |
| <700 | 0.84 | 76.0 | 76.4 | <0.001 |
| <800 | 0.83 | 70.2 | 77.3 | <0.001 |
| Pre-transplant oliguria | 0.62 | 41.5 | 81.9 | <0.05 |
| (B) DGF during the first 7 days post transplantation excluding those dialyzed during the first 24 h | ||||
| (A) Total urine output (mL) over 2 h | ||||
| <50 | 0.73 | 61.5 | 79.2 | <0.001 |
| <100 | 0.78 | 74.4 | 70.8 | <0.001 |
| <150 | 0.79 | 76.9 | 69.0 | <0.001 |
| <200 | 0.76 | 74.4 | 67.0 | <0.001 |
| <300 | 0.79 | 76.9 | 67.0 | <0.001 |
| (B) Total urine output (mL) over 6 h | ||||
| <300 | 0.80 | 71.8 | 78.3 | <0.001 |
| <400 | 0.84 | 84.6 | 75.5 | <0.001 |
| <500 | 0.86 | 84.6 | 76.4 | <0.001 |
| <600 | 0.85 | 87.2 | 74.5 | <0.001 |
| <700 | 0.85 | 79.5 | 76.4 | <0.001 |
| <800 | 0.84 | 76.9 | 77.4 | <0.001 |
Unadjusted and adjusted area under the receiver operator curves (AUC) were calculated for the prediction of DGF at all UO values at 2h and 6 h. Adjusted AUC that predicted DGF for different values of UO at 2 and 6h were generated based on the result of the multiple logistical regression (MLR) analysis for each UO value. Known predictors of DGF used in the MLR analysis were donor age, donor race, donor terminal creatinine, cold ischemic time, recipient weight and baseline urinary flow rate (UFR) (defined as non-oliguria UO > 400 mL/24 h versus oliguria UO < 400 mL/24 h). Hour 2 is the sum of the first 2 h after the furosemide stress test (FST) and is the sum of the urine output from the first and second hour after the FST. Hour 6 is the first 6 h after the FST and is the sum of the urine output from the first, second, third, fourth, fifth, and sixth hour after the FST. Adjusted for terminal creatinine, donor age, donor race, recipient baseline UFR (oliguria versus no oliguria), cold ischemic time, Recipient weight. UFR-raw: pre-furosemide baseline UFR; UFR-ABW: pre-furosemide baseline UFR adjusted for actual body weight. ROC AUC: receiver-operating characteristic AUC. Oliguria is defined as <400 mL/UO in 24 h.
FST characteristics and modified definition of DGF
We repeated MLR analysis excluding all patients dialyzed in the first 24 h and the performance of the FST at 2-h UO of <150 mL was modest with an AUC of 0.79. The 6-h UO of <500 mL in response to the FST performed best and was associated with the development of DGF with an AUC 0.86, p < 0.001 (Table 2). After excluding those patients dialyzed in the first 24 h, the 2-h UO of <150 mL and 6-h UO of <500 mL after FST were associated with the development of DGF with an odds ratio of 8.41 (CI 4.43–15.96, p < 0.001) and 10.22 (CI 5.27–19.82, p = 0.001), respectively.
Short- and long-term outcomes: FST responders and FST non-responders
We assessed short-and long-term transplant-associated out-comes in those that responded to the FST (defined as FST responders: those who produced UO > 600 mL at 6 h post furosemide). FST non-responders were defined as those patients who produced UO < 600 mL at 6 h post furosemide. This cohort included all patients dialyzed within the first week post DDKT including those patients dialyzed in the first 24 h post transplantation. There was a higher rate of DGF in the FST non-responders (72 patients, 77%) compared to the FST responders (22 patients, 23%, p < 0.001) (Table 3). When we excluded those patients dialyzed within the first 24 h post transplantation, the higher rate of DGF persisted in the FST non-responder group (32 patients, 82%) compared to the FST responder group (7 patients, s18%, p < 0.001). The FST was well tolerated with no hypotension (defined as mean arterial pressure (MAP) less than 60 mmHg), mean lowest MAP within the first 24 h post FST of 75.1 mmHg ± SD 15 mmHg in the FST non-responders and 82 mmHg ± SD 16 mmHg, in the FST responders (p < 0.01). Similarly there was no significant difference in the median (IQR) potassium levels in the FST responders and the FST non responders [4.1 (IQR 3.8–4.6) mEq/L vs 4.1 (3.8–4.5) mEq/L, p = 0.98 (Table 3). There was a higher length of hospital stay in the FST non-responders (median stay of 12 days (IQR 9–19)), compared to the FST responders, median stay of 8 days (IQR 6–11), p < 0.001). There was no significant difference in the prevalence of graft loss, death with functional graft death in those patients who were FST non-responders compared to those who were FST responders at a median follow-up of 1.76 years (Table 3).
Table 3.
Clinical outcomes in FST responders and FST non-responders using urine output greater than or less than 600 mL, respectively.
| Outcome | All patients (n=200) | FST Responders* (n= 93) | FST Non-responders** (n= 107) | p-Value |
|---|---|---|---|---|
| DGF (HD within one week post transplantation) | 94 (47%) | 22 (23%) | 72 (77%) | <0.001 |
| Lowest serum potassium first 24h, mEq/L | 4.1 (3.8–4.5) | 4.1 (3.8–4.6) | 4.1 (3.8–4.5) | 0.98 |
| Lowest MAP first 24h, mmHg | 78 (±16) | 82 (±16) | 75 (±15) | 0.006 |
| Graft Loss, n (%) | 9 (5%) | 3 (3%) | 6 (6%) | 0.31 |
| Graft loss due to rejection (acute or chronic) | 4 (2%) | 2 (2%) | 2 (2%) | 1.0 |
| Length of Hospital stay, days | 10 (7–15) | 8 (6–11) | 12 (9–17) | <0.001 |
| Death, n (%) | 7 (4%) | 2 (2%) | 5 (5%) | 0.25 |
| Death with graft function, n (%) | 5 (3%) | 2 (2%) | 3 (3%) | 0.65 |
Continuous variables are presented as mean (± standard deviation) or median (interquartile range) unless otherwise stated. Parametric data were compared with an unpaired t-test. Non-parametric data were compared using the Wilcoxon–Mann–Whitney test. Binomial data were compared using a Persons coefficient.
UO > 600 mL 6 h post transplantation.
UO < 600 mL 6 h post transplantation.
FST: Furosemide stress test; K+: potassium; MAP: mean arterial pressure; HD: dialysis; SE: standard error.
Discussion
In this retrospective single centre study, we assessed the ability of UO after a standardized intraoperative furosemide challenge to predict DGF in patients undergoing DDKT. The performance of the FST to predict DGF in patients undergoing DDKT in this study was robust, with a ROC AUC of 0.85 at 6 h post FST. The FST had an improved ROC AUC for predicting DGF in comparison to patients’ baseline UFR (0.85 vs. 0.62 respectively, p < 0.05). This finding suggests that the FST offers additional clinical information not captured by baseline UFR alone.
Furosemide tightly bound to albumin is actively secreted into the proximal tubule via the human organic anion transporter system before inhibiting luminal cation–chloride cotransport in the thick ascending limb of Henle resulting in natriuresis(Dirks and Seely 1970, Burg et al. 1973, Hasannejad et al. 2004). The physiological rationale for using a furosemide challenge during DDKT is to create a brisk urine flow, a method for assessing renal tubular integrity and evaluating the renal handling of furosemide by the allograft. This line of reasoning has led to the idea that a diminished urinary flow after furosemide administration in the setting of a renal insult such as ischemic injury, may inform clinicians how functionally intact the proximal and distal tubules are in the renal graft. Reduced graft function during DDKT may be due to ischemic damage to the graft before or during harvesting, and is further aggravated by the reperfusion syndrome and may be an ideal method for assessing the FST (Cugini et al. 2005).
The FST has been shown in preliminary studies to predict patients who will progress to advanced stage AKI in the intensive care unit (Chawla et al. 2013). In a pilot study of 77 clinically euvolemic patients with early AKI (AKIN stage 1), the 2-h UO after a standardized high-dose FST had the predictive capacity to identify those patients with severe and progressive AKI (AKIN stage 3) (Chawla et al. 2013). In that study, the ideal cut-off for predicting progressive AKI during these first 2 h was a urine volume of 200 mL (100 mL/h), with a sensitivity and specificity of 87.1% and 84.1%, respectively, and hence the reason for selecting the 2-h urinary output time point post DDKT in our study. However, we also selected the 6-h urinary output time point since some deceased donor kidneys display some degree of allograft stunning very early after allograft revascularization and the 6-h time point might capture this effect. In our study, the cut-off for predicting DGF was most optimal at 6-h post anastomosis of the renal vessels with a UO of greater than 600 mL with a sensitivity, specificity and ROC of 83%, 74% and 0.85, respectively. The ability of the FST to predict DGF persisted even after adjusting for those patients who were dialyzed in the first 24 h after transplantation. The AUC of 0.85 for the prediction of DGF at 6 h is robust and is higher than most urinary biomarkers (especially neutrophil gelatinase-associated lipocalin [NGAL]) in the prediction of DGF post DDKT(Hall et al. 2010, Hollmen et al. 2011, Koo et al. 2016). The 2 h FST did not perform as well as the 6 h FST, with the best ROC curve at 2 h post FST of 0.79 (UO < 150 mL). The improved performance of the FST at 6 h may highlight some degree of allograft stunning that exists very early after allograft revascularization as a result of ischemia injury and can cause a spectrum of injury from a subtle decrease in the expected decline in creatinine to the requirement of dialysis (Johnston et al. 2006).
Renal transplantation is a clinical setting of ischemia-reperfusion injury and acute tubular injury post transplantation that includes many features of AKI. However, several complex discrepancies exist between DGF and AKI including donor factors, perioperative factors and recipient factors. It is challenging to predict which patients will develop allograft dys-function after DDKT especially given the interplay of these complex multifactorial variables. Nonetheless, ischemia reperfusion injury and acute tubular injury both play a significant role in the immediate post-transplant course similar to AKI. Our findings may have important implications for the clinical management of patients who develop kidney insufficiency post DDKT. The FST could provide a “window of opportunity” to identify the potential need for RRT as early as 6 h post KT. Dialysis is not a benign process especially post KT and is affiliated not only with the risks of dialysis catheter placement but also complications associated with the dialytic procedure itself. There is also some evidence that haemodialysis may impair the immune response which could interfere antirejection therapies(Pertosa et al. 2000). Therefore, any test that can predict renal tubular integrity in the allograft as early as 6 h post revascularization could help prepare nephrologists, transplant surgeons and ICU physicians about the impeding need for dialysis. As with other forms of AKI also caused by ischemia reperfusion injury, the lag in diagnosis of DGF with serum creatinine, like in AKI, has greatly hampered efforts to prevent or treat renal injury in human clinical trials (Hall et al. 2010). This information could prompt decisions surrounding use of less nephrotoxic immunosuppressive regimens, removal or placement of RRT access, or timing of dialysis initiation post KT while awaiting later recovery.
To our knowledge, this study is the first to use the FST to predict DGF in patients undergoing DDKT. There is significant variation in clinical practice of use of intraoperative diuretics during or immediately after KT by transplant surgeons (Hanif et al. 2011). In one survey of 40 transplant surgeons from the 18 transplant centres in the UK, 13 surgeons did not use any intraoperative diuretics, 10 used mannitol, 6 used furosemide and 11 surgeons used a combination of both mannitol and furosemide (Hanif et al. 2011). There is a traditional view that diuretics are employed in conjunction with intravenous hydration during KT as a strategy to prevent acute tubular injury (Luciani et al. 1979, Carlier et al. 1982, Karajala et al. 2009). In theory, a good urine flow avoids tubular obstruction, keeps the tubules open and flushes out debris and prevents back leak of glomerular filtrate into the renal interstitium (Tiggeler et al. 1985). The evidence to support the use of intraoperative diuretics during DDKT in order to prevent acute tubular injury is limited and subjective(Tiggeler et al. 1985).
In in vivo rat models of AKI, loop diuretics have been shown to reduce the metabolic requirements and oxygen consumption of renal tubular cells and theoretically protect the renal tubular cells from ischemic injury (Heyman et al. 1994, Heyman et al. 1997). On the contrary, in one systematic review in non-transplant patients, diuretics have been shown to be ineffective in the prevention of AKI or for improving outcomes once AKI is established (Karajala et al. 2009). At our institution, most patients undergoing DDKT receive a one-time dose of furosemide after revascularization, not to counteract intravenous hydration received during transplantation as most patients are maintained euvolemic, but to assess the physiological response of the new allograft to furosemide and hence to risk stratify the need for RRT.
Previous investigators have developed DGF scoring systems for the risk stratification of patients undergoing KT. One such DGF scoring system is called DGFS, from a French multicentre and prospective cohort of 1844 adult recipients of deceased donor kidneys with the highest discriminatory power to predict DGF post DDKT with modest ROC AUC of 0.73 (Chapal et al. 2014). These DGF scores rely on common clinical parameters (such as, donor age, CIT) to predict DGF but fail to use functional markers such as the FST in their model.
Our data demonstrate that the FST in DDKT did not have any significant effect on blood pressure or potassium homeostasis (Table 3). Although clinicians often use furosemide in AKI to facilitate fluid management, furosemide is not without side effects. Patients need to be euvolemic before embarking on the FST and volume replacement is required in patients who are not obviously volume overloaded. Clinical details of volume status were not assessed in this retrospective study. Even though diuretics have been tested for the prevention and treatment of AKI, no clinical trials have shown convincing benefit (Mehta et al. 2002, Karajala et al. 2009, Ho and Power 2010). Loop diuretic use in patients with established AKI typically show a rise in UO without changes in patientcentred clinical outcomes such as need for dialysis, and renal recovery mortality (Shilliday et al. 1997, Cantarovich et al. 2004). One observational study comparing the outcome of renal transplant recipients who received intraoperative diuretics (n = 80) or no diuretics (n = 69) and followed them for over a 1-year period. There was no significant overall difference in 1-year graft survival (94% vs. 94%, p = 0.08) or the rate of DGF (23 vs. 26%, p = 0.07) in patients who received intraoperative diuretics versus no diuretics (Hanif et al. 2011). Although we did not have a cohort who did not receive furosemide, there was no difference in the rate of graft survival or rejection in the FST responders versus the FST nonresponders.
Significant variation in the incidence of DGF occurs across all United States centres with a range of 3.2% to 63.3%, even after adjusting for patient-level and centre-level characteristics (Orandi et al. 2015). The incidence of DGF in our study is higher than that seen in other institutions and may be related to the higher CITs and the willingness to transplant more marginal allografts at our institution (Johnston et al. 2006, Orandi et al. 2015). Patients in our study were monitored for median follow-up period of 1.76 years (IQR 1.08–2.70 years) post-cadaveric transplantation for assessment of graft loss, rejection, death with functional graft or death. There was no difference in the incidence of either of these outcomes between the FST responders or FST non-responders. Consistent with other studies, DGF was associated with increased and the length of hospital stay (Muth et al. 2016).
The study has several limitations. First, there is no control group to measure the amount of urine without receiving furosemide. Baseline UFRs prior to transplantation were taken into consideration and furosemide did not appear to influence the prediction of DGF. All patients at our institution receive intraoperative furosemide during DDKT and we do not have the ability to provide a cohort with the same care who did not receive the FST. This study is therefore bound by many of the limitations of a single-centre retrospective observational study and a larger prospective study of the value of FST during cadaveric KT is warranted in order to fully understand the benefits and drawbacks of this functional test. Furthermore, some data points to early UO not being as predictive as late UO (Lai et al. 2010). Second, there is no consensus in the literature about how to best define DGF (Ojo et al. 1997, Yarlagadda et al. 2008). We chose the need for dialysis in the first week following transplantation as the definition for DGF in this study, this is by far the mostused definition for DGF in the literature (Yarlagadda et al. 2009, Yarlagadda et al. 2008, Decruyenaere et al. 2016). Current means of diagnosing DGF, using serum creatinine and UO-based criteria can take a number of days to confirm and can delay the diagnoses of DGF. A recent study compared 22 different definitions for DGF including dialysis-, serum creatinine-, UO-based criteria or different combinations of these criteria (Decruyenaere et al. 2016). These criteria performed similarly to the definition of the need for dialysis within 7 days post transplantation. Third, we do not have any information on the Kidney Donor Risk Index (KDRI) and the Kidney Donor Profile Index (KDPI). The KDPI formulae were generated to predict the hazard risk for allograft survival over time and was not designed for prediction of DGF and is not necessarily linked to acute tubular injury which is the basis of our study with FST (Tso 2014).
Finally, we did not have any information on whether donors were from the extended donor criteria or whether patients were diuretic naïve or not, this may contribute to the predictive power of the FST. However, the cohort of patients undergoing KT who are diuretic naive is likely to be very small given the degree of advanced kidney failure in this group of patients. The primary strength of this study is the protocolized administration of intravenous furosemide and the subsequent hourly recording of UO in consecutive patients undergoing DDKT at our institution, limiting bias in patient selection for this study.
Conclusions
In conclusion, this study has demonstrated that the FST has the potential to improve early risk stratification for patients who may develop DGF following DDKT. This finding has the potential to guide physicians on the timing of the initiation of dialysis post DDKT. Ultimately, a systematic approach to the risk stratification of patients undergoing cadaveric KT who are considered high risk for development of DGF is needed, this approach may incorporate a combination of risk factors, biomarkers (functional and damage) and the FST to identify patients who are likely to benefit from an early intervention in the setting of DGF (Pianta et al. 2015, McMahon and Koyner 2016, Chapal et al. 2014). Further validation studies of the FST in patients undergoing DDKT are necessary.
Clinical significance.
The furosemide stress test (FST) is a novel test that may predict the need for dialysis post deceased donor kidney transplantation (DDKT).
A furosemide challenge can create a brisk urine flow, a method to evaluate the renal handling of furosemide by the allograft. A diminished urinary flow after furosemide administration in the setting of organ procurement may inform clinicians how functionally intact the tubules are.
We assess if the FST could identify the need for dialysis as early as 2 and 6 h post DDKT and prompt decisions surrounding the early use of less nephrotoxic immunosuppressive regimens and dialysis.
Acknowledgements
The authors declare no acknowledgements and no relevant financial interests.
Footnotes
Disclosure statement
The authors of this manuscript have no conflicts of interest to disclose
References
- Brater DC, Anderson SA, and Strowig S, 1979. Azosemide, a “loop diuretic, and furosemide”. Clinical pharmacology and therapeutics, 25 (4), 435–439. [DOI] [PubMed] [Google Scholar]
- Burg M, et al. , 1973. Furosemide effect on isolated perfused tubules. The American journal of physiology, 225 (1), 119–124. [DOI] [PubMed] [Google Scholar]
- Cantarovich F, et al. , 2004. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. American journal of kidney diseases, 44 (3), 402–409. [PubMed] [Google Scholar]
- Carlier M, et al. , 1982. Maximal hydration during anesthesia increases pulmonary arterial pressures and improves early function of human renal transplants. Transplantation, 34 (4), 201–204. [DOI] [PubMed] [Google Scholar]
- Chapal M, et al. , 2014. A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney international, 86 (6), 1130–1139. [DOI] [PubMed] [Google Scholar]
- Chawla LS, et al. , 2013. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Critical care (London, England), 17 (5), R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini D, et al. , 2005. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney international, 67 (5), 1753–1761. [DOI] [PubMed] [Google Scholar]
- Debout A, et al. , 2015. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney international, 87 (2), 343–349. [DOI] [PubMed] [Google Scholar]
- Decruyenaere P, et al. , 2016. A Single-Center Comparison of 22 Competing Definitions of Delayed Graft Function After Kidney Transplantation. Annals of transplantation, 21, 152–159. [DOI] [PubMed] [Google Scholar]
- Dirks JH, and Seely JF, 1970. Effect of saline infusions and furosemide on the dog distal nephron. The American journal of physiology, 219 (1), 114–121. [DOI] [PubMed] [Google Scholar]
- Dominguez J, et al. , 2009. Duration of delayed graft function is an important predictor of 1-year serum creatinine. Transplantation proceedings, 41 (1), 131–132. [DOI] [PubMed] [Google Scholar]
- Gaudry S, et al. , 2016. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med, 375, 122–133. [DOI] [PubMed] [Google Scholar]
- Gibney N, et al. , 2008. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clinical journal of the American society of nephrology: CJASN, 3 (3), 876–880. [DOI] [PubMed] [Google Scholar]
- Giral-Classe M, et al. , 1998. Delayed graft function of more than six days strongly decreases long-term survival of transplanted kidneys. Kidney international, 54 (3), 972–978. [DOI] [PubMed] [Google Scholar]
- Hall IE, et al. , 2010. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. Journal of the American society of nephrology: JASN, 21 (1), 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif F, et al. , 2011. Outcome of renal transplantation with and without intra-operative diuretics. International journal of surgery (London, England), 9 (6), 460–463. [DOI] [PubMed] [Google Scholar]
- Hasannejad H, et al. , 2004. Interactions of human organic anion transporters with diuretics. The journal of pharmacology and experimental therapeutics, 308 (3), 1021–1029. [DOI] [PubMed] [Google Scholar]
- Heyman SN, et al. , 1997. Renal microcirculation and tissue damage during acute ureteral obstruction in the rat: effect of saline infusion, indomethacin and radiocontrast. Kidney international, 51 (3), 653–663. [DOI] [PubMed] [Google Scholar]
- Heyman SN, et al. , 1994. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney international, 45 (4), 981–985. [DOI] [PubMed] [Google Scholar]
- Ho KM, and Power BM, 2010. Benefits and risks of furosemide in acute kidney injury. Anaesthesia, 65 (3), 283–293. [DOI] [PubMed] [Google Scholar]
- Hollmen ME, et al. , 2011. Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney international, 79 (1), 89–98. [DOI] [PubMed] [Google Scholar]
- Jayaram D, et al. , 2012. Delayed graft function requiring more than one-time dialysis treatment is associated with inferior clinical outcomes. Clinical transplantation, 26 (5), E536–E543. [DOI] [PubMed] [Google Scholar]
- Johnston O, et al. , 2006. Reduced graft function (with or without dialysis) vs immediate graft function–a comparison of long-term renal allograft survival. Nephrology dialysis transplantation, 21 (8), 2270–2274. [DOI] [PubMed] [Google Scholar]
- Karajala V, Mansour W, and Kellum JA, 2009. Diuretics in acute kidney injury. Minerva anestesiologica, 75 (5), 251–257. [PubMed] [Google Scholar]
- Karvellas CJ, et al. , 2011. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Critical care, 15 (1), R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo TY, et al. , 2016. Pre-transplant evaluation of donor urinary biomarkers can predict reduced graft function after deceased donor kidney transplantation. Medicine (Baltimore), 95 (11), e3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Q, et al. , 2010. Early urine output predicts graft survival after kidney transplantation. Transplantation proceedings, 42 (4), 1090–1092. [DOI] [PubMed] [Google Scholar]
- Luciani J, et al. , 1979. Early anuria prevention in human kidney transplantation. Advantage of fluid load under pulmonary arterial pressure monitoring during surgical period. Transplantation, 28 (4), 308–312. [PubMed] [Google Scholar]
- Marek C, et al. , 2014. The prognostic value of time needed on dialysis in patients with delayed graft function. Nephrology dialysis transplantation, 29 (1), 203–208. [DOI] [PubMed] [Google Scholar]
- McMahon BA, and Koyner JL, 2016. Risk stratification for acute kidney injury: are biomarkers enough? Advances in chronic kidney disease, 23 (3), 167–178. [DOI] [PubMed] [Google Scholar]
- Mehta RL, et al. , 2002. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA, 288 (20), 2547–2553. [DOI] [PubMed] [Google Scholar]
- Motamed C, and Bourgain JL, 2016. An anaesthesia information management system as a tool for a quality assurance program: 10years of experience. Anaesthesia critical care & pain medicine, 35, 191–195. [DOI] [PubMed] [Google Scholar]
- Muth BL, et al. , 2016. Outpatient Management of Delayed Graft Function Is Associated With Reduced Length of Stay Without an Increase in Adverse Events. American journal of transplantation, 16 (5), 1604–1611. [DOI] [PubMed] [Google Scholar]
- Ojo AO, et al. , 1997. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation, 63 (7), 968–974. [DOI] [PubMed] [Google Scholar]
- Orandi BJ, et al. , 2015. Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation, 99 (5), 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico N, et al. , 2004. Delayed graft function in kidney transplantation. Lancet (London, England), 364 (9447), 1814–1827. [DOI] [PubMed] [Google Scholar]
- Pertosa G, et al. , 2000. Clinical relevance of cytokine production in hemodialysis. Kidney international supplement, 76, S104–S111. [DOI] [PubMed] [Google Scholar]
- Pianta TJ, et al. , 2015. Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transplant international: official journal of the european society for organ transplantation, 28 (12), 1392–1404. [DOI] [PubMed] [Google Scholar]
- Roodnat JI, et al. , 2003. Ischemia times and donor serum creatinine in relation to renal graft failure. Transplantation, 75 (6), 799–804. [DOI] [PubMed] [Google Scholar]
- Shilliday IR, Quinn KJ, and Allison ME, 1997. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrology dialysis transplantation, 12 (12), 2592–2596. [DOI] [PubMed] [Google Scholar]
- Tapiawala SN, et al. , 2010. Delayed graft function and the risk for death with a functioning graft. Journal of the American society of nephrology: JASN, 21 (1), 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiggeler RG, et al. , 1985. Prevention of acute tubular necrosis in cadaveric kidney transplantation by the combined use of mannitol and moderate hydration. Annals of surgery, 201 (2), 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso PL, 2014. Access to renal transplantation for the elderly in the face of new allocation policy: a review of contemporary perspectives on “older” issues. Transplantation reviews (Orlando), 28 (1), 6–14. [DOI] [PubMed] [Google Scholar]
- Vaara ST, et al. , 2014. Timing of RRT based on the presence of conventional indications. Clinical journal of the American society of nephrology: CJASN, 9 (9), 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald R, et al. , 2015. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney international, 88 (4), 897–904. [DOI] [PubMed] [Google Scholar]
- Yarlagadda SG, et al. , 2009. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrology dialysis transplantation, 24 (3), 1039–1047. [DOI] [PubMed] [Google Scholar]
- Yarlagadda SG, et al. , 2008. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrology, dialysis, transplantation: official publication of the European dialysis and transplant association-European renal association, 23 (9), 2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, et al. , 2016. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA, 315 (20), 2190–2199. [DOI] [PubMed] [Google Scholar]